- Open access
- Published: 27 May 2023

Gastric cancer treatment: recent progress and future perspectives
- Wen-Long Guan 1 , 2 na1 ,
- Ye He 1 , 2 na1 &
- Rui-Hua Xu 1 , 2
Journal of Hematology & Oncology volume 16 , Article number: 57 ( 2023 ) Cite this article
22k Accesses
53 Citations
11 Altmetric
Metrics details
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become the standard treatment. Ongoing investigations are exploring the potential benefits of targeted therapy or immunotherapy in the perioperative or adjuvant setting. For metastatic disease, there have been notable advancements in immunotherapy and biomarker-directed therapies recently. Classification based on molecular biomarkers, such as programmed cell death ligand 1 (PD-L1), microsatellite instability (MSI), and human epidermal growth factor receptor 2 (HER2), provides an opportunity to differentiate patients who may benefit from immunotherapy or targeted therapy. Molecular diagnostic techniques have facilitated the characterization of GC genetic profiles and the identification of new potential molecular targets. This review systematically summarizes the main research progress in systemic treatment for GC, discusses current individualized strategies and presents future perspectives.
Gastric cancer (GC) is the fifth most common malignant tumor and the fourth leading cause of cancer-associated death worldwide [ 1 , 2 ]. The incidence varies geographically across the globe, with the highest incidence in Eastern Asia (Japan and Mongolia) and Eastern Europe, whereas incidence rates in Northern Europe and Northern America are generally low, comparable to African regions [ 2 ]. Notably, the incidence of gastric cancer among young adults (aged < 50 years) in recent years has been progressively rising in both low-risk and high-risk countries. Aside from Helicobacter Pylori infection, the occurrence of GC has been linked to genetic risk factors as well as lifestyle factors, such as alcohol consumption and smoking [ 3 , 4 , 5 , 6 ].
Despite the high incidence of GC, most patients are unfortunately diagnosed at advanced stages with dismal prognoses due to the lack of distinguishing clinical indications [ 7 , 8 ]. Systemic chemotherapy is the mainstay treatment for metastatic GC (mGC), with a median overall survival (OS) of ~ 12 months for patients treated with conventional chemotherapy [ 9 ]. Intratumoral and intertumoral heterogeneity are the prominent features of GC that partly contribute to its poor prognosis. However, histological classifications alone are insufficient to effectively stratify patients for individualized treatment and improve patients’ clinical outcomes [ 10 ]. Therefore, cutting-edge diagnostic techniques and drugs are of fundamental importance for better characterizing molecular profiles and identifying potential novel therapeutic targets for GC patients [ 11 , 12 , 13 ].
Trastuzumab, a monoclonal antibody targeting Human Epidermal Receptor 2 (HER2), was the first approved targeted therapy for GC. However, after the ToGA study, progress in the development of treatments for gastric cancer stalled for nearly a decade [ 14 ]. Emerging advances in immunotherapy, particularly in anti-HER2 therapy, and various biomarker-directed therapies in GC have recently broken this trend. For example, anti-programmed cell death 1 (PD-1) antibodies have demonstrated impressive efficacy and prolonged survival in untreated MSI-H/dMMR mGC patients [ 15 ]. Substantial breakthroughs in the treatment of gastric cancer have been achieved with novel anti-HER2 therapeutic agents, such as T-DXd and disitamab vedotin (RC48) [ 16 ]. In addition, in light of the success of immunotherapy and targeted therapy as first-line treatments for advanced gastric cancer, ongoing research is investigating their potential to advance the treatment of patients with locally advanced stage GC.
The treatment landscape of gastric cancer has evolved significantly in the past few years, with the emergence of new immunotherapy and targeted therapies for patients at various stages of the disease (Fig. 1 ). In this review, we systematically summarize the pivotal clinical trials in GC treatment and provide an update on the management of localized and metastatic gastric cancer. We also discuss the developments in immunotherapy and targeted therapy and highlight current individualized treatments and future perspectives.
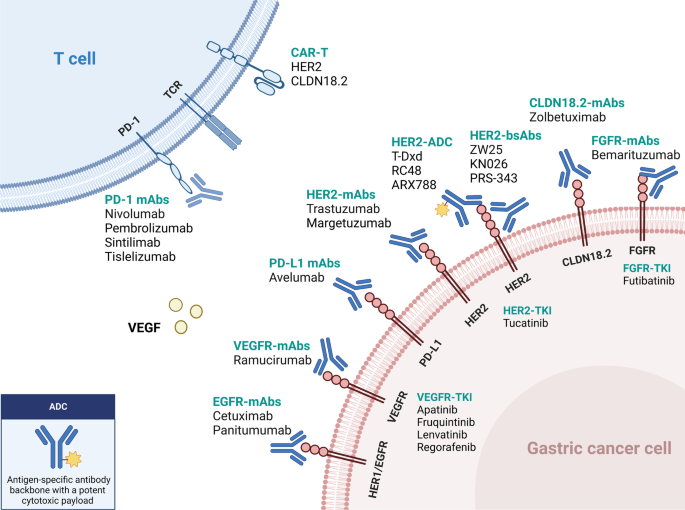
Updated immunotherapy and targeted therapy for gastric cancer. This algorithm provides guidance for selecting currently available immunotherapy and targeted therapy based on different biomarkers
Management for localized GC
Radical surgery is the primary treatment for resectable gastric cancer. Several therapeutic approaches have been established to lower the risk of recurrence and improve long-term survival, including perioperative chemotherapy, adjuvant chemotherapy, and adjuvant chemoradiotherapy (Table 1 ). They are listed as the recommended treatments for resectable localized GC in current guidelines[ 5 , 17 , 18 ]. Further, the addition of targeted therapy and/or immune checkpoint inhibitors (ICIs) is currently being studied in the neoadjuvant/adjuvant setting.
- Perioperative chemotherapy
Perioperative chemotherapy has become the standard treatment for resectable localized GC. Several clinical trials have demonstrated that perioperative chemotherapy could improve the prognosis of patients with resectable GC compared to surgery alone.
The MAGIC trial marked a significant advancement in the field of perioperative chemotherapy for resectable GC. In this phase 3 study, 503 patients were enrolled with resectable gastric, gastroesophageal junction (GEJ), or lower esophageal adenocarcinoma. Patients in the experimental group received three preoperative and three postoperative cycles of epirubicin, cisplatin, and fluorouracil (ECF) [ 19 ]. The results showed that the perioperative ECF regimen could decrease tumor stage and significantly improve progression-free survival (PFS, HR 0.66; 95% CI 0.53–0.81, P < 0.001) and overall survival (OS, HR 0.75; 95% CI 0.60–0.93, P = 0.009). Another phase III trial conducted in 28 French centers compared radical surgery with or without perioperative cisplatin and fluorouracil (CF) chemotherapy and showed that perioperative chemotherapy led to a higher 5-year overall survival rate versus surgery alone (38% versus 24%, respectively; HR 0.69; 95% CI 0.50–0.95, P = 0.02) [ 20 ]. Recently, the randomized phase II/III FLOT4-AIO study compared perioperative FLOT regimen (fluorouracil, leucovorin, oxaliplatin, and docetaxel) with previous standard ECF/ECX (epirubicin, cisplatin, and fluorouracil/capecitabine) regimen in gastric or GEJ cancer patients who had cT2 or higher and nodal positive (cN +) disease [ 21 ]. The results suggested that the FLOT regimen could improve overall survival (50 months versus 35 months), confirming the role of the FLOT regimen as the new standard perioperative treatment for resectable gastric cancer [ 5 , 18 ].
Since most of the clinical trials mentioned above were conducted in western countries, these perioperative regimens (ECF, CF, and FLOT) are less frequently used in Asia. In the phase III PRODIGY trial, 530 Korean patients with cT2-3N + or cT4N any gastric or GEJ cancer were randomly randomized to the neoadjuvant or adjuvant group. Patients in the neoadjuvant arm underwent preoperative DOS (docetaxel, oxaliplatin, and S-1) followed by surgery and S-1 adjuvant chemotherapy, while those in the adjuvant arm received upfront radical surgery followed by S-1 chemotherapy [ 22 ]. The perioperative chemotherapy group had significantly higher rates of R0 resection and pathological complete response (pCR) (95% and 10.4%, respectively). Moreover, PFS was improved in the perioperative arm compared to the adjuvant arm (HR 0.70; 95% CI 0.52–0.95; P = 0.023). The major criticism of this study was that the adjuvant S-1 monotherapy was insufficient for stage III patients, considering another phase III study had demonstrated the superiority of docetaxel plus S-1 to S-1 for 3-year relapse-free survival (RFS) in stage III gastric cancer [ 23 ]. Recently, the phase III RESOLVE trial conducted in China investigated the role of perioperative S-1 plus oxaliplatin (SOX) chemotherapy versus upfront surgery followed by adjuvant chemotherapy [ 24 ]. This study recruited over 1,000 patients with cT4aN + or cT4bN any gastric or GEJ adenocarcinoma, of whom over 60% had gastric cancer. Patients in the intervention group received perioperative SOX (three preoperative cycles and five postoperative cycles followed by three cycles of S-1 monotherapy). The two adjuvant groups received surgery followed by SOX or CAPOX (capecitabine and oxaliplatin) chemotherapy. These results suggested that the perioperative SOX chemotherapy could improve the 3-year disease-free survival (DFS) rate compared to adjuvant CAPOX therapy (59.4% vs. 51.1%, respectively, P = 0.028).
Based on the evidence shown above, perioperative chemotherapy has become the standard treatment in many countries. The FLOT regimen is the most commonly used in Western countries according to the evidence from the FLOT4-AIO study[ 21 ], while the SOX regimen is more recommended in China, based on the results of the RESOLVE study[ 24 ]. However, perioperative chemotherapy is less recommended in Japan, since evidence of the superiority of neoadjuvant chemotherapy is still lacking among Japanese patients[ 25 ].
Adjuvant chemotherapy
Adjuvant chemotherapy is recommended for patients who undergo primary surgery and have stage II or stage III disease due to improvement in survival demonstrated by several clinical trials, particularly in Asian patients. The multi-center phase III CLASSIC trial undertaken in South Korea, China, and Taiwan compared upfront D2 surgery followed by CAPOX adjuvant chemotherapy versus D2 gastrectomy alone in patients with stage II-IIIB gastric cancer [ 26 , 27 ]. Adjuvant CAPOX chemotherapy significantly improved both 5-year DFS (68% vs. 53%; HR 0.58; 95% CI, 0.47 to 0.72; P < 0.0001) and OS (78% vs. 69%; HR 0.66; 95% CI, 0.51 to 0.85; P = 0.0015) compared with surgery alone. Another similar phase III ACTS-GC trial from Japan randomly assigned 1,059 stage II or III GC patients to undergo D2 surgery followed by S-1 monotherapy or D2 surgery alone and showed that adjuvant S-1 monotherapy for one year led to a better 3-year OS than surgery alone (80.1% vs. 70.1%; HR 0.68; 95% CI, 0.52 to 0.87; P = 0.003). The survival benefit persisted after five years of follow-up [ 28 ]. Moreover, the phase III JACCRO GC-07 trial investigated the superiority of adjuvant docetaxel plus S-1 over S-1 monotherapy for pathological stage III gastric cancer [ 23 ]. The addition of docetaxel to S-1 after surgery showed a better 3-year RFS (66% vs. 50%; HR 0.632; 99.99% CI, 0.400 to 0.998; P < 0.001) in the second interim analysis, and the study was terminated as recommended by the independent data and safety monitoring committee. The RESOLVE trial also investigated the non-inferiority of adjuvant SOX chemotherapy compared with the CAPOX regimen. The 3-year DFS was statistically comparable between the two groups (56.5% vs. 51.1%; HR 0.86; 95% CI, 0.68 to 1.07; P = 0.17) [ 24 ]. Based on the results of the phase III trials presented above, several cytotoxic regimens could be used as adjuvant treatments for stage II-III GC after radical surgery, including S-1, CAPOX, SOX, and DS. The choice of regimens depends on many factors, including the pathological staging, patient performance status, and toxicity profile. In general, S-1 monotherapy is more recommended for stage II disease or for patients with poor performance status. Combination therapies such as CAPOX, SOX, or DS are often recommended for pathological stage III disease[ 17 , 25 ].
GC with microsatellite instability-high (MSI-H) or mismatch-repair deficiency (dMMR) is a distinct subtype [ 11 ]. Recently, an individual-patient-data meta-analysis including data from four large phase III studies (CLASSIC, ARTIST, MAGIC, and ITACA-S trial) explored the role of adjuvant chemotherapy in the MSI-H subtype [ 29 ]. It showed that for resectable MSI-H/dMMR GC patients, the prognosis of patients who received surgery alone was better than those who underwent surgery followed by adjuvant chemotherapy, even though the sample size of MSI-H/dMMR in this meta-analysis was very modest (N = 121). Based on this result, adjuvant chemotherapy is not recommended for resectable MSI-H/dMMR GC patients in the latest ESMO guideline [ 5 ]. Additionally, the updated CSCO guidelines suggest that either observation or adjuvant chemotherapy could be considered after a thorough discussion with the patients regarding the possible risks and benefits [ 17 ].
Adjuvant chemoradiotherapy
Unlike chemotherapy, the role of radiotherapy for resectable GC in the adjuvant setting is controversial. Adjuvant chemoradiotherapy (CRT) was once adopted in North America, according to the results of the phase III INT-0116 trial [ 30 ]. In this study, 556 patients with resectable GC or GEJ adenocarcinoma were randomly assigned to the upfront surgery plus adjuvant CRT group or the surgery group. Patients in the experimental arm received adjuvant fluorouracil chemotherapy plus 4500 cGy of radiation (5 × 5). Overall, CRT did prolong the OS compared to surgery alone (36 vs. 27 months, respectively; P = 0.005). However, most patients in this study received D0 or D1 lymphadenectomy and only 10% had D2 lymphadenectomy. The extent of dissection might affect the outcome of the surgery-only group. The phase III ARTIST trial from Korea evaluated the role of postoperative CRT based on the D2 dissection backbone [ 31 ]. Four hundred fifty-eight patients who received D2 lymphadenectomy and R0 resection were enrolled and randomly assigned to the adjuvant chemotherapy arm (capecitabine plus cisplatin, XP) or the adjuvant CRT arm (XP-XRT-XP). Unfortunately, the addition of radiotherapy postoperatively did not improve their DFS ( P = 0.0862). However, in the subgroup analysis, DFS was significantly prolonged in the CRT arm in the patients with lymph node-positive (N +) disease (3-year DFS rate: 77.5% vs.72.3%, HR 0.69, 95% CI 0.474–0.995, P = 0.0365). Based on these findings, the subsequent ARTIST II trial further explored the role of adjuvant CRT in patients with lymph node-positive GC [ 32 ]. Five hundred forty-six patients after D2 dissection were randomly assigned to adjuvant S-1, adjuvant SOX, and adjuvant SOX plus radiotherapy (SOXRT) in a 1:1:1 ratio. However, there was no significant difference in DFS between the adjuvant SOX and SOXRT treatments (3-year DFS rate: 72.8% vs.74.3%; HR 0.97, 95% CI 0.66–1.42, P = 0.879). Therefore, according to current results from these clinical trials, adjuvant CRT is not recommended in patients who received D2 lymphadenectomy and R0 resection.
Novel perioperative therapies
Perioperative targeted therapy.
Anti-HER2 and anti-vascular endothelial growth factor (VEGF) therapies have been recommended as the standard treatments for advanced GC in the first- and second-line setting, respectively. However, the role of targeted therapy in the perioperative or adjuvant setting is still unclear and is currently under investigation.
Anti-HER2 therapy
According to the ToGA study, adding trastuzumab to chemotherapy improved the OS in patients with metastatic HER2-positive GC [ 14 ]. However, the role of anti-HER2 therapy in resectable GC was unclear. In the multicenter phase II HER-FLOT study, patients with HER2-positive esophagogastric adenocarcinoma received perioperative FLOT chemotherapy for four cycles preoperatively and four cycles postoperatively, followed by 9 cycles of trastuzumab monotherapy [ 33 ]. The pCR rate was 21.4%, and the median DFS was 42.5 months. The phase II randomized PETRARCA study investigated the efficacy of adding trastuzumab and pertuzumab to perioperative FLOT chemotherapy in patients with ≥ cT2 or cN + resectable GC [ 34 ]. The pCR rate was significantly improved with trastuzumab and pertuzumab (35% vs. 12%, P = 0.02), and the R0 resection rate and surgical morbidity were comparable between both groups. However, adding targeted therapy to perioperative chemotherapy did not improve DFS or OS and caused more severe adverse events (≥ grade 3), especially diarrhea (41% vs. 5%) and leukopenia (23% vs. 13%). Based on these results, the trial did not proceed to phase III. Another phase II NEOHX study recruited 36 HER2-positive GC patients who received perioperative CAPOX plus trastuzumab treatment, followed by 12 cycles of trastuzumab maintenance therapy [ 35 ]. The pCR rate, 18-month DFS rate, and 5-year OS rate were 9.6%, 71%, and 58%, respectively. The randomized phase II INNOVATION trial assigned patients to 3 groups: perioperative chemotherapy, chemotherapy plus trastuzumab, and chemotherapy plus trastuzumab and pertuzumab [ 36 ]. According to the investigators' choice, the chemotherapy could be FLOT, CAPOX, FOLFOX, or XP. The primary endpoint was major pathological response (MPR) rate, and the result is pending. In general, adding anti-HER2 therapy to chemotherapy showed certain efficacy in the perioperative setting, but the associated survival benefit should be further investigated in a larger randomized trial.
Anti-VEGF therapy
As for anti-VEGF therapy, the randomized, open-label, phase II/III ST03 trial recruited 1,063 resectable esophagogastric adenocarcinoma patients and randomly assigned them to perioperative chemotherapy (ECX) group or perioperative chemotherapy plus bevacizumab group [ 37 ]. The result showed that adding bevacizumab did not improve the 3-year OS (48.1% vs. 50.3% for chemotherapy alone; HR 1.08; 95% CI, 0.91 to 1.29; P = 0.36). Besides, adding bevacizumab was associated with higher rates of postoperative anastomotic leak (24% vs. 10%). Ramucirumab, a VEGF receptor 2 inhibitor, has become one of the standard choices in the second-line treatment of GC [ 5 , 17 , 18 ]. The RAMSES/FLOT7 evaluated the efficacy of adding ramucirumab to perioperative FLOT for resectable GC [ 38 ]. The R0 resection rate in the intervention group was improved compared to the chemotherapy group (96% vs. 82%, P = 0.0093). The median DFS was prolonged in the FLOT plus ramucirumab group (32 months vs. 21 months), while the OS was similar in both groups (46 months vs. 45 months).
Perioperative immunotherapy
Based on several phase III clinical trials, programmed death 1 (PD-1) inhibitors were approved for first- and third-line treatment of unresectable/metastatic GC in different countries [ 5 , 17 , 18 ]. However, the role of ICI in resectable GC remains unclear and is being investigated in various clinical trials. In the randomized phase II DANTE trial, patients with resectable GC were assigned to the experimental arm with the PD-L1 inhibitor atezolizumab plus FLOT chemotherapy and the control arm with standard FLOT chemotherapy [ 39 ]. The R0 resection rate, surgical morbidity and mortality were comparable in both groups. Atezolizumab combined with chemotherapy was associated with tumor downstage and pathological regression, which were more pronounced in patients with a higher PD-L1 combined positive score (CPS).
Several single-arm phase II clinical trials explored the efficacy of perioperative ICIs combined with different treatments (chemotherapy, targeted therapy, or radiotherapy) in resectable GC [ 40 , 41 , 42 , 43 , 44 ]. The pCR rates ranged from 10 to 41%. In the phase III ATTRACTION-5 trial (NCT03006705), the use of nivolumab in the adjuvant setting was investigated. Patients who have undergone D2 surgery will receive either S-1 for one year or CAPOX for six months, with nivolumab added to the adjuvant therapy in the intervention arm. The primary endpoint of the study is relapse-free survival (RFS). The result was announced recently. Unfortunately, the addition of nivolumab did not extend the RFS compared with adjuvant chemotherapy alone. Additionally, the role of pembrolizumab in combination with perioperative chemotherapy for resectable GC is being examined in the phase III clinical trial, KEYNOTE-585 [ 45 ]. The chemotherapy regimens under investigation are XP, FP, or FLOT, and the primary endpoints of the study are OS, event-free survival (EFS), and pCR rate. The potential survival benefits and efficacy of ICI are also being evaluated in the double-blind, randomized phase III MATTERHORN study, which is based on the FLOT backbone [ 46 ]. Patients with resectable GC will receive either perioperative FLOT or FLOT plus durvalumab (a PD-L1 antibody). The primary endpoint of the study is EFS, with secondary endpoints including OS and pCR rate.
For the dMMR/MSI-H subgroup, as discussed above, the value of chemotherapy was controversial. Considering dMMR/MSI-H is a predictive biomarker for immunotherapy in advanced GC, treatment with immune checkpoint inhibitors in the perioperative setting has the potential to improve the response rate and survival. The phase II GERCOR NEONIPIGA study evaluated the response rate and safety of the combination of neoadjuvant nivolumab and low-dose ipilimumab followed by adjuvant nivolumab in patients with dMMR/MSI-H locally advanced G/GEJ adenocarcinoma. Among 29 patients who underwent surgery, 17 (58.6%; 90% CI, 41.8–74.1) achieved pCR[ 47 ]. Similarly, the pCR rate of tremelimumab plus durvalumab was 60% in the neoadjuvant setting (cohort 1) in the phase II INFINITY study[ 48 ]. Based on these encouraging results, it is possible for patients who achieved pCR after neoadjuvant immunotherapy to avoid surgery. Cohort 2 of the INFINITY study has started enrollment to investigate the activity of tremelimumab plus durvalumab as the definitive treatment for dMMR/MSI-H locally advanced GC.
Management for unresectable/metastatic GC
Chemotherapy.
Cytotoxic agents, including fluoropyrimidine, platinum, taxanes and irinotecan, are the main treatment in advanced gastric cancer. Generally, fluoropyrimidine (fluorouracil, capecitabine, and S-1) combined with platinum is used as the backbone therapy in the first line. Oxaliplatin is considered to be as effective as cisplatin. In the phase III SOX-GC trial, the SOX regimen showed improved survival compared to the SP regimen in diffuse or mixed-type GC[ 49 ]. For patients who are not fit for intensive chemotherapy (older age or poor performance status), the phase III GO2 trial showed that a modified dose of two-drug chemotherapy (60% of the full dose) provided a better tolerance but did not compromise the clinical outcome[ 50 ]. Paclitaxel, docetaxel, and irinotecan are commonly used in the second line of chemotherapy. In the ABSOLUTE phase III clinical trial conducted in Japan, weekly use of albumin-bound paclitaxel (nab-paclitaxel) was not inferior to weekly solvent-based paclitaxel in terms of overall survival[ 51 ]. In third-line treatment, trifluridine-tipiracil (TAS-102), an oral cytotoxic agent, has been proven in the phase III TAGS trial to improve overall survival compared with placebo (5.7 vs.3.6 months, HR 0.69, 95% CI 0.56–0.85)[ 52 ].
Immune Checkpoint Inhibitors (ICIs) in unresectable/metastatic GC
Immune checkpoint inhibitors (ICIs) (monotherapy or combined with other treatments) have shown anti-tumor effects across a spectrum of solid tumors, including gastrointestinal tumors. Here, we present an overview of current evidence of ICI treatment in GC (Table 2 ) and discuss different predictive biomarkers for ICIs.
KEYNOTE-062 was the first global, randomized phase III trial to compare the efficacy and safety of immuno-monotherapy (pembrolizumab) or immunotherapy plus chemotherapy versus standard chemotherapy in HER2-negative advanced GC in the first-line setting [ 53 ]. According to the last update in ASCO 2022, it was suggested that pembrolizumab monotherapy was non-inferior to chemotherapy alone (cisplatin and fluorouracil/capecitabine) in patients with PD-L1 CPS ≥ 1 (median OS 10.6 vs. 11.1 months, HR 0.90, 95% CI 0.75–1.08) but was superior in the CPS ≥ 10 population (median OS 17.4 vs. 10.8 months; HR, 0.62; 95% CI, 0.45–0.86) [ 54 ]. However, the combination of pembrolizumab and chemotherapy did not bring OS benefit compared to chemotherapy alone in either CPS ≥ 1 (12.5 vs. 11.1 months; HR, 0.85; 95% CI, 0.71–1.02) or CPS ≥ 10 (12.3 vs. 10.8 months; HR, 0.76; 95% CI, 0.56–1.03) subgroup [ 54 ]. In another double-blind, placebo-controlled phase III KEYNOTE-859 study, the addition of pembrolizumab to chemotherapy (FP or CAPOX) demonstrated slight survival benefit compared with chemotherapy alone (OS 12.9 vs. 11.5 months, HR, 0.78; 95% CI, 0.70–0.87. PFS 6.9 vs. 5.6 months, HR, 0.76; 95% CI, 0.67–0.85). The results were generally consistent in different PD-L1 CPS subgroups[ 55 ].
CheckMate-649 is another global, randomized, phase III trial investigating the effects of ICIs (nivolumab plus ipilimumab, a CTLA-4 inhibitor) or ICI (nivolumab) plus chemotherapy versus chemotherapy (CAPOX or FOLFOX) alone in metastatic HER2-negative GC patients [ 56 ]. One thousand five hundred eighty-one patients were assigned to nivolumab plus chemotherapy arm or chemotherapy arm. The addition of nivolumab to chemotherapy improved the OS (14.4 vs. 11.1 months; HR 0.71; 98.4% CI, 0.59 to 0.86; P < 0.0001) and PFS (7.7 vs. 6.05 months; HR 0.68; 98% CI, 0.56 to 0.81; P < 0.0001) for the patients with PD-L1 CPS ≥ 5; therefore both primary endpoints were met. For all-randomized patients, nivolumab combined with chemotherapy also improved OS (13.8 vs. 11.6 months; HR 0.80; 99.3% CI, 0.68 to 0.94; P = 0.0002). Moreover, all CPS subgroups exhibited an increased objective response rate in the nivo-chemotherapy arm. However, the chemo-free treatment with nivolumab and ipilimumab did not show OS improvement compared to chemotherapy alone [ 57 ]. Based on these findings, nivolumab combined with chemotherapy was listed as one of the recommended first-line treatments in the NCCN, ESMO, and CSCO guidelines [ 5 , 17 , 18 ].
ATTRACTION-04 was a randomized, double-blind, placebo-controlled, multicenter phase II/III trial that evaluated the effects of nivolumab plus chemotherapy (SOX or CAPOX) compared with chemotherapy alone in the first-line treatment for HER2-negative advanced GC in the Asian population, regardless of PD-L1 expression [ 58 ]. The combination therapy significantly improved the PFS (HR 0·68; 98·51% CI 0·51–0·90; P = 0·0007) but not the OS (both groups achieved > 17 months of median OS). One of the possible reasons for the different results of OS between ATTRACTION-04 and CheckMate-649 could be the subsequent anticancer therapies, whereby the proportion of patients who received subsequent anticancer treatments or ICIs therapy was much higher in ATTRACTION-04 (66% vs. 39% in CheckMate-649).
The efficacy of immunotherapy plus chemotherapy was further confirmed in the Asian phase III ORIENT-16 trial, which compared sintilimab plus chemotherapy (CAPOX) to chemotherapy alone as the first-line treatment [ 59 ]. The pre-specified interim result was reported at ESMO 2021. Sintilimab plus chemotherapy showed a survival benefit versus chemotherapy alone in patients with CPS ≥ 5 (18.4 vs. 12.9 months; HR 0.660; 95% CI 0.505–0.864) and all randomized patients (15.2 vs. 12.3 months; HR 0.766; 95% CI 0.626–0.936). Another PD-1 antibody, tislelizumab, is currently being investigated in the phase III RATIONALE-305 trial [ 60 ]. Advanced GC patients are randomized to receive tislelizumab plus chemotherapy (CAPOX/FP regimen) or chemotherapy alone. The primary endpoints are PFS and OS. Results from the interim analysis of the PD-L1 + (i.e., PD-L1 TAP score ≥ 5%) population were represented at 2023 ASCO-GI, showing that tislelizumab plus chemotherapy led to significant OS (17.2 vs. 12.6 months; HR 0·74; 95% CI 0·59–0·94) and PFS (7.2 vs. 5.9 months; HR 0·67; 95% CI 0·55–0·83) improvement compared to chemotherapy alone[ 61 ]. The ITT population outcomes will be reported after the final analysis.
In summary, in first-line treatment for HER2-negative advanced GC, the addition of anti-PD-1 therapy could improve clinical outcomes in patients with high PD-L1 expression, according to the results from CheckMate-649, ORIENT-16, and RATIONALE-305. For patients with low PD-L1 expression or unknown PD-L1 status, the survival benefit of adding PD-1 antibodies is still controversial (discussed below), and the risk–benefit balance of ICIs treatment should be considered, and decisions should be discussed case by case.
The role of maintenance therapy with ICIs after first-line chemotherapy was evaluated in the phase III JAVELIN Gastric 100 trial [ 62 ]. Patients with HER2-negative advanced GC without progression after at least 12 weeks of first-line chemotherapy (oxaliplatin plus fluoropyrimidine) were randomly assigned to avelumab (a PD-L1 inhibitor) maintenance or continued chemotherapy. Avelumab maintenance did not show OS benefit compared to chemotherapy (24-month OS rate: 22.1% versus 15.5%; HR 0.91; 95% CI, 0.74–1.11; P = 0.1779).
Second line and beyond
The randomized, open-label, phase III KEYNOTE-061 trial compared pembrolizumab monotherapy with paclitaxel in patients with advanced GC or GEJ cancer in the second-line setting [ 53 ]. Though the primary endpoints (the OS and PFS in patients with PD-L1 CPS ≥ 1) were not met, it was suggested that the efficacy of pembrolizumab monotherapy was associated with the PD-L1 CPS level. Patients with CPS ≥ 10 had a better outcome in the pembrolizumab group than in the chemotherapy group.
KEYNOTE-059 was a phase II study that explored the effect of pembrolizumab in patients with advanced GC after progression from ≥ 2 lines of treatment [ 63 ]. Among the 259 patients enrolled, the ORR and median duration of response (DoR) was 11.6% and 8.4 months, respectively. Moreover, pembrolizumab showed higher efficacy in the subgroup with PD-L1-positive cancer (CPS ≥ 1) compared to PD-L1-negative cancers (ORR 15.5% vs. 6.4%; DoR 16.3 vs. 6.9 months, respectively). The phase III ATTRACTION-2 study compared nivolumab monotherapy versus placebo in advanced GC patients after two lines of therapy, regardless of the PD-L1 expression [ 64 ], and survival benefit was observed in the nivolumab group (OS 5.3 vs. 4.1 months; HR 0·63, 95% CI 0·51–0·78; P < 0·0001). Based on the results of this study, nivolumab is recommended as monotherapy in third-line treatment for GC in the CSCO guideline but not in the ESMO or NCCN guidelines due to the patients enrolled being exclusively Asian. The role of avelumab in the third-line treatment for advanced GC was investigated in the phase III JAVELIN Gastric 300 trial [ 65 ]. Though avelumab showed a more manageable safety than the physician's choice of chemotherapy, it did not improve OS (primary endpoint, 4.6 vs. 5.0 months; HR 1.1, 95% CI 0·9–1.4; P = 0.81), PFS, or ORR.
Molecular Biomarkers of Immunotherapy in GC
HER2-positive GC, defined as immunohistochemical (IHC) expression 3+ or 2 + combined with positive fluorescent in situ hybridization (FISH) verification, accounts for approximately 15–20% of gastric or gastroesophageal cancer. The phase III ToGA study has established trastuzumab combined with chemotherapy as the standard first-line treatment for HER2-positive advanced GC [ 14 ]. In preclinical models, HER2 signaling could regulate the recruitment and activation of tumor-infiltrating immune cells [ 66 ]. Besides, trastuzumab has been shown to upregulate the expression of PD-1 and PD-L1 [ 67 , 68 ], and anti-PD-1 antibodies could significantly increase the therapeutic activity of HER2 inhibitors [ 69 ]. Several phase I/II studies demonstrated the promising efficacy of the addition of ICIs to trastuzumab and chemotherapy in HER2-positive GC. In the phase Ib Ni-HIGH study conducted in Japan, patients with HER2-positive advanced GC received nivolumab, trastuzumab, and chemotherapy (CAPOX or SOX regimen) in the first-line setting, and the ORR was 75%, as reported at ASCO 2020 [ 70 ]. The multi-institutional phase Ib/II PANTHERA trial explored the efficacy and safety of the combination of pembrolizumab, trastuzumab and chemotherapy as first-line therapy for HER2-positive advanced GC [ 71 ]. The updated data at ASCO-GI 2021 showed that the ORR was 76.7% (CR 16.3%, PR 60.5%), the PFS was 8.6 months (95% CI 7.2–16.5 months), and the OS was 19.3 months (95% CI 16.5-NR). The striking efficacy was also reported in another phase II study, in which patients with HER2-positive GC received pembrolizumab, trastuzumab and chemotherapy (oxaliplatin/cisplatin + capecitabine/5-FU) [ 72 ]. Overall, the ORR was 91% and DCR was 100%. The median PFS and OS was 13·0 months and 27·3 months, respectively, which was much better than the OS reported in the ToGA study. Recently, the randomized, double-blind, placebo-controlled phase III KEYNOTE-811 trial reported the results of its first interim analysis [ 73 ], in which patients with metastatic HER2-positive GC or GEJ cancer received pembrolizumab or placebo plus trastuzumab and chemotherapy. The results showed that adding pembrolizumab to trastuzumab and chemotherapy could markedly increase the ORR (74.4% vs. 51.9%; the estimated difference between the two groups was 22.7%; 95% CI, 11.2–33.7%; P = 0.00006). Based on this result, the FDA approved pembrolizumab combined with trastuzumab and chemotherapy as the first-line treatment for advanced HER2-positive gastric or GEJ adenocarcinoma. The results of the primary endpoints (PFS and OS) are still immature.
MSI-H tumor is one of the four subtypes of GC according to The Cancer Genome Atlas (TCGA) Research Network [ 11 ]. The incidence of MSI-H status in GC was reported to range from 8 to 25%, which was much lower in metastatic disease [ 74 ]. Mismatch repair (MMR) proteins are supposed to fix the errors that occur during DNA replication. When MMR proteins are deficient, the defects of DNA replication will lead to the accumulation of mutations and the expression of neoantigens, which may act as potential targets of immune cells [ 75 ]. Hence, it is reasonable that tumors with MSI-H/dMMR status may attract more immune cell infiltration and enhance the effect of immune checkpoint inhibitors. A post hoc analysis of KEYNOTE-059 (third-line treatment), KEYNOTE-061 (second-line treatment), and KEYNOTE-062 (first-line treatment) was conducted to evaluate the efficacy of pembrolizumab versus chemotherapy in the patients with MSI-H advanced G/GEJ adenocarcinoma [ 15 ]. Overall, 7 of 174 patients (4.0%) in KEYNOTE-059, 27 of 514 (5.3%) in KEYNOTE-061, and 50 of 682 (7.3%) in KEYNOTE-062 with MSI-H status were enrolled. By the time of analysis, the OS of the patients with MSI-H was not reached for pembrolizumab monotherapy in KEYNOTE-059, 061 and 062, or for pembrolizumab combined with chemotherapy in KEYNOTE-062, compared with an OS of around 8 months for chemotherapy alone. Besides, the ORR was much higher in the immunotherapy groups. In another meta-analysis including four phase III trials (KEYNOTE-062, CheckMate-649, JAVELIN Gastric 100, and KEYNOTE-061), 2545 patients with known MSI status were enrolled, and the proportion of MSI-H was 4.8% [ 76 ]. In the MSI-H group, the HR for OS benefit with immunotherapy was 0.34 (95% CI 0.21–0.54), compared to 0.85 (95% CI 0.71–1.00) for the MSS group. Among the patients with MSI-H status, the HR for PFS was 0.57 (95% CI 0.33–0.97; P = 0.04), and the odds ratio (OR) for ORR was 1.76 (95% CI 1.10–2.83; P = 0.02). Altogether, these findings suggested that MSI-H status was a predictive biomarker for immune checkpoint inhibitor treatments, regardless of the line of therapy.
Epstein-Barr virus-associated GC (EBVaGC) is another distinct molecular subtype of the TCGA classification [ 11 ], accounting for about 9% of GC in the TCGA cohort and approximately 5% in China [ 77 , 78 ]. EBV has been linked to CD8 + T cell infiltration and increased expression of PD-L1 and PD-L2 [ 11 , 79 ], making it a potential biomarker for ICI treatment. While a Korean study with a small sample size (n = 6) once reported a 100% response rate in EBV-positive advanced GC [ 80 ], several other studies did not demonstrate a high response rate [ 81 , 82 , 83 ]. Differences in response rates across studies may be attributed to confounding factors such as tumor mutational burden (TMB) and PD-L1 expression. Therefore, the role of EBV positivity in immunotherapy for GC remains unclear and requires further investigation.
As discussed earlier, the level of PL-L1 expression, especially the CPS score, has been considered a predictive biomarker for response to ICIs. However, the reliable cut-off value to predict the benefit of immunotherapy is needed to be determined. The cut-off points often used in clinical trials are 1, 5 and 10. In the KEYNOTE-059 trial, CPS ≥ 1 was used to separate the patients that could benefit from third-line pembrolizumab treatment [ 63 ]. However, this benefit was not seen compared to chemotherapy in the KEYNOTE-061/062 trials [ 53 , 84 ]. In KEYNOTE-061/062, CPS ≥ 10 effectively differentiated the response to pembrolizumab. Patients with CPS ≥ 10 had better OS benefits than those with CPS ≥ 1. A comprehensive analysis of patients with CPS ≥ 10 in KEYNOTE-059, 061 and 062 also showed consistent improvement toward better outcomes with pembrolizumab in different lines of treatment in this subgroup [ 85 ]. In the CheckMate-649 and ORIENT-16 studies, CPS ≥ 5 was used as the cut-off value for the primary endpoint OS. Though the OS benefit of nivolumab plus chemotherapy was also observed in all randomized patients in CheckMate-649, the subgroup analysis suggested that the benefit was insignificant in the CPS < 5 or < 1 group [ 86 ]. A recent study reconstructed unreported Kaplan–Meier plots of PD-L1 CPS subgroups of three phase III trials (CheckMate-649, KEYNOTE-062, and KEYNOTE-590) and investigated the outcome of low CPS subgroup [ 87 ]. The result suggested that patients with low PD-L1 expression (CPS 1–4 and CPS 1–9) did not benefit from adding ICIs to chemotherapy. In summary, although the predictive role of PD-L1 CPS for immunotherapy efficacy has been demonstrated in multiple clinical trials, there is still a need to determine the optimal cut-off value for CPS and to develop further classifications for patients with low CPS scores. Recently, the result of the phase III RATIONALE-305 trial suggested that the TAP score > 5% also had predictive value for ICI treatment in gastric cancer[ 61 ], and further exploration is needed.
Tumor mutation burden (TMB)
It is hypothesized that a high TMB status results in the high expression of neoantigens, which are immunogenic and can induce the response of the immune system and potentially increase the efficacy of ICI treatment. In a phase Ib/II study that explored the efficacy of the PD-1 antibody toripalimab in patients with advanced GC, patients with TMB-high (TMB-H, TMB ≥ 12 mut/Mb) showed a higher ORR and better OS compared with patients with TMB-L status (ORR 33.3% vs. 7.1%, P = 0.017; OS 14.6 vs. 4.0 months, P = 0.038)[ 88 ]. In the subgroup analysis of the KEYNOTE-061 study, the TMB status (≥ 10 or < 10 mut/Mb) was associated with response rate, PFS, and OS in patients treated with pembrolizumab. In the TMB-H subgroup, pembrolizumab demonstrated a better OS compared with paclitaxel, and this benefit remained even when MSI-H patients were excluded[ 89 ]. Though FDA granted approval for the use of pembrolizumab in patients with TMB-H (i.e., TMB ≥ 10 mutations/Mb) advanced solid tumors that progressed after standard treatments, according to the subgroup analysis of KEYNOTE-158 study[ 90 ], the evidence is still not enough for the use of ICIs in TMB-H gastric cancer, and phase III studies to illustrate the predictive value of TMB are needed.
Molecular targeted therapy in unresectable/metastatic GC
Molecular targeted therapy remains an essential treatment option for patients with advanced GC, aimed to inhibit tumor proliferation and increase survival rates. Targeted therapies, including anti-HER2, anti-angiogenesis, and other biomarker-directed therapies, have demonstrated promising efficacy in treating GC, with significant benefits observed in biomarker-enriched patients (Table 3 ). Therefore, next-generation sequencing or ctDNA detection is crucial for mGC patients to establish a comprehensive molecular profile, including the status of HER2, fibroblast growth factor receptor (FGFR), Claudin18.2 (CLDN18.2), PD-L1 and EGFR.
HER2, also known as ERBB2, is a member of the ERBB protein families that includes the epidermal growth factor receptor (EGFR or HER1), HER3, and HER4 [ 91 ]. HER2 overexpression or amplification has been found in a range of 7.3% to 20.2% in advanced gastric and gastroesophageal junction adenocarcinomas, with the overexpression rate varying globally [ 92 ]. In addition, intestinal-type gastric cancers and those arising from the proximal stomach or gastroesophageal junction are more likely to exhibit HER2 positivity. [ 11 , 93 ].
Trastuzumab is a humanized monoclonal antibody that targets HER2 extracellular domain 4, then inhibits downstream signal activation and cancer cell proliferation. Trastuzumab plus chemotherapy has been established as the standard first-line treatment for HER2-positive advanced GC. The landmark ToGA trial revealed that trastuzumab plus chemotherapy significantly improved the overall survival of patients with advanced GC [ 14 ], especially for patients with HER2 positivity, who were identified as having HER2 immunohistochemistry (IHC) scores of 2 + and fluorescence in situ hybridization (FISH)-positive or HER2 IHC 3 + based on a post-hoc exploratory analysis [ 92 ]. The EVIDENCE trial has demonstrated that combining first-line trastuzumab with chemotherapy was associated with improved clinical outcomes in Chinese patients with HER2-positive metastatic GC, providing real-world evidence. [ 94 ].
However, subsequent attempts of HER2-targeted therapy in advanced GC were not as successful as expected. Even though pertuzumab [ 95 , 96 ], trastuzumab emtansine (T-DM1) [ 97 ], and lapatinib [ 98 , 99 ] were all investigated in several first-line and second-line trials, no survival improvement was observed in any of these trials. Additionally, trastuzumab beyond progression also failed to show a survival benefit in pre-treated HER2-positive GC patients in the T-ACT trial [ 100 ].
Potential resistance mechanisms of HER2-targeted therapy
Primary or acquired resistance is a major impediment to the management of mGC patients, while mechanisms underlying the poor efficacy of HER2-directed therapy in GC are not fully understood. Multiple potential resistance mechanisms have been researched, as listed below, and further studies are warranted to improve treatment resistance in GC patients treated with HER2-targeted therapy in clinical settings.
HER2 heterogeneity
Intratumoral HER2 heterogeneity is observed in 23% to 79% of GC patients and is associated with patients’ survival [ 101 , 102 , 103 ]. Specifically, Shusuke et al. reported prolonged survival in homo-HER2 positive GC patients, defined as all tumor cells overexpressing HER2 in biopsy specimens [ 101 ]. Tumor cells with HER2 overexpression or amplification are killed during HER2-targeted therapy, while residual drug-resistant colonies keep proliferating and eventually take control, leading to tumor recurrence. As a result, resistance to HER2-targeted therapy has been associated with the heterogeneity of HER2 expression [ 101 , 104 , 105 ]. Discordance between next-generation sequencing and FISH/IHC may also indicate intratumoral heterogeneity and result in an unfavorable treatment outcome. In addition, there still exist discrepancies in HER2 status between primary tumor and metastatic sites, which increases the risk of HER2-targeted therapy failure due to false-positive HER2 detection [ 106 , 107 ].
Loss of HER2 expression
For mGC patients experiencing progression on trastuzumab, 29–69% of them may experience loss of HER2 expression, which is an important factor responsible for resistance [ 108 , 109 , 110 ]. Given the risk of HER2 expression loss during treatment, patients should re-evaluate HER2 status upon progression after anti-HER2 therapy to determine the most optimal treatment.
Gene amplification
Receptor tyrosine kinase (RTK) amplification was commonly detected in MET-amplified metastatic GC, with 40% to 50% of cases exhibiting co-amplification of either HER2 or EGFR. These patients did not usually respond to HER2-targeted therapy, but MET and HER2 combination inhibition could sometimes bring extra clinical benefit [ 111 ]. CCNE1, which encodes the cell cycle regulator cyclin E1, is another oncogene co-amplified with HER2 in metastatic GC. CCNE1 co-amplification has been found to be more strongly related to HER2-positive AGC than to HER2-positive breast cancer [ 112 ]. In a phase II study of lapatinib with capecitabine and oxaliplatin in HER2-positive AGC patients, CCNE1 amplification was demonstrated to play a role in resistance to HER2-targeted therapy [ 113 ]. A high level of copy number variation for CCNE1 has also been associated with worse survival in patients with HER2-positive metastatic GC treated with trastuzumab [ 114 ]. Other studies have also reported that deletion of ErbB2 16 exon and co-mutation and/or amplification of KRAS, HER3, EGFR, PI3K or PTEN could contribute to the resistance of anti-HER2 therapy [ 109 , 113 , 115 , 116 ].
Alterations in intracellular signaling
HER2-targeted therapy suppresses downstream signaling pathways by blocking the binding of HER2 receptors and ligands, which inhibits the migration and proliferation of tumor cells and leads to apoptosis. RTK/RAS/PI3K signaling alterations have been shown to be involved in the development of resistance to trastuzumab. [ 109 ]. Furthermore, activation of the bypass pathway might also result in resistance. Sampera et al. discovered that SRC-mediated persistent activation of the MAPK-ERK and PI3K-mTOR pathways was connected to the treatment resistance in HER2-positive GC cell lines [ 117 ]. NRF2 has also been associated with HER2 resistance by activating the PI3K-mTOR signaling pathway [ 118 ].
Newer HER2-targeted agents
To overcome intrinsic and acquired resistance to trastuzumab, various clinical trials have explored newer agents and combinations. The following innovative HER2-targeted agents for advanced metastatic GC are currently under investigation (Table 4 ): monoclonal antibodies (mAbs) (e.g., margetuximab), bispecific antibodies (BsAbs) (e.g., ZW25, KN026), antibody–drug conjugates (ADCs) (e.g., T-DXd, Disitamab vedotin, ARX788), tyrosine kinase inhibitors (TKIs) (e.g., tucatinib), and other novel therapeutic approaches.
Monoclonal antibodies
Margetuximab
Margetuximab is an Fc-engineered anti-HER2 mAb that targets the same epitope as trastuzumab but with a higher affinity for single-nucleotide polymorphisms of the activating Fc receptor (CD16A) [ 119 , 120 ]. Margetuximab can recruit CD16A-expressing natural killer cells, macrophages and monocytes and further promote antibody-dependent cell-mediated cytotoxicity (ADCC) [ 119 ]. The first phase I study of margetuximab in humans illustrated that margetuximab was well-tolerated with promising efficacy in relapsed HER2-overexpressing carcinoma [ 121 ]. Later in the phase Ib/II CP-MGAH22-05 study, patients with previously treated HER2-positive GC responded effectively to a chemotherapy-free treatment consisting of margetuximab plus pembrolizumab. Patients with HER2 IHC3 + and PD-L1 positive (CPS ≥ 1, by IHC) had an ORR of 44% and a DCR of 72% [ 122 ]. More recently, the phase II/III MAHOGANY trial has reported the efficacy of margetuximab plus anti-PD-1 antibody retifanlimab (Cohort A) for the first-line treatment of patients with G/GEJ adenocarcinoma, with an ORR and a DCR of 53% and 73% [ 123 ]. The ORR reported in this trial was superior to the ORR observed with other history chemotherapy-free treatments; nonetheless, given that chemotherapy-based regimens remain the predominant treatment for GC, the MAHOGANY trial has been halted for commercial reasons.
Bispecific antibodies (BsAbs)
Zanidatamab (ZW25)
Zanidatamab (ZW25) is a novel HER2-targeted bispecific antibody that binds to HER2 extracellular domain (ECD) II and IV. According to a phase I study, ZW25 was well tolerated with durable response in heavily pretreated GEA patients (including prior HER2-targeted therapy) [ 86 ]. Later in a phase II trial involving patients with advanced/metastatic HER2-positive GEA, zanidatamab plus chemotherapy (CAPOX or FP) showed a confirmed ORR of 75%, mDOR of 16.4 months and mPFS of 12.0 months in the first-line setting [ 124 ]. Based on these findings, a global phase III study (HERIZON-GEA-01) has been designed to assess the efficacy and safety profiles of zanidatamab plus chemotherapy with or without tislelizumab versus standard of care (trastuzumab plus chemotherapy) for patients with metastatic HER2-positive GEAs in first-line settings [ 125 ].
KN026 mimics the dual effects of trastuzumab and pertuzumab by simultaneously binding to HER2 ECD II and IV [ 126 ]. In a phase II clinical study, KN026 showed favorable results in patients with HER2-overexpressing G/GEJ adenocarcinoma (IHC3 + or IHC 2 + ISH +) with an ORR of 56% [ 127 ]. The ongoing phase II/III trial (KN026-001) is planned to evaluate the survival benefit of KN026 plus chemotherapy in patients with HER2-positive unresectable or advanced G/GEJ adenocarcinoma upon progression after trastuzumab-containing treatment (NCT05427383). Most recently, the preliminary data presented at ESMO 2022 illustrated that KN026 plus KN046, a recombinant humanized PD-L1/CTLA-4 bispecific antibody, had remarkable efficacy and tolerable safety in HER2-positive G/GEJ patients without prior systemic treatment [ 128 ]. In this phase II study, the ORR was 77.8%, and the DCR was 92.6%, indicating the need for a future randomized clinical trial to confirm the efficacy of KN026 plus KN046 treatment versus standard of care.
Other BsAbs
PRS-343 is a BsAb that targets HER2 and the costimulatory immunoreceptor 4-1BB on immune cells. In patients with advanced HER2-positive solid tumors, including GC, PRS-343 showed anticancer efficacy both alone and in combination with the anti-PD-L1 antibody atezolizumab in a phase I clinical study [ 129 ]. A phase II study (NCT05190445) is ongoing to investigate the efficacy of PRS-343 in combination with ramucirumab and paclitaxel in patients who have already received treatment for HER2-high (IHC 3+ or IHC 2+ with HER2/neu gene amplification) G/GEJ adenocarcinoma and in combination with tucatinib in HER2-low (IHC 1+ or IHC 2+ without HER2/neu gene amplification) G/GEJ adenocarcinoma.
Antibody–drug conjugates (ADCs)
Trastuzumab deruxtecan (T-DXd)
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate (ADC) composed of an anti-HER2 antibody connected to a cytotoxic topoisomerase I inhibitor via a cleavable tetrapeptide-based linker [ 130 ]. Different from T-DM1, T-DXd has a bystander effect on nearby cells, including those not expressing HER2, thus greatly enhancing the antitumor effect [ 131 ]. This action method is inspiring, particularly for advanced GC patients with diverse intratumoral HER2 expression. In the Asia DESTINY-Gastric01 trial, T-DXd significantly improved overall survival in patients with HER2 + advanced GC compared with chemotherapy in the later-line settings [ 132 ]. Interestingly, the efficacy and safety of T-DXd were also evaluated in exploratory cohorts of patients with HER2-low G/GEJ cancers in the DESTINY-Gastric01 trial (cohort 1, IHC 2 + /ISH–; cohort 2, IHC 1 +). The confirmed ORR was 26.3% in Cohort 1 and 9.5% in Cohort 2. The median OS was 7.8 months in cohort 1 and 8.5 months in cohort 2[ 133 ]. These results provide initial evidence that T-DXd has clinical benefits in patients with heavily pretreated HER2-low G/GEJ cancers.
Similarly, T-Dxd in the DESTINY-Gastric02 trial also achieved encouraging results in 2L western GC patients with a cORR of 41.8% and a median PFS of 5.6 months [ 134 ]. Other trials, such as phase III 2L DESTINY-Gastric04 and phase III 1L DESTINY-Gastric03, are also in progress (NCT04379596, NCT04704934).
Disitamab vedotin (RC48)
Disitamab vedotin (RC48) is a novel HER2-ADC drug independently developed in China, which is composed of three parts: anti-HER2 extracellular domain antibody, MC-Val-Cit-PAB linker, and cytotoxin monomethyl auristatin E (MMAE) [ 135 ]. This novel antibody has a stronger affinity to HER2 than the standard of care. Unlike T-DM1, disitamab vedotin has a bypass-killing effect on nearby tumor cells regardless of HER2 status, which could help overcome spatial heterogeneity and enhance anti-tumor effects. RC48 was well tolerated and showed promising antitumor activity in patients with HER2-positive advanced GC in a phase I trial [ 136 ]. The phase II RC48-C008 trial revealed a significant benefit of RC48 with HER2-overexpressing GC patients who had undergone at least two prior lines of therapy, in which the ORR was 24.8%, mPFS was 4.1 months and mOS was 7.9 months [ 137 ]. Of note, the ORR of RC48 in patients with HER2 IHC2 + /FISH- was 16.7%, slightly lower than in HER2-positive patients. These findings indicated that RC48 exerted considerable anti-tumor effectiveness and tolerable safety in patients with HER2-positive GC, as well as in those with HER2 low expression GC. In June 2021, disitamab vedotin was approved in China for the treatment of patients with HER2-overexpressing advanced or metastatic G/GEJ adenocarcinoma who received at least two systemic chemotherapy regimens. The ongoing phase III RC48-C007 (NCT04714190) trial aims to evaluate the efficacy and safety of RC48 as a third-line treatment and beyond in patients with advanced HER2-positive GC.
ARX788 is another investigational anti-HER2 antibody–drug conjugate consisting of HER2-targeted monoclonal antibody (mAb) coupled with a highly effective tubulin inhibitor (AS269). ARX788 was well tolerated and had a promising anti-tumor effect in HER2-positive GC patients previously treated with trastuzumab-based regimens in a phase I multicenter dosage expansion trial [ 138 ]. The ORR was confirmed to be 37.9%, and the DCR was 55.2%. With a median follow-up period of 10 months, the mPFS and OS were 4.1 and 10.7 months, respectively. On March 18, 2021, the FDA granted ARX788 as an orphan drug for treating HER2-positive GC. A randomized controlled, multicenter, open-label phase II/III study is underway to assess the efficacy of ARX788 as second-line treatment for HER2-positive advanced G/GEJ adenocarcinoma (Chinadrugtrials.org.cn: CTR20211583).
Tyrosine kinase inhibitors
Tucatinib, a highly selective HER2-directed tyrosine kinase inhibitor (TKI), was approved by FDA for HER2-positive metastatic breast cancer in 2020 and is under exploration in GC. In preclinical studies, tucatinib plus trastuzumab demonstrated superior activity compared to a single agent in GEC xenograft models [ 139 ]. Recently, the phase II/III MOUNTAINEER-02 (NCT04499924) was initiated to evaluate the efficacy of tucatinib, trastuzumab combined with ramucirumab, and paclitaxel in previously treated HER2 + advanced G/GEJ adenocarcinoma [ 140 ].
Other novel therapeutic approaches are being under investigation, including anti-HER2 CAR-T-cell therapy (NCT04511871, NCT04650451), CAR-natural killer cell (NK) therapy [ 141 ], and CAR-macrophage (CAR-M) therapy (NCT04660929), B-cell and monocyte-based immunotherapeutic vaccines (BVAC-B), BAY2701439 and CAM-H2 targeted HER2 radiotherapy (NCT04147819, NCT04467515). These widespread attempts at HER2-targeted CAR cell therapy in solid tumors may hopefully lead to the development of new drug candidates in patients with HER2-positive GC.
Antiangiogenic therapy
Blocking angiogenesis is a key strategy in GC therapy, including anti-VEGF monoclonal antibodies, VEGF-binding proteins, and VEGF receptor TKIs (Table 5 ) [ 142 ]. Ramucirumab, a typical antiangiogenic monoclonal antibody, targets VEGFR-2 and is approved by the FDA for treating advanced GC [ 143 ]. In the second-line REGARD trial, ramucirumab demonstrated significant improvement in patient OS and PFS versus best supportive care in metastatic GC [ 144 ]. In the RAINBOW trial, when coupled with paclitaxel, ramucirumab significantly prolonged overall survival compared to paclitaxel alone [ 145 ]. Similarly, results from RAINBOW-Asia bridging study also supported the application of ramucirumab plus paclitaxel as second-line therapy in a predominantly Chinese population with advanced gastric or GEJ adenocarcinoma [ 146 ]. However, neither ramucirumab nor bevacizumab brought extra survival benefits when added to platinum or fluoropyrimidine chemotherapy in GC patients in the first-line settings [ 147 , 148 ].
Regorafenib is an oral multi-kinase inhibitor targeting angiogenic, stromal and oncogenic receptor tyrosine kinases (RTK). Results from a phase III trial (INTEGRATE IIa) presented at ASCO GI 2023 demonstrated that regorafenib significantly improved OS (4.5 months vs. 4.0 months; HR = 0.52; P = 0.011) in patients with advanced gastro-oesophageal cancer (AGOC) in later-line settings [ 149 ]. Meanwhile, other studies exploring the efficacy of anti-VEGF and anti-PD1 combination in GC populations are also under investigation. The combination of regorafenib and nivolumab had a manageable safety profile and effective antitumor activity in a phase I trial for the GC subgroup [ 150 ]. INTEGRATE IIb ((NCT0487936)), an international randomized phase 3 trial, is ongoing to compare regorafenib plus nivolumab to standard chemotherapy in pre-treated patients with AGOC. Besides, lenvatinib plus pembrolizumab showed promising anti-tumor activity with an ORR of 69% in the first-line and second-line treatment of advanced GC [ 151 ].
Apatinib is a small molecule VEGFR inhibitor with China Food and Drug Administration (CFDA) approval for the treatment of advanced or metastatic chemotherapy-refractory GC. Apatinib improved median PFS and OS versus placebo in Chinese patients with advanced gastric or gastroesophageal junction adenocarcinoma in the third line and beyond[ 152 ]. Most of the patients in this trial did not receive prior antiangiogenic therapies since they were not standard treatments in China at that time, so clinical evidence is still lacking for the use of apatinib in patients who previously received ramucirumab. Unfortunately, no significant improvements were observed in overall survival (OS) in western populations in the phase III ANGEL clinical trial [ 153 ].
Fruquintinib is a highly selective VEGFR family kinase inhibitor that targets VEGFR1, 2 and 3 and is independently developed in China. Fruquintinib was approved in China by the NMPA in September 2018 and commercially launched in late November 2018 as a third-line treatment for patients with metastatic colorectal cancer. In a phase Ib/II study, adding fruquintinib to paclitaxel as second-line treatment for mGC patients at recommended phase 2 dose (RP2D) showed an mPFS of 4 months and mOS of 8.5 months. In the 4 mg dose cohort of 27 patients with evaluable tumor response, the ORR was 25.9% and the DCR was 66.7%[ 154 ]. A randomized phase III FRUTIGA study has investigated fruquintinib plus paclitaxel versus paclitaxel alone in patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma who had progressed after first-line standard chemotherapy (NCT03223376). Initial results from FRUTIGA showed that fruquintinib combined with paclitaxel showed significant improvements in PFS, ORR and DCR. Full detailed results are still being analyzed and will be revealed soon.
Other biomarker-targeted therapy
Novel diagnostic techniques have contributed to characterizing the genetic profile of GC and identifying new potential molecular targets. Recently, researchers have looked into Claudin-18.2-targeted therapy, fibroblast growth receptor (FGFR) pathway inhibitors, and EGFR inhibitors as effective targeted therapies to treat advanced GC (Table 5 ). Although emerging innovative drugs have made remarkable progress in GC treatments, extensive clinical explorations are needed to advance precision medicine.
CLAUDIN 18.2-targeted therapy
Claudin 18.2 (CLDN18.2), a component of intercellular junctions [ 155 ], is exclusively detected in gastric mucosa and absent from other healthy tissues. Upon malignant transformation, CLDN18.2 expression can be retained in various tumor tissues, including G/GEJ cancer and especially diffuse-type GC [ 156 ]. The prevalence of CLDN18.2 overexpression in GC varies wildly among studies ranging from 14.1% to 72% [ 157 , 158 , 159 ].
Zolbetuximab is a chimeric IgG1 monoclonal antibody that binds to CLDN18.2 and induces antibody-dependent and complement-dependent cytotoxicity [ 160 ]. To date, zolbetuximab has shown great potential to become a valuable target in GC. In the phase II MONO study, single-agent zolbetuximab achieved an ORR of 9% and a disease control rate of 23% in 43 patients with previously treated oesophageal or G/GEJ cancers [ 161 ]. A randomized phase II study (FAST) indicated that zolbetuximab plus first-line chemotherapy significantly improved PFS and OS in patients with CLDN18.2-positive G/GEJ cancer [ 159 ]. Subgroup analysis indicated a correlation between moderate-to-strong CLDN18.2 expression and a better overall survival rate. In the phase III SPOTLIGHT trial, zolbetuximab plus mFOLFOX6 significantly improved mPFS (10.61 vs 8.67 months, HR 0.751, P = 0.0066) and mOS (18.23 vs 15.54 months, HR 0.750, P = 0.0053) in patients with CLDN18.2-positive and HER-2-negative advanced G/GEJ cancer[ 162 ].
GLOW (NCT03653507) is another phase III trial investigating zolbetuximab plus CAPOX as first-line treatment in patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric or GEJ cancer. In this study, zolbetuximab plus CAPOX showed a significant improvement in mPFS (8.21 vs 6.80 months, HR 0.687, P = 0.0007) and mOS (14.39 vs 12.16 months, HR 0.771, P = 0.0118) compared to placebo plus CAPOX[ 163 ]. Additionally, zolbetuximab is also being studied in combination with immunotherapy in patients with CLDN18.2-positive advanced gastric or GEJ cancer in the ILUSTRO study (NCT03505320).
Another promising therapeutic approach targeting CLDN18.2 employs CLDN18.2-specific chimeric antigen receptor (CAR) T cells. CLDN18.2-specific CAR T cells achieved partial or complete tumor regression in CLDN18.2-positive PDX models [ 164 ]. A phase I study of CLDN18.2-specific CAR T cells in gastrointestinal cancers conducted by Prof. Shen Lin's team demonstrated that in GC patients, the ORR and DCR were 57.1% and 75.0%, respectively, and the 6-month overall survival rate was 81.2% [ 165 ]. Claudin 18.2 served as a new target for the later-line treatment of GC, with considerable ORR improvement achieved in Claudin 18.2 CAR-T therapy, which has become a hallmark event for cellular immunotherapy in solid tumors. Currently, several new drugs focusing on Claudin 18.2, such as Claudin 18.2 bispecific antibodies (Claudin 18.2/CD3, Claudin 18.2/PD-L1) and ADC analogs, are being developed. Although these drugs have not been approved for clinical applications, some of them showed promising preclinical data and are being widely studied in different clinical trials. Since Claudin 18.2 is also expressed on the normal gastric mucosal epithelial surface, the risk of adverse reactions and whether ADC drugs may aggravate normal mucosal damage should also be a concern.
FGFR-targeted therapy
FGFR1 mutations, FGFR2 amplifications, and FGFR3 rearrangements are the most common FGFR alterations in GC [ 166 ]. Different types of FGFR targeting agents were explored or developed in GC, including multikinase inhibitors, pan-FGFR inhibitors, FGFR1-3 inhibitors, selective FGFR inhibitors and ADC. Nevertheless, most multikinase inhibitor studies were preclinical or single case reports in GC without robust clinical evidence [ 167 ]. Futibatinib, an irreversible and highly selective FGFR1–4 inhibitor that permanently disables FGFR2, has been tested in a phase II trial involving patients with advanced-stage solid tumors harboring FGFR alterations, including those with FGFR2-amplified G/GEJ cancers [ 168 ]. Although the ORR was reported to be 22.2% in the GC cohort [ 169 ], more data are needed to support the efficacy of multiple FGFR inhibitors in different FGFR gene alterations in GC.
Currently, bemarituzumab has shown some promising results in the treatment of mGC [ 170 ]. It is a first-in-class afucosylated monoclonal antibody against the FGFR2b splice variant frequently overexpressed in FGFR2- amplified G/GEJ cancers. In a phase I trial, 17.9% of patients with FGFR2 amplifications had a confirmed response to bemarituzumab [ 171 ]. Based on the safety and activity profile of bemarituzumab monotherapy in GC, the phase II FIGHT trial was designed to evaluate the efficacy of bemarituzumab plus mFOLFOX6 regimen in previously untreated, FGFR2b-overexpressing advanced-stage G/GEJ cancers [ 172 ]. The trial showed a 2-month improvement in PFS, and the OS was not reached (NR) in the experimental arm (bemarituzumab + mFOLFOX6). However, the experimental arm had a higher incidence of adverse events than the control chemotherapy arm, particularly in regard to ocular toxicity.
EGFR-targeted therapy
Approximately 5–10% of patients with G/GEJ cancers have EGFR amplifications or EGFR overexpression, both of which are associated with poor prognosis [ 173 ]. Previous large randomized clinical trials have failed to demonstrate any significant survival benefit with EGFR-targeted agents [ 92 , 174 ], perhaps because most of the studies were performed in unselected patient populations regardless of EGFR status. Besides, biomarker analysis of the EXPAND and COG trials suggests activity in patients with tumors expressing high levels of EGFR, thus supporting the significance of patient selection for future trials [ 175 , 176 ]. In a prospective cohort, patients with metastatic gastroesophageal adenocarcinoma were screened for EGFR amplification and subsequently treated with anti-EGFR therapy (cetuximab). The ORR was 58% (4 of 7 patients), and the DCR was 100% (7 of 7 patients), implying that EGFR inhibition should be further studied in selected patients [ 177 ]. Many of the ongoing EGFR inhibitor studies should test EGFR alterations in the GC patients prior to enrollment to overcome resistance to EGFR-targeted therapies.
MET/HGF pathway inhibitors
c-Mesenchymal-Epithelial Transition (c-MET) is a tyrosine kinase receptor from MET families, and hepatocyte growth factor (HGF) is the common ligand to c-MET [ 178 ]. MET/HGF pathway activation is associated with tumor invasiveness and poor disease prognosis. The anti-MET monoclonal antibody, onartuzumab, has been studied in a phase III trial of onartuzumab plus mFOLFOX6 vs placebo plus mFOLFOX6 in patients with metastatic HER2-negative G/GEJ cancers. However, the addition of onartuzumab to mFOLFOX6 did not improve clinical outcomes in the ITT population or in the MET-positive population [ 179 ]. Rilotumumab is a humanized monoclonal antibody targeting HGF. Two phase III trials (RILOMET-1 and RILOMET-2) investigated rilotumumab plus chemotherapy in advanced MET-positive G/GEJ cancers. Unfortunately, both studies were terminated due to increased number of deaths in the rilotumumab group[ 180 , 181 ]. Additionally, several selective/non-selective c-MET TKIs, such as tinvatinib, AMG 337 and foretinib, have also been tested in MET-positive GC, but no significant benefit was seen in clinical trials[ 182 , 182 , 184 ].
Challenges and future perspectives
Even though substantial advances have been made in the treatment of GC, further research and development are still necessary. Improving early detection, reducing recurrence and optimizing treatment strategies are the primary challenges and prospects for GC management. To increase GC early detection and promote patients’ overall survival, endoscopic screening programs should be implemented in high-risk regions, and more precise early detection technologies are of great value. In a previous study, we demonstrated an artificial intelligence (AI) diagnostic platform, GRAIDS, to detect upper gastrointestinal cancers using real-world endoscopic imaging data from six Chinese hospitals with varying experience in the endoscopic diagnosis of upper gastrointestinal cancer [ 185 ]. GRAIDS provided both real-time and retrospective assistance for enhancing the effectiveness of upper gastrointestinal cancer screening and diagnosis, with high diagnostic accuracy and sensitivity in detecting upper gastrointestinal cancers. In the near future, the AI system will help many physicians in community-based hospitals identify upper gastrointestinal cancers more efficiently and accurately [ 186 ].
In addition, recurrence of GC remains common despite the multimodality treatment, so many studies in progress aim to identify individuals at risk of recurrence after treatment. Circulating tumor DNA (ctDNA) can be detected in the circulation of cancer patients and has the potential to predict minimal residual disease [ 187 ]. Liquid biopsies can detect a broader spectrum of abnormalities in a heterogeneous tumor compared to conventional tissue biopsies. According to a study investigating perioperative therapies in patients in the CRITICS trial with resectable GC, the presence of ctDNA could predict recurrence when analyzed within nine weeks after preoperative treatment and after surgery in patients eligible for multimodal treatment [ 187 ]. These findings highlight the significance of ctDNA as a biomarker for predicting patient outcomes following perioperative cancer treatment and surgical resection in patients with GC. In another 1630-patient cohort of ctDNA results, genomic alterations were correlated with clinicopathologic characteristics and outcomes and provided prognostic and predictive information [ 188 ]. As for advanced GC, ctDNA also serves as a potential biomarker of immunotherapy response, and its potential role in predicting irAEs is worth further investigation [ 189 ]. Further research aimed at prospectively collecting ctDNA is needed to confirm these findings. The existence of persistent ctDNA following curative-intent treatment of GC may indicate minimal residual disease, and trials are underway to determine whether additional adjuvant therapy can result in the clearance of ctDNA.
Intratumoral, intrapatient, and interpatient heterogeneity in GC is the major barrier to drug development for systemic therapies. Most GC patients are not susceptible to immune checkpoint inhibitor monotherapies. Thus, one of the major challenges in systemic treatments for GC is overcoming resistance to ICI therapy. One strategy is to develop novel ICIs with better efficacy. Recently, many novel immune checkpoint modulators have been widely investigated, including LAG-3, VISTA, TIM-3, TIGIT, CD38, CD39, and CD73[ 190 ]. Another key strategy is combining ICI and other therapies, such as other ICI, targeted therapies, other immune-modulating agents, chemotherapy (as discussed above), and radiotherapy [ 191 ]. As mentioned above, in the CheckMate-649 study, the combination of anti-PD-1 and anti-CTLA-4 agents (nivolumab plus ipilimumab) failed to improve treatment outcomes compared to traditional chemotherapy [ 57 ]. In the EPOC1706 study, lenvatinib, an anti-angiogenic multiple receptor tyrosine kinase inhibitor, combined with pembrolizumab showed an exciting activity with an ORR of 69% in the first-line and second-line treatment of advanced GC[ 151 ]. ICI combined with other anti-immunosuppressive factor agents, such as anti-transforming growth factor-β (TGF-β), is also being investigated in clinical trials (NCT04856774). To fully understand the mechanism of resistance to immunotherapy, factors such as epigenetics, metabolism, immune suppression, and microbiota must be considered. Therefore, the development of combined therapies should be based on understanding the underlying mechanisms of immune modulation and resistance, rather than simply combining available therapies in a haphazard manner.
Rapid developments are ongoing in the clinical use of ADCs and are now considered one of the current hot spots for antitumor drug development. In particular, ADCs have emerged as a new era of targeted therapy in the field of GC treatment. The latest generation of ADCs has expanded the treatment population to include novel targets and demonstrated superior clinical outcomes compared to traditional chemotherapy drugs. Nevertheless, certain aspects of ADCs remain to be addressed. Firstly, it is necessary to explore ways to advance ADCs as first-line therapy to benefit a larger number of patients. Secondly, to make better use of medical resources, a more differentiated target layout needs to be established, moving beyond the focus on distinct targets such as HER2. To address these challenges, optimization of the toxin, linker and toxicity of ADCs is essential, along with the development of ADC-combination therapies to improve efficacy. We anticipate the discovery of more potential ADC drugs and expect a breakthrough in first-line treatment.
Currently, many clinical trials have complex treatment regimens, including mono-immunotherapy, double-checkpoint inhibitors, anti-angiogenic drugs, and biomarker-directed therapies [ 190 , 192 ]. However, the challenge of determining the optimal treatment strategy and the appropriate timing of molecular biomarker screening has yet to be resolved. We expect that extensive translational research, preclinical investigations, and multi-omics-based clinical trials will lead to breakthroughs in the diagnosis and treatment of GC. Therefore, we eagerly anticipate future studies that have the potential to improve clinical practice in the coming years.
Availability of data and materials
Not applicable.
Abbreviations
Antibody–drug conjugates
Antibody-dependent cell-mediated cytotoxicity
Advanced gastro-oesophageal cancer
Artificial intelligence
American Society of Clinical Oncology
Bispecific antibodies
Best supportive care
Capecitabine and oxaliplatin
Chimeric antigen receptor
Cisplatin and fluorouracil
China Food and Drug Administration
Confidence interval
Claudin18.2
Combined positive score
Chemoradiotherapy
Chinese Society for Clinical Oncology
Circulating tumor DNA
Cytotoxic T lymphocyte antigen-4
Disease control rate
Disease-free survival
Mismatch-repair deficiency
Duration of response
Docetaxel, oxaliplatin, and S-1
Epstein-Barr virus
Epstein-Barr virus-associated gastric cancer
Extracellular domain
Epirubicin, cisplatin, and fluorouracil
Event-free survival
Epidermal growth factor receptor
Epirubicin and oxaliplatin
Erythroblastic leukemia viral oncogene homolog
Extracellular regulated protein kinase
European Society for Medical Oncology
Food and Drug Administration
Fibroblast growth factor receptor
Fluorescent in situ hybridization
Fluorouracil, leucovorin, oxaliplatin, and docetaxel
Fluorouracil, leucovorin, and oxaliplatin
Fluorouracil and cisplatin
- Gastric cancer
Gastroesophageal junction adenocarcinoma
Gastroesophageal junction
Human epidermal growth factor receptor 2
Hazard ratio
Immune checkpoint inhibitor
Immunohistochemistry
Kirsten rats sarcomaviral oncogene homolog
Lymphocyte-activation gene 3
Mitogen-activated protein kinase
Mesenchymal epithelial transition
Cytotoxin monomethyl auristatin E
Microsatellite instability
Mammalian target of rapamycin
National Comprehensive Cancer Network
Not evaluable
Natural killer
Objective response rate
Overall survival
Pathological complete response
Programmed cell death 1
Programmed cell death ligand 1
Progression-free survival
Phosphatidylinositol-3-kinase
Phosphatase and tensin homolog
Relapse-free survival
Recommended phase 2 dose
Receptor tyrosine kinase
S-1 and oxaliplatin
S-1, oxaliplatin and radiotherapy
S-1 and cisplatin
Tumor area positivity
The Cancer Genome Atlas
Trastuzumab emtansine
Trastuzumab deruxtecan
Transforming growth factor-β
T cell immunoreceptor with Ig and ITIM domain
T cell immunoglobulin and mucin domain 3
Tumor mutational burden
Vascular endothelial growth factor
V-domain Ig suppressor of T cell activation
Capecitabine and cisplatin
Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Article PubMed Google Scholar
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–62.
Article CAS PubMed Google Scholar
Tramacere I, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28–36.
Lordick F, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Lu L, et al. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). 2021;41(11):1137–51.
Pennathur A, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48.
Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
PubMed Google Scholar
Korfer J, Lordick F, Hacker UT. Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers (Basel), 2021;13(20).
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature, 2014;513(7517): 202–9.
Salem ME, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319–27.
Article CAS PubMed PubMed Central Google Scholar
Wang J, et al. Large-scale analysis of KMT2 mutations defines a distinctive molecular subset with treatment implication in gastric cancer. Oncogene. 2021;40(30):4894–905.
Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Chao J, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021;7(6):895–902.
Article PubMed PubMed Central Google Scholar
Nakamura Y, et al. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18(8):473–87.
Wang FH, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95.
Ajani JA, et al. Gastric cancer, version 2, 2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(2):167–92.
Article CAS Google Scholar
Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Ychou M, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
Al-Batran SE, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Kang YK, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39(26):2903–13.
Yoshida K, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Zhang X, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92.
Japanese Gastric Cancer, A.Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer, 2023;26(1): 1–25.
Bang YJ, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Noh SH, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96.
Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93.
Pietrantonio F, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37(35):3392–400.
Macdonald JS, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Lee J, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–73.
Park SH, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol. 2021;32(3):368–74.
Hofheinz RD, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149(6):1322–31.
Hofheinz RD, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trial of the AIO. J Clin Oncol. 2020;38(15_suppl):4502–4502.
Article Google Scholar
Rivera F, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67.
Wagner AD, et al. EORTC-1203-GITCG - the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19(1):494.
Cunningham D, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017;18(3):357–70.
Goetze TO, et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7) with high rate of signet cell component: final results of the multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J Clin Oncol. 2022;40(16_suppl):4042–4042.
Al-Batran S-E, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022;40(16_suppl):4003–4003.
Liu Y, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: updated results of efficacy and safety. J Clin Oncol. 2021;39(15_suppl):4036.
Li H, et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4050–4050.
Alcindor T, et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: preliminary results. J Clin Oncol. 2021;39(15_suppl):4046–4046.
Li S, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib, S-1 ± oxaliplatin for locally advanced cT4a/bN+ gastric cancer. J Clin Oncol. 2021;39(15_suppl):4061.
Wei J, et al. SHARED: Efficacy and safety of sintilimab in combination with concurrent chemoradiotherapy (cCRT) in patients with locally advanced gastric (G) or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4040–4040.
Bang YJ, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943–52.
Janjigian YY, et al. MATTERHORN: efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer—a randomized, double-blind, placebo-controlled, phase 3 study. J Clin Oncol. 2021;39(15):TPS4151
Andre T, et al. neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41(2):255–65.
Pietrantonio F, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41(4_suppl):358–358.
Xu R-H, et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: a randomized, phase 3 trial. J Clin Oncol. 2019;37(15_suppl):4017–4017.
Hall PS, et al. efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the go2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–77.
Shitara K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–87.
Shitara K, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48.
Shitara K, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80.
Wainberg ZA, et al. Pembrolizumab with or without chemotherapy versus chemotherapy alone for patients with PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma: Update from the phase 3 KEYNOTE-062 trial. J Clin Oncol. 2022;40(4_suppl):243–243.
Rha SY, et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;34(3):319–20.
Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Janjigian YY, et al. LBA7 Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study. Ann Oncol. 2021;32:S1329–30.
Kang YK, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47.
Xu J, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331.
Xu R-h, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. J Clin Oncol 2020;38(4_suppl): TPS458
Moehler MH, et al. Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 2023;41(4):286–286.
Moehler M, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–77.
Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5): e180013.
Kang YK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Bang YJ, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60.
Triulzi T, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. 2019;8(1): e1512942.
Varadan V, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperativetrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22(13):3249–59.
Chaganty BKR, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56.
Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142–7.
Takahari D, et al. A phase Ib study of nivolumab plus trastuzumab with S-1/capecitabine plus oxaliplatin for HER2-positive advanced gastric cancer (Ni-HIGH study): safety evaluation. J Clin Oncol. 2020;38(15_suppl): 4525
Rha SY, et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): Molecular profiling and clinical update. J Clin. Oncol.2021;39(3_suppl):218.
Janjigian YY, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31.
Janjigian YY, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30.
Guan WL, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: a multicenter analysis. Front Oncol. 2021;11: 712760.
Vanderwalde A, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–56.
Pietrantonio F, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6(1): 100036.
Huang SC, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145(12):3218–30.
Qiu MZ, et al. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer. 2020;146(1):272–80.
Derks S, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32.
Kim ST, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Sun YT, et al. PD-1 antibody camrelizumab for Epstein-Barr virus-positive metastatic gastric cancer: a single-arm, open-label, phase 2 trial. Am J Cancer Res. 2021;11(10):5006–15.
CAS PubMed PubMed Central Google Scholar
Xie T, et al. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43(4):139–44.
Mishima S, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7(1):24.
Shitara K, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Wainberg ZA, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score >/=10. Clin Cancer Res. 2021;27(7):1923–31.
Moehler MH, et al. First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): expanded efficacy and safety data from CheckMate 649. J Clin Oncol. 2021;39(15_suppl): 4002
Zhao JJ, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402.
Wang F, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479–86.
Shitara K, et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol. 2020;38(15_suppl): 4537
Marabelle A, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Van Cutsem E, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84.
Qin S, et al. Treatment patterns and outcomes in chinese patients with gastric cancer by HER2 status: a noninterventional registry study (EVIDENCE). Oncologist. 2021;26(9):e1567–80.
Tabernero J, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84.
Liu T, et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond). 2019;39(1):38.
Thuss-Patience PC, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Hecht JR, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol. 2016;34(5):443–51.
Satoh T, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Makiyama A, et al. Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J Clin Oncol. 2020;38(17):1919–27.
Yagi S, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22(3):518–25.
Nishida Y, et al. A novel gene-protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2015;18(3):458–66.
Lee HJ, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2-positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144(4):570–8.
Kim KC, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10):2833–40.
Haffner I, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. 2021;39(13):1468–78.
Palle J, et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15.
Park SR, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Seo S, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22(3):527–35.
Janjigian YY, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8(1):49–58.
Shen L. Liquid biopsy: a powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun (Lond). 2018;38(1):72.
Kwak EL, et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 2015;5(12):1271–81.
Kim J, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124(12):5145–58.
Kim ST, et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29(4):1037–48.
Lee JY, et al. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci Rep. 2015;5:9289.
Sanchez-Vega F, et al. EGFR and MET amplifications determine response to HER2 Inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019;9(2):199–209.
Wang DS, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68(7):1152–61.
Sampera A, et al. HER-family ligands promote acquired resistance to trastuzumab in gastric cancer. Mol Cancer Ther. 2019;18(11):2135–45.
Gambardella V, et al. NRF2 through RPS6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25(5):1639–49.
Nordstrom JL, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123.
Shinde A, et al. Can immunotherapy replace radiotherapy in melanoma brain metastases? J Clin Oncol. 2019;37(12):1030–1.
Bang YJ, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61.
Catenacci DVT, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76.
Catenacci DVT, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7(5)
Ku G, et al. 1380P Phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. 2021;32:S1044–5.
Tabernero J, et al. HERIZON-GEA-01: Zanidatamab + chemo +/- tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18(29):3255–66.
Zhang J, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I Study. Clin Cancer Res. 2022;28(4):618–28.
Xu J, et al. A phase II study evaluating KN026 monotherapy in patients (pts) with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancers (GC/GEJC). J Clin Oncol. 2022;40(16_suppl):4040–4040.
Shen L, et al. 1210P The preliminary efficacy and safety of KN026 combined with KN046 treatment in HER2-positive locally advanced unresectable or metastatic gastric/gastroesophageal junction cancer without prior systemic treatment in a phase II study. Ann Oncol. 2022;33:S1102.
Piha-Paul S, et al. O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J Immunother Cancer. 2020;8(Suppl 1):A1.
Google Scholar
Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14(1):20.
Ogitani Y, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Yamaguchi K, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II Trial. J Clin Oncol. 2023;41(4):816–25.
Ku GY, et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol. 2022;33:S1100.
Dai L, et al. Efficacy of disitamab vedotin in treating HER2 2+/FISH- gastric cancer. Onco Targets Ther. 2022;15:267–75.
Xu Y, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25.
Peng Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41(11):1173–82.
Zhang Y, et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep Med. 2022;3(11): 100814.
Kulukian A, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Catenacci DVT, et al. MOUNTAINEER-02: Phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—Trial in progress. J Clin Oncol. 2022;40(4_suppl):371.
Wu X, Huang S. HER2-specific chimeric antigen receptor-engineered natural killer cells combined with apatinib for the treatment of gastric cancer. Bull Cancer. 2019;106(11):946–58.
Smyth EC, et al. Gastric cancer. Lancet. 2020;396(10251):635–48.
Casak SJ, et al. FDA approval summary: ramucirumab for gastric cancer. Clin Cancer Res. 2015;21(15):3372–6.
Fuchs CS, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
Wilke H, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Xu RH, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1015–24.
Fuchs CS, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–76.
Pavlakis N, et al. INTEGRATE IIa: a randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—a study led by the Australasian Gastro-intestinal Trials Group (AGITG). J Clin. Oncol. 2023;41(4):LBA294.
Fukuoka S, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61.
Kawazoe A, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057–65.
Li J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54.
Kang YK, et al. Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed & 2 prior chemotherapy regimens. Ann Oncol. 2019;30:v877–8.
Zhang Y, et al. A phase Ib/II study of fruquintinib in combination with paclitaxel as the second-line therapy for advanced gastric cancer. Cancer Commun (Lond), 2022.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021
Sahin U, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624–34.
Hong JY, et al. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. 2020;9(5):3367–74.
Rohde C, et al. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–6.
Sahin U, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609–19.
Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10(1):105.
Tureci O, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30(9):1487–95.
Shitara K, et al. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) withclaudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: primary results from phase 3 SPOTLIGHT study. J Clin Oncol 2023;41(4_suppl): LBA292
Shitara K, et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol. 2023;41(36_suppl):405736–405736.
Jiang H, et al. Claudin182-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111(4):409–18.
Qi C, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189–98.
Helsten T, Schwaederle M, Kurzrock R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 2015;34(3):479–96.
Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J Hematol Oncol. 2021;14(1):23.
Bahleda R, et al. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann Oncol. 2020;31(10):1405–12.
Meric-Bernstam F, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12(2):402–15.
Lengyel CG, et al. FGFR Pathway Inhibition in Gastric Cancer: The Golden Era of an Old Target? Life (Basel), 2022;12(1)
Catenacci DVT, et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J Clin Oncol. 2020;38(21):2418–26.
Catenacci DVT, et al. FIGHT: a randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). J Clin Oncol. 2021;39(15_suppl):4010–4010.
Nagatsuma AK, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18(2):227–38.
Dutton SJ, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904.
Petty RD, et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279–87.
Lordick F, et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. J Clin Oncol. 2013;31(15_suppl):4021–4021.
Maron SB, et al. Targeted therapies for targeted populations: anti-EGFR Treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696–713.
Raj S, et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol Cancer. 2022;21(1):31.
Shah MA, et al. A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist. 2016;21(9):1085–90.
Catenacci DVT, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–82.
Doi T, et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the RILOMET-2 trial. J Clin Oncol. 2015;33(3_suppl): TPS226
Kang YK, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32(2):355–61.
Hong DS, et al. Phase I Study of AMG 337, a highly selective small-molecule MET inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2019;25(8):2403–13.
Shah MA, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS ONE. 2013;8(3): e54014.
Luo H, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20(12):1645–54.
Chen ZH, et al. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond). 2021;41(11):1100–15.
Leal A, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020;11(1):525.
Maron SB, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25(23):7098–112.
Jin Y, et al. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19(1):154.
Wang Y, et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol. 2022;15(1):111.
Upadhaya S, et al. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20(3):168–9.
Zhao H, et al. Emerging immunological strategies: recent advances and future directions. Front Med. 2021;15(6):805–28.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82203678 to Y.H.), the Science and Technology Program of Guangdong (Grant No. 2019B020227002 to R.-H.X.), the CAMS Innovation Fund for Medical Sciences (Grant No. 2019-I2M-5-036 to R.-H.X.).
Author information
Wen-Long Guan and Ye He have contributed equally.
Authors and Affiliations
Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China
Wen-Long Guan, Ye He & Rui-Hua Xu
Research Unit of Precision Diagnosis and Treatment for Gastrointestinal Cancer, Chinese Academy of Medical Sciences, Guangzhou, 510060, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Rui-Hua Xu designed this review. Rui-Hua Xu, Wen-Long Guan, and Ye He drafted the manuscript and prepared the figures. Rui-Hua Xu, Wen-Long Guan, and Ye He collected the related references and participated in the discussion. All authors contributed to this manuscript and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rui-Hua Xu .
Ethics declarations
Ethical approval and consent to participate, competing interests.
All authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guan, WL., He, Y. & Xu, RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 16 , 57 (2023). https://doi.org/10.1186/s13045-023-01451-3
Download citation
Received : 13 March 2023
Accepted : 10 May 2023
Published : 27 May 2023
DOI : https://doi.org/10.1186/s13045-023-01451-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- Targeted therapy
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
Groundbreaking research offers early clues to stomach cancer development
In a breakthrough study published in the journal Cancer Cell , researchers have decoded critical genetic factors in intestinal metaplasia patients, shedding light on early signs and prevention strategies for stomach cancer -- often a "ticking time bomb" as patients experience no or only mild symptoms in the early stages.
Intestinal metaplasia, which is a change in the cells of the mucous membrane lining the stomach that often stems from chronic gastritis and manifests with symptoms akin to acid reflux, is also a sinister link to stomach cancer. Individuals afflicted with intestinal metaplasia cells face a sixfold increased risk of succumbing to this lethal cancer.
In Singapore alone, stomach cancer ranks as the fourth leading cause of cancer deaths in men and the fifth among women, claiming 300 to 500 lives annually, largely due to late detection. Two thirds of stomach cancer patients are only diagnosed at an advanced stage.
Unveiling early indicators through collaborative breakthrough research
The longitudinal study, which represents the world's largest genomic survey of patients with intestinal metaplasia, examines more than 1,100 tissue samples using powerful technologies such as single-cell RNA sequencing and spatial transcriptomics*. Based on this extensive survey, researchers identified 26 'driver genes' that play a pivotal role in the transition to stomach cancer. This landmark finding provides a glimpse into the mechanisms governing the transformation and offers a critical window for early detection and targeted prevention.
"Advances in DNA sequencing have made it possible for us to uncover diverse cell populations within these stomach changes, hinting at their potential transformation into cancerous cells influenced by various factors. It's akin to understanding the ticking mechanism of a time bomb," explained Dr Huang Kie Kyon, co-first author and Senior Research Fellow with the Cancer & Stem Cell Biology Programme at Duke-NUS Medical School (Duke-NUS).
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS and a professor with the School's Cancer & Stem Cell Biology Programme said: "The comprehensive dataset we've assembled provides unprecedented insights into the progression of cell changes in the stomach to cancer. By using both clinical information and genetic data from advanced molecular technologies, we can better predict which stomach conditions might turn into stomach cancer compared to using only clinical information. This can help in the development of new and more precise ways to prevent and stop stomach cancer." Prof Tan is also a member of the Genome Institute of Singapore, Cancer Science Institute of Singapore, and Precision Health Research Singapore (PRECISE).
The multi-institutional effort by researchers from Duke-NUS, National University Hospital (NUH), National University of Singapore's Yong Loo Lin School of Medicine (NUS Medicine) and Seoul National University Hospital reflects the strengths of Singapore's multi-institutional cancer research ecosystem and its strong links with global partners. This study was supported by the Singapore Gastric Cancer Consortium (SGCC), a national translational research group comprising clinicians and scientists working in stomach cancer research from academic medical centres, universities, hospitals and research institutes across Singapore. The published work is derived from the prospective Gastric Cancer Epidemiology Programme cohort.
The study offers clues into whether intestinal metaplasia cells directly transform into stomach cancer. It was revealed that a subpopulation of intestinal stem-like cells in patients with intestinal metaplasia closely resembles early stomach cancer cells, pointing to a possible early origin and potential of its malignant future. This discovery highlights the importance of screening for intestinal metaplasia in managing stomach cancer risk.
Co-senior author Professor Jimmy So, Head & Senior Consultant, Division of General Surgery (Upper Gastrointestinal Surgery), NUH commented on the clinical implications: "This molecular roadmap of disease progression from intestinal metaplasia offers many translational opportunities. We can now explore more targeted surveillance for patients at highest risk, as well as anti-inflammatorial or antibiotic agents to intercept premalignant clones before they evolve into cancer, potentially leading to improved patient outcomes through early detection." He is also a professor at the Department of Surgery, NUS Medicine.
More efficient and targeted preventive measures for populations
At the population level, the findings hold promise for refining screening strategies and allocating resources more effectively to intercept the development of gastric cancer in high-risk individuals, ultimately contributing to more efficient and targeted preventive measures. This is especially relevant in countries such as Singapore, where the incidence of stomach cancer is moderate compared to Japan and South Korea where stomach cancer incidence is high enough to warrant mass screening.
"Encouragingly, our results revealed that combining genomic data with clinical check-ups can make predictions about stomach cancer more accurate. This means we might use genetic tests, including simple and inexpensive blood tests, to identify people who are at a very high risk of getting stomach cancer. With this approach, we can divide people into groups based on their risk using either regular check-ups or these affordable blood tests. This helps to save resources by making sure those at the highest risk get the right tests and care they need," added Professor Khay Guan Yeoh, Lead Principal Investigator of the Singapore Gastric Cancer Consortium and Senior Consultant in the Division of Gastroenterology & Hepatology, National University Hospital. Prof Yeoh is also the Kishore Mahbubani Professor in Medicine and Health Policy, Department of Medicine, NUS Medicine.
Senior author Associate Professor Chung Hyunsoo from Seoul National University Hospital stressed the clinical ramifications: "This breakthrough may refine screening protocols, enabling early interventions for high-risk patients, while sparing others unnecessary procedures."
The research was funded by the Open Fund-Large Collaborative Grant that is supported by the National Research Foundation, Singapore and administered by the Singapore Ministry of Health's National Medical Research Council. The team also received support from Singapore's Ministry of Education, Cancer Science Institute of Singapore under the National University of Singapore and the Francis Crick Institute. In addition, the project could not have been made possible without the contributions of researchers from Tan Tock Seng Hospital, Singapore General Hospital, Changi General Hospital, Nihon University School of Medicine, Yonsei University Wonju College of Medicine and The Chinese University of Hong Kong.
* Single-cell RNA sequencing (scRNA-seq) is a powerful technology that allows scientists to study individual cells' genetic material (RNA) one cell at a time. Spatial transcriptomics is another technique used to study gene expression in tissues but with an added dimension of spatial information. It allows scientists to see where specific genes are being expressed within tissues or organs, providing insight into the organisation and communication between cells in their actual location within the tissue.
- Gastrointestinal Problems
- Breast Cancer
- Lung Cancer
- Colon Cancer
- Diseases and Conditions
- Prostate Cancer
- Brain Tumor
- Alzheimer's disease
- Cervical cancer
- Malignant melanoma
- Stomach cancer
- Mammography
- Breast cancer
Story Source:
Materials provided by Duke-NUS Medical School . Note: Content may be edited for style and length.
Journal Reference :
- Kie Kyon Huang, Haoran Ma, Roxanne Hui Heng Chong, Tomoyuki Uchihara, Benedict Shi Xiang Lian, Feng Zhu, Taotao Sheng, Supriya Srivastava, Su Ting Tay, Raghav Sundar, Angie Lay Keng Tan, Xuewen Ong, Minghui Lee, Shamaine Wei Ting Ho, Tom Lesluyes, Hassan Ashktorab, Duane Smoot, Peter Van Loo, Joy Shijia Chua, Kalpana Ramnarayanan, Louis Ho Shing Lau, Takuji Gotoda, Hyun Soo Kim, Tiing Leong Ang, Christopher Khor, Jonathan Wei Jie Lee, Stephen Kin Kwok Tsao, Wei Lyn Yang, Ming Teh, Hyunsoo Chung, Jimmy Bok Yan So, Khay Guan Yeoh, Patrick Tan. Spatiotemporal genomic profiling of intestinal metaplasia reveals clonal dynamics of gastric cancer progression . Cancer Cell , 2023; 41 (12): 2019 DOI: 10.1016/j.ccell.2023.10.004
Cite This Page :
Explore More
- Simple Brain-Computer Link: Gaming With Thoughts
- Clinical Reasoning: Chatbot Vs Physicians
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
Trending Topics
Strange & offbeat.
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Stomach Cancer: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about this type of cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about stomach cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials . Always talk with your doctor about the best diagnostic and treatment options for you.
Chemoprevention. Chemoprevention is the use of drugs or nutrients to lower a person’s risk of developing cancer. Early research suggests that using antibiotics to treat H. pylori infections (see Risk Factors ) can prevent changes to stomach cells that may lead to cancer.
Combination therapy. The combination of chemotherapy, radiation therapy, and surgery may reduce the chance that stomach cancer will return. Doctors may give chemotherapy before surgery, called neoadjuvant therapy, or after surgery, called adjuvant therapy. In addition, doctors may also combine radiation therapy and chemotherapy after surgery. Doctors are also looking at giving both radiation therapy and chemotherapy before surgery.
Newer chemotherapy treatments. Chemotherapy with multiple combinations of drugs is being increasingly used for people with stomach cancer. Drug combinations work slightly better than single drugs. As outlined in the Types of Treatment section, drugs such as 5-FU, paclitaxel, docetaxel, irinotecan, oxaliplatin, as well as oral medications such as capecitabine are being studied in combination with other types of chemotherapy.
Molecular testing of the tumor . Researchers are looking at the genetic changes in tumor cells to identify specific genes, proteins, and other factors unique to the tumor. Patients with different types of tumors with the same genetic change are able to participate in clinical trials, called “basket trials” , with the goal of finding treatments that target that genetic change.
Targeted therapy. Previous research has shown that several types of targeted therapy do not work well for stomach cancer. These include drugs that target the gene c-MET , bevacizumab (Avastin, Mvasi), and drugs that block epidermal growth factor receptor (EGFR). However, research continues on this type of treatment approach (see Molecular testing of the tumor, above).
Immunotherapy. Immunotherapy is an expanding area of research for stomach cancer. Researchers are looking at different types of immunotherapy that block the CTLA4 and/or PD-1 pathways. A tumor can use these pathways to hide from the body’s immune system. Immunotherapy that blocks these pathways allow the immune system to identify and destroy the cancer.
Palliative and supportive care . Clinical trials are underway to find better ways of reducing symptoms and side effects of current stomach cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in stomach cancer, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to read about research announced in ASCO's Clinical Cancer Advances report from 2021 .
Listen to a podcast from ASCO experts discussing highlights from 2022 Congress of the European Society for Medical Oncology (ESMO).
Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Stomach Cancer Guide
Cancer.Net Guide Stomach Cancer
- Introduction
- Medical Illustrations
- Risk Factors
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
- Introduction
- Article Information
Patients who were pathologically diagnosed as having gastric adenocarcinoma or adenocarcinoma of the esophagogastric junction, underwent curative gastrectomy with D2 lymphadenectomy, and had confirmed Helicobacter pylori infection from January 1, 2010, to December 31, 2018, were enrolled in the study. Propensity score matching (PSM) was performed based on initially imbalanced variables.
HR indicates hazard ratio.
eTable 1. Univariable and Multivariable Survival Analysis of Overall Survival in the Overall Cohort (Before PSM: n = 1293)
eTable 2. Univariable and Multivariable Survival Analysis of Disease-Free Survival in the Overall Cohort (Before PSM: n = 1293)
eFigure 1. Subgroup Analyses for Overall Survival According to Clinical Indicators Including Age, Gender, Tumor Differentiation, Etc
eFigure 2. Subgroup Analyses for Disease-Free Survival According to Clinical Indicators Including Age, Gender, Tumor Differentiation, Etc
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zhao Z , Zhang R , Chen G, et al. Anti– Helicobacter pylori Treatment in Patients With Gastric Cancer After Radical Gastrectomy. JAMA Netw Open. 2024;7(3):e243812. doi:10.1001/jamanetworkopen.2024.3812
Manage citations:
© 2024
- Permissions
Anti– Helicobacter pylori Treatment in Patients With Gastric Cancer After Radical Gastrectomy
- 1 Department of Gastric Surgery, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2 Department of Medical Oncology, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
Question Is anti– Helicobacter pylori treatment associated with enhanced postgastrectomy survival prospects for patients diagnosed with gastric cancer who have a preoperative confirmation of H pylori infection?
Findings In this cohort study of 1293 patients, the anti– H pylori treatment group had a significant survival advantage compared with the non–anti– H pylori treatment group in terms of overall survival and disease-free survival. After propensity score matching, the survival advantage for both overall and disease-free survival remained.
Meaning The findings indicated that for patients with gastric cancer who have H pylori infections, undergoing anti– H pylori treatment may lead to improved survival outcomes.
Importance Whether anti– Helicobacter pylori treatment can provide survival benefits for patients with gastric cancer who are diagnosed with H pylori infection is an area with limited research.
Objective To explore the potential survival benefits of anti– H pylori treatment after radical gastrectomy in patients with gastric cancer and presurgical confirmation of H pylori infection.
Design, Setting, and Participants This retrospective cohort study was conducted using data from patients with gastric cancer treated between January 1, 2010, and December 31, 2018, and followed up for outcome ascertainment until May 19, 2021. Propensity score matching was performed in patients treated with or without anti– H pylori treatment. This study involved a single institute in a comprehensive cancer treatment and research center located in Guangzhou, Guangdong Province, China. The study included patients with gastric or esophagogastric junction adenocarcinoma who underwent curative gastrectomy with D2 lymphadenectomy and tested positive for H pylori infection. Data were analyzed from March to June 2023.
Exposure Anti– H pylori treatment, which primarily includes triple therapy regimens consisting of amoxicillin, clarithromycin, and omeprazole for 14 days.
Main Outcomes and Measures Clinical outcomes, including overall survival (OS) and disease-free survival (DFS), were analyzed by Kaplan-Meier method, log-rank test, and Cox proportional hazards regression model. Subgroup analysis based on crucial clinical information was also conducted.
Results All 1293 patients (median [IQR] age, 59 [50-65] years; 860 [66.5%] male) were divided into 2 groups, with 125 patients in the anti– H pylori treatment group and 1168 patients in the non–anti– H pylori treatment group based on whether they received anti– H pylori treatment during the perioperative period and the follow-up. Survival analysis showed that the 5-year OS rates were 94.1% (95% CI, 89.3%-99.2%) in the anti– H pylori group and 73.8% (95% CI, 70.7%-77.0%) in the non–anti– H pylori group, and the hazard ratio (HR) of these 2 groups was 0.33 (95% CI, 0.18-0.60; P < .001). The survival benefit remained after propensity score matching (HR, 0.50; 95% CI, 0.26-0.99; P = .048). Multivariable analysis for OS and DFS further showed the survival benefit of anti– H pylori treatment, with HRs of 0.38 (95% CI, 0.17-0.87; P = .02) and 0.48 (95% CI, 0.28-0.83; P = .008), respectively. Among patients with TNM stage II/III disease who received adjuvant chemotherapy, anti– H pylori treatment was associated with survival benefits (OS: HR, 0.49; 95% CI, 0.24-0.99; P = .046), whereas among those who did not receive adjuvant chemotherapy, anti– H pylori treatment was not associated with survival benefits (OS: HR, 0.29; 95% CI, 0.04-2.08; P = .22).
Conclusions and Relevance This cohort study indicates that anti– H pylori treatment may be associated with improved survival in patients with gastric cancer who have H pylori infections. The study reinforces the importance of including H pylori screening and treatment in the surgical treatment of these patients.
Despite a recent decrease in incidence in the past few years, gastric cancer (GC) continues to be a significant global health concern. It ranks as the fifth most common cancer and stands as the fourth leading cause of cancer-related deaths worldwide, accounting for more than 1.08 million new cases and 0.76 million deaths in 2020. 1 It has been acknowledged that Helicobacter pylori infection is a major cause of chronic gastritis, peptic ulcer, and GC. 2 It is estimated that 4.4 billion people worldwide are infected with H pylori . Similar to the incidence of GC, the rate of H pylori infection tends to be higher in East Asian populations. 3
Numerous studies have provided substantial evidence that eradicating H pylori in healthy individuals can reduce the incidence of precancerous lesions for GC, subsequently decreasing the likelihood of developing GC. 4 - 8 In addition, several studies have reported that patients with H pylori– positive GC have better survival outcomes after GC surgery compared with those with H pylori– negative GC. 9 - 12 Helicobacter pylori– negative status is identified as an independent prognostic factor for poor outcomes. 10 - 12 Although several high quality randomized clinical trials (RCTs) demonstrated that eradication of H pylori after endoscopic resection of early GC can significantly reduce the incidence of the development of metachronous gastric carcinoma (MGC), 13 , 14 the effect of H pylori eradication in patients after gastrectomy have not yet been clarified. Two previous observational studies from Korea reported conflicting results in this setting. Kim et al 15 found no significant difference in overall survival (OS), GC-specific death, and cancer recurrence rates between the anti– H pylori treatment group and the placebo group in patients with GC after distal gastrectomy. However, Choi et al 16 observed a statistically significant advantage in OS and GC-specific survival for the eradication group compared with the noneradication group, with H pylori positivity identified as an independent risk factor for GC-specific death. The relationship between H pylori infection and survival rates with GC is not yet fully elucidated, limited by contradictory results, small sample size, and a paucity of high-quality related research. Thus, our study seeks to provide a deeper understanding of how anti– H pylori treatment influences postoperative survival outcomes in a larger, more diverse group of patients with GC, enhancing the scope of existing research findings. We conducted a retrospective cohort study from a high-volume institution to further explore the survival benefit of anti– H pylori treatment in patients with H pylori –positive GC after radical gastrectomy.
Retrospective collection of medical data was conducted for patients who underwent curative surgical treatment for GC at Sun Yat-sen University Cancer Center (Guangzhou, China) between January 1, 2010, and December 31, 2018. Patients were included in this cohort study if they were pathologically diagnosed as having gastric adenocarcinoma or adenocarcinoma of the esophagogastric junction, underwent curative gastrectomy with D2 lymphadenectomy, and had confirmed H pylori infection. Patients were excluded if they had other malignant tumors, were pathologically diagnosed with stage IV disease, underwent R1/2 resection, had uncertain or negative H pylori status, or had previously received anti– H pylori treatment within 3 months before the diagnosis of GC. Pathological staging, including depth of tumor invasion, lymph node involvement, and resection status, was evaluated according to the eighth edition of the American Joint Committee on Cancer’s Cancer Staging Manual . 17
A total of 1293 patients who met the above criteria were included in the study ( Figure 1 ). Patients were grouped into the anti– H pylori treatment group (125 cases) and the non–anti– H pylori treatment group (1168 cases) based on whether they received anti– H pylori treatment during the perioperative period and the follow-up. The following demographic and clinicopathologic characteristics of these patients were recorded: age, sex, comorbidities (including hypertension, coronary heart disease, and diabetes), body mass index, history of smoking, histologic grade, Borrmann classification, Lauren classification, tumor depth of invasion, lymph node metastasis, TNM staging, maximum tumor diameter, type of gastrectomy, postoperative complications, preoperative carcinoembryonic antigen level, and adjuvant chemotherapy. Written informed consent to use the samples for research purposes was obtained from all the patients before surgery. This study was performed in accordance with the Declaration of Helsinki 18 and was approved by the institutional review board at the Sun Yat-sen University Cancer Center. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
All included patients in the study underwent histologic examination with the Giemsa staining method, 13C urea breath test, and/or rapid urease test to ascertain their H pylori infection status before the gastrectomy. Patients who tested negative for H pylori infection before the gastrectomy were categorized as H pylori negative and were consequently excluded from the analysis, whereas those with 1 or more positive results were confirmed to be infected with H pylori .
According to the World Gastroenterology Organization global H pylori guidelines, the first-line treatment includes triple or quadruple therapy regimens, and the recommended treatment duration is 7 to 14 days. 19 In clinical practice, variations in antibiotic selection may occur based on patients’ condition and physicians’ preferences. During data collection, 2 independent investigators carefully reviewed the recorded medications of each patient and confirmed whether the patients received an appropriate anti– H pylori treatment. According to the data collected retrospectively, the patients in our institution primarily received a triple anti– H pylori therapy consisting of amoxicillin, clarithromycin, and omeprazole for 14 days, and they were often advised by clinicians to start anti– H pylori treatment during their postoperative follow-up, particularly 4 to 6 weeks after gastrectomy, when a positive H pylori test result was observed.
Patients received regular follow-up care through outpatient or inpatient visits or through telephone calls every 3 to 6 months during the initial 2 years after surgery, every 6 to 12 months during the 3 to 5 years after surgery, and annually thereafter. In this study, the median (range) follow-up time was 40.4 (0.2-131.3) months. The main follow-up examinations included physical examinations (eg, palpation of left supraclavicular lymph node and digital rectal examination), tumor marker measurement, computed tomography examination, ultrasonographic examination, and endoscopy.
In this study, the primary end point was OS, defined as the time from the date of surgery to the date of death from any cause. The secondary end point was disease-free survival (DFS), defined as the time from the date of surgery to disease progression, relapse, or death, whichever came first. The follow-up period for outcome ascertainment in our study extended until May 19, 2021.
The χ 2 or Fisher test was used to compare differences between groups for categorical variables. Propensity score matching (PSM) was conducted to minimize the selection biases between the 2 groups in the overall cohort, with a ratio of 1:3 and a caliper value of 0.02, using the nearest matching method. The variables used for PSM included Borrmann classification, pathological T and N stages, TNM stage, tumor maximum diameter, type of gastrectomy, and adjuvant chemotherapy. This selection focused on variables that were initially imbalanced or potentially related to clinical outcomes. Kaplan-Meier analysis was used to generate the survival curves, and log-rank tests were used to compare the survival differences between groups. To explore the potential survival benefits of anti– H pylori treatment across specific patient populations, we conducted exploratory subgroup analyses based on the majority of clinical information collected, such as sex, age, and TNM staging, while taking into account the sample size. Then, in the overall cohort, the variables with statistical significance in the univariable analysis were entered into the multivariable Cox proportional hazards regression analysis for both OS and DFS. All statistical analyses were 2-sided, with P < .05 considered statistically significant. Data analysis and graphical visualization were performed using R software, version 4.2.2 (R Foundation for Statistical Computing). Data were analyzed from March to June 2023.
In this study, 1293 patients (median [IQR] age, 59 [50-65] years; 860 [66.5%] male and 433 [33.5%] female) with preoperatively confirmed H pylori infection were included. These participants had a median (IQR) body mass index of 22.0 (19.6-24.0) (calculated as weight in kilograms divided by height in meters squared), and 829 (64.1%) underwent distal gastrectomy. Postoperatively, 600 patients (46.4%) were pathologically diagnosed with TNM stage III disease and 808 (62.5%) received adjuvant chemotherapy. However, as indicated in the Table , only 125 (9.7%) had received anti– H pylori treatment. There were no statistically significant differences between the anti– H pylori treatment group and the non–anti– H pylori treatment group in terms of sex, age, body mass index, smoking history, and comorbidities. However, the anti– H pylori treatment group had a higher proportion of distal gastrectomy (102 of 125 [81.6%] vs 727 of 1168 [62.2%]; P < .001) and received less adjuvant chemotherapy (54 of 125 [43.2%] vs 754 of 1168 [64.6%]; P < .001) compared with the non–anti– H pylori treatment group. Of note, patients in the non–anti– H pylori treatment group had advanced pathological T stage, N stage, and TNM stage as well as larger tumor maximum diameter, which indicated the potential selection bias between the 2 groups.
To minimize the potential influence of selection bias, we used PSM. After PSM, a total of 124 patients in the anti– H pylori treatment group and 364 patients in the non–anti– H pylori treatment group were included, and the clinical characteristics between the 2 groups were well balanced ( Table ).
We conducted a survival analysis to compare OS of the 2 groups ( Figure 2 A). The OS rates were 95.9% (95% CI, 92.5%-99.5%) at 3 years and 94.1% (95% CI, 89.3%-99.2%) at 5 years in the anti– H pylori treatment group compared with 81.4% (95% CI, 79.0%-83.8%) at 3 years and 73.8% (95% CI, 70.7%-77.0%) at 5 years in the non–anti– H pylori treatment group. The OS difference between the 2 groups was significant (hazard ratio [HR], 0.33; 95% CI, 0.18-0.60; P < .001). After PSM, the difference in OS between the 2 groups remained statistically significant (HR, 0.50; 95% CI, 0.26-0.99; P = .048) ( Figure 2 B).
Subsequently, survival analysis on the DFS of the 2 groups was conducted. The DFS rates were 94.5% (95% CI, 90.3%-98.9%) at 3 years and 84.9% (95% CI, 75.6%-95.4%) at 5 years in the anti– H pylori treatment group compared with 70.0% (95% CI, 67.1%-73.1%) at 3 years and 59.2% (95% CI, 55.4%-63.3%) at 5 years in the non–anti– H pylori treatment group. As shown in Figure 2 C, the anti– H pylori treatment group also had a significant advantage in DFS compared with the non–anti– H pylori treatment group (HR, 0.29; 95% CI, 0.17-0.50; P < .001). After PSM, the survival difference in DFS remained statistically significant (HR, 0.48; 95% CI, 0.27-0.87; P = .02) ( Figure 2 D).
Next, we conducted subgroup analysis of OS and DFS (eFigures 1 and 2 in Supplement 1 ). The relative treatment benefit of anti– H pylori was consistent across most of the subgroups, specifically with relation to age, sex, and tumor differentiation. In terms of TNM stage, we found that the differences in OS (HR, 0.86; 95% CI, 0.19-4.00; P = .85) ( Figure 3 A) and in DFS (HR, 0.62; 95% CI, 0.18-2.11; P = .44) ( Figure 3 B) between the anti– H pylori treatment group and non–anti– H pylori treatment group were not significant in patients with stage I disease. However, in patients with stage II/III disease, the differences in OS (HR, 0.43; 95% CI, 0.22-0.85; P = .01) ( Figure 3 C) and DFS (HR, 0.39; 95% CI, 0.21-0.71; P = .002) ( Figure 3 D) between the 2 groups were significant. We also explored the survival benefit of anti– H pylori treatment for patients with TNM stage II/III disease who did or did not undergo adjuvant chemotherapy. The results indicated that among patients who received adjuvant chemotherapy, anti– H pylori treatment conferred survival benefits for both OS (HR, 0.49; 95% CI, 0.24-0.99; P = .046) ( Figure 4 A) and DFS (HR, 0.41; 95% CI, 0.22-0.78; P = .006) ( Figure 4 B), whereas among those who did not receive adjuvant chemotherapy, anti– H pylori treatment did not provide survival benefits for either OS (HR, 0.29; 95% CI, 0.04-2.08; P = .22) ( Figure 4 C) or DFS (HR, 0.29; 95% CI, 0.04-2.07; P = .22) ( Figure 4 D).
Furthermore, univariable and multivariable analyses using the Cox proportional hazards regression model were performed. As detailed in eTable 1 in Supplement 1 , age, Lauren classification, TNM stage, tumor maximum diameter, adjuvant chemotherapy, and anti– H pylori treatment were associated with the OS in the univariable analysis. In the multivariable analysis, age (≥60 years [HR, 1.47; 95% CI, 1.10-1.96; P = .009]), Lauren classification (intestinal type [HR, 0.67; 95% CI, 0.48-0.95; P = .02] and mixed type [HR, 0.62; 95% CI, 0.44-0.87; P = .007]), TNM stage II/III (HR, 8.87; 95% CI, 3.99-19.75; P < .001), tumor maximum diameter (>4 cm [HR, 2.33; 95% CI, 1.72-3.18; P < .001]), adjuvant chemotherapy (HR, 0.63; 95% CI, 0.46-0.85; P = .003), and anti– H pylori treatment (HR, 0.38; 95% CI, 0.17-0.87; P = .02) were identified as independent factors associated with OS. In eTable 2 in Supplement 1 , univariable and multivariable analyses for DFS showed that TNM stage II/III (HR, 4.61; 95% CI, 2.84-7.49; P < .001), tumor maximum diameter (>4 cm [HR, 2.37; 95% CI, 1.87-3.01; P < .001]), and anti– H pylori treatment (HR, 0.48; 95% CI, 0.28-0.83; P = .008) remained associated with DFS.
To the best of our knowledge, this is the largest study to investigate the survival benefit of anti– H pylori treatment in patients with H pylori –positive GC after radical gastrectomy. Our study found that postgastrectomy anti– H pylori treatment may provide substantial survival benefits in terms of both OS and DFS in a large cohort subjected to a long-term follow-up period of up to 10 years. The aforementioned conclusions remained consistent after PSM. The positive association of anti– H pylori treatment remained consistent across most subgroups, with significant OS and DFS benefits particularly pronounced in the TNM stage II/III subgroup. In contrast, the TNM stage I subgroup did not exhibit such benefits. Notably, although the patients with TNM stage II/III disease who received adjuvant chemotherapy derived benefits from anti– H pylori treatment, those who did not undergo such chemotherapy did not experience these advantages. Furthermore, Cox proportional hazards regression multivariable analysis demonstrated that anti– H pylori treatment is independently associated with both OS and DFS.
The worldwide prevalence of H pylori infection is significant. In 2015, approximately 4.4 billion individuals were infected with H pylori , constituting approximately 50% of the total population. 3 Notably, H pylori infection increases the risk of GC by approximately 3 times and is the most significant risk factor for GC. 20 The role of H pylori infection in facilitating the development of GC is well established. However, the correlation between H pylori infection and the prognosis of patients with GC is yet to be clearly defined. Some studies have reported that patients who test negative for H pylori before undergoing curative GC surgery seem to have a worse prognosis compared with H pylori –positive patients. 2 , 10 , 11 , 21 Furthermore, a previous study 12 indicated that H pylori –negative GC is associated with worse OS and has intrinsic correlations with adverse pathological and clinical characteristics.
In contrast, there is evidence suggesting that H pylori infection may play a role in the progression of gastric mucosal carcinogenesis in the remnant stomach after gastrectomy for GC. 22 In the Korean guidelines for H pylori , eradication of H pylori is recommended to prevent gastric mucosal dysplasia and even GC recurrence in patients who undergo distal gastrectomy. 23 Furthermore, several high-quality RCTs have demonstrated that prophylactic eradication of H pylori after endoscopic resection of early GC could prevent the development of MGC. Fukase et al 13 reported that the odds ratio for developing MGC was 0.353 (95% CI, 0.161-0.775; P = .009) in the full intention-to-treat population, whereas the hazard ratio for developing MGC was 0.339 (95% CI, 0.157-0.729; P = .003) in the modified intention-to-treat population when comparing the eradication group with the control group. The study performed by Choi et al, 14 which aimed to assess the long-term effects of H pylori eradication treatment on histologic improvement and the prevention of MGC in patients after endoscopic resection for early GC or high-grade adenoma, reported that MGC developed in 14 patients (7.2%) in the treatment group and 27 patients (13.4%) in the placebo group, with an HR of 0.50 in the treatment group (95% CI, 0.26-0.94; P = .03).
In the clinical setting, both the actual rate of anti– H pylori treatment use and the H pylori eradication rate remain low. In our study, few H pylori –positive patients (9.7%) opted for anti –H pylori treatment, and it remains an exploratory and unresolved question whether H pylori eradication is beneficial to the prognosis of patients after gastrectomy. To the best of our knowledge, only a few researchers have conducted exploratory studies in this area, and the findings are in dispute. The study conducted by Kim et al, 15 which was a retrospective outcome event analysis extracted from a prospective cohort of an RCT study involving 169 patients, reported that among patients with GC after distal gastrectomy, there were no significant differences between the anti– H pylori treatment group and the placebo group in the 5-year OS, 5-year cumulative GC-specific death, and 5-year cancer recurrence rates. However, the study had certain limitations; it originated from an RCT cohort initially focused on evaluating H pylori eradication effects on mucosal atrophy and intestinal metaplasia in the remnant stomach after distal gastrectomy. Additionally, the study lacked patient stratification and was constrained by a relatively small sample size. In another study of 1031 patients with GC who underwent subtotal gastrectomy, Choi et al 16 reported that the eradication group had a statistically significant advantage in terms of OS and GC-specific survival compared with the noneradication group, and this advantage persisted after PSM. However, the study included patients who tested positive for H pylori during the first postoperative gastroscopy follow-up, which occurred annually after the gastrectomy, and who underwent H pylori eradication treatment within 2 years. As a result, the interval between surgery and undergoing the test for H pylori infection in this study may be too long, and the infection status of H pylori may vary after surgery.
We designed this large-scale study to provide relevant evidence for future clinical practice for patients with GC and H pylori infection. We found that patients with H pylori infection who received anti– H pylori treatment experienced survival advantages in terms of OS and DFS compared with those who did not receive the treatment, and these advantages persisted even after PSM, similar to the previous study mentioned above. 16 Furthermore, detailed stratified analyses revealed that most subgroups experienced the survival benefit of anti– H pylori treatment. In our Cox proportional hazard regression models, anti– H pylori treatment stood out as an independent prognostic factor for both OS and DFS.
Imbalances in factors related to survival between the 2 patient groups could impact the accuracy of the analysis. Therefore, it may be crucial to perform the subgroup analysis and use PSM. The previous study conducted by Choi et al 16 found that H pylori eradication treatment has statistically significant survival benefits in both patients with early GC and those with advanced GC after subtotal gastrectomy. However, our subgroup analysis based on TNM stage indicated that the anti– H pylori treatment group had a significant advantage in both OS and DFS compared with the non–anti– H pylori treatment group only in patients with TNM stage II/III GC, indicating that anti– H pylori treatment may provide survival benefits only in patients with advanced-stage GC.
Another intriguing observation is that although anti– H pylori treatment offered survival benefits to the patients with TNM stage II/III who underwent adjuvant chemotherapy, no such difference was noted in those patients without adjuvant chemotherapy. This finding suggests that combining anti– H pylori treatment with adjuvant chemotherapy might offer enhanced benefits to patients. To further substantiate our conjecture, we also designed an RCT that aimed to corroborate whether anti– H pylori treatment can bring survival benefits to patients with surgically resected GC. 24 We hope that with the emergence of more relevant research, more effective treatment strategies will be available for patients with H pylori infection and GC.
We acknowledge that the current study has several limitations. First, it is a retrospective analysis based on clinical data from a single institution, which may introduce selection bias. Second, the study period is relatively long, during which the details of surgical procedures and postoperative treatments received by patients may have varied. Third, the sample size is relatively small in certain subgroup analyses, which may result in limited statistical power.
This study’s findings indicate that for patients with GC and H pylori infection before surgery, undergoing anti– H pylori treatment may be associated with notable survival advantages. We suggest expanding the scope of future H pylori treatment guidelines and implementing thorough screening and treatment for H pylori in patients undergoing surgical treatment for GC.
Accepted for Publication: January 31, 2024.
Published: March 28, 2024. doi:10.1001/jamanetworkopen.2024.3812
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Zhao Z et al. JAMA Network Open .
Corresponding Authors: Runcong Nie, MD, PhD ( [email protected] ), and Yingbo Chen, MD ( [email protected] ), Department of Gastric Surgery, Sun Yat-sen University Cancer Center, No. 651 Dongfeng Eastern Rd, Guangzhou 510060, Guangdong, China.
Author Contributions: Drs Y. Chen and R. Nie had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Zhao, R. Zhang, G. Chen, and M. Nie contributed equally to this work.
Concept and design: Zhao, G. Chen, F. Lin, Zheng, Y. Chen, R. Nie.
Acquisition, analysis, or interpretation of data: R. Zhang, M. Nie, F. Zhang, X. Chen, J. Lin, Z. Chen, F. Lin, Wei, Zheng, Ruan, Huang.
Drafting of the manuscript: Zhao, R. Zhang, G. Chen, M. Nie, X. Chen, J. Lin, Wei, Zheng, Ruan.
Critical review of the manuscript for important intellectual content: Zhao, F. Zhang, Z. Chen, F. Lin, Huang, Y. Chen, R. Nie.
Statistical analysis: Zhao, R. Zhang, G. Chen, M. Nie, F. Zhang, X. Chen, J. Lin, Wei, Zheng, Ruan, Huang.
Obtained funding: R. Zhang, Y. Chen, R. Nie.
Administrative, technical, or material support: Zheng.
Supervision: Y. Chen, R. Nie.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by grant 202201010885 from Guangzhou Science and Technology Plan Projects (Dr R. Nie), grant 81772589 from National Natural Science Foundation of China (Dr R. Nie), grants 2114050000603 (Dr Y. Chen) and SL2024A04J01162 (Dr R. Zhang) from Guangzhou Basic and Applied Basic Research Foundation, and grants Y-tongshu2021/qn-0227 and Y-Young2022–0281 from Beijing Xisike Clinical Oncology Research Foundation (Dr R. Nie).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Advertisement
Gastric cancer: a comprehensive review of current and future treatment strategies
- Non-Thematic Review
- Published: 07 September 2020
- Volume 39 , pages 1179–1203, ( 2020 )
Cite this article
- Rachel E. Sexton 1 ,
- Mohammed Najeeb Al Hallak 1 ,
- Maria Diab 1 &
- Asfar S. Azmi ORCID: orcid.org/0000-0003-1178-9505 1
10k Accesses
285 Citations
11 Altmetric
Explore all metrics
Gastric cancer remains a major unmet clinical problem with over 1 million new cases worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. A major fraction of gastric cancer has been linked to variety of pathogenic infections including but not limited to Helicobacter pylori ( H. pylori ) or Epstein Barr virus (EBV). Strategies are being pursued to prevent gastric cancer development such as H. pylori eradication, which has helped to prevent significant proportion of gastric cancer. Today, treatments have helped to manage this disease and the 5-year survival for stage IA and IB tumors treated with surgery are between 60 and 80%. However, patients with stage III tumors undergoing surgery have a dismal 5-year survival rate between 18 and 50% depending on the dataset. These figures indicate the need for more effective molecularly driven treatment strategies. This review discusses the molecular profile of gastric tumors, the success, and challenges with available therapeutic targets along with newer biomarkers and emerging targets.
This is a preview of subscription content, log in via an institution to check access.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Institutional subscriptions
Similar content being viewed by others

Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition)
Japanese Gastric Cancer Association

Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers
Yu Wang & Baocheng Deng

Molecular pathological classification of colorectal cancer—an update
Philip D. Dunne & Mark J. Arends
Prashanth, R., & Barsouk, A. (2019). Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad Gastroenterologiczny., 14 (1), 26–38. https://doi.org/10.5114/pg.2018.80001 .
Article CAS Google Scholar
American Cancer Society. Key statistics about stomach cancer. Cancer.org . Accessed 3/6/2020.
Chen, Y. C., Fang, W. L., Wang, R. F., Liu, C. A., Yang, M. H., Lo, S. S., Wu, C. W., Li, A. F., Shyr, Y. M., & Huang, K. H. (2016). Clinicopathological variation of Lauren classification in gastric cancer. Pathology Oncology Research, 22 (1), 197–202. https://doi.org/10.1007/s12253-015-9996-6 .
Article CAS PubMed Google Scholar
Hu, B., Hajj, N., Sittler, S., Lammert, N., Barnes, R., & Meloni-Ehrig, A. (2012). Gastric cancer: classification, histology and application of molecular pathology. Journal of Gastrointestinal Oncology, 3 (3), 251–261. https://doi.org/10.3978/j.issn.2078-6891.2012.021 .
Article PubMed PubMed Central Google Scholar
Li, Y; Xue, X.W.; Luo, Y.F.; Wu, H.W.; Chen, J; Zhou, W.X. Clinicopathologic features of gastric adenocarcinoma based on the revised Lauren’s classification. Zhonghua Bing Li Xue Za Zhi 2018; 47(7): 486–491. DOI: https://doi.org/10.3760/cma.j.issn.0529-5807.2018.07.002 .
Miaozhen, Q., Zhou, Y., Zhang, X., Wang, Z., Wang, F., Shao, J., Lu, J., Jin, Y., Wei, X., Zhang, D., et al. (2014). Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer, 14 (823). https://doi.org/10.1186/1471-2407-14-823 .
Liang, Y. X., Deng, J. Y., Guo, H. H., et al. (2013). Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World Journal of Gastroenterology, 19 (39), 6568–6578. https://doi.org/10.3748/wjg.v19.i39.6568 .
Petrelli, F., Berenato, R., Turati, L., Mennitto, A., Steccanella, F., Caporale, M., Dallera, P., de Braud, F., Pezzica, E., Di Bartolomeo, M., et al. (2017). Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. Journal of Gastrointestinal Oncology., 8 (1).
Rugge, M., Fassan, M., & Graham, D. Y. (2015). Epidemiology of Gastric Cancer. Gastric Cancer , 23–34.
Han, J. P., Hong, S. J., & Kim, H. K. (2014). Long-term outcomes of early gastric cancer diagnosed as mixed adenocarcinoma after endoscopic submucosal dissection. JGH., 30 (2), 316–320. https://doi.org/10.1111/jgh.12838 .
Article Google Scholar
Stahl, P., Seeschaaf, C., Lebok, P., et al. (2015). Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol, 15 , 7. Published 2015 Feb 5. https://doi.org/10.1186/s12876-015-0231-4 .
Article CAS PubMed PubMed Central Google Scholar
Bae, J. M., & Kim, E. H. (2016). Epstein-Barr virus and gastric cancer risk: a meta-analysis with meta-regression of case-control studies. Journal of Preventive Medicine and Public Health, 49 (2), 97–107. https://doi.org/10.3961/jpmph.15.068 .
Wroblewski, L. E., Peek, R. M., & Wilson, K. T. (2010). Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical Microbiology Reviews, 23 (4), 713–739. https://doi.org/10.1128/CMR.00011-10 .
Cats, A., Meuwissen, S. G., Forman, D., Craanen, M. E., & Kuipers, E. J. (1998). Helicobacter pylori: a true carcinogen? European Journal of Gastroenterology & Hepatology, 10 (6), 447–450. https://doi.org/10.1097/00042737-199806000-00001 .
Conteduca, V., Sansonno, D., Lauletta, G., Russi, S., Ingravallo, G., & Dammacco, F. H. (2012). Pylori infection and gastric cancer: state of the art (Review). International Journal of Oncology . https://doi.org/10.3892/ijo.2012.1701 .
Rick, J. R., Goldman, M., Semino-Mora, C., Liu, H., Olsen, C., Rueda-Pedraza, E., Sullivan, C., & Dubois, A. (2010). In situ expression of cagA and risk of gastroduodenal disease in Helicobacter pylori infected children. Journal of Pediatric Gastroenterology and Nutrition, 50 (2), 167–172. https://doi.org/10.1097/MPG.0b013e3181bab326 .
Hatakeyama, M. (2004). Oncogenic mechanisms of the helicobacter pylori CagA protein. Nature Reviews. Cancer, 4 (9), 688–694. https://doi.org/10.1038/nrc1433 .
Wroblewski, L. E., Peek Jr., R. M., & Wilson, K. T. (2010). Helicobacter pylori and gastric Cancer: factors that modulate disease risk. Clinical Microbiology Reviews , 713–739. https://doi.org/10.1128/CMR.00011-10 .
Jones, K. R., Joo, Y. M., Jang, S., Yoo, Y. J., Lee, H. S., Chung, I. S., Olsen, C. H., Whitmire, J. M., Merrell, S., & Cha, J. H. (2009). Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. Journal of Clinical Microbiology , 959–968. https://doi.org/10.1128/JCM.02330-08 .
Kim, K. E. (2003). Gastric cancer in Korean Americans: risks and reductions. Korean Korean Am Stud Bull, 13 (1/2), 84–90 PMCID: 1592326.
PubMed PubMed Central Google Scholar
Lee Y. Association between helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology (New York, N.Y. 1943) . 05/2016;150(5):1113–1124.e5 https://doi.org/10.1053/j.gastro.2016.01.028 .
Iizas, H., Nanbo, A., Nishikawa, J., & Yoshiyama, H. (2012). Epstein Barr virus (EBV)-associated gastric carcinoma. Viruses., 4 (12), 3420–3439. https://doi.org/10.3390/v4123420 .
Kim, Y. S., Paik, S. R., Kim, H. K., Yeom, B. W., Kim, I., & Lee, D. (1998). Epstein Barr virus and CD21 expression in gastrointestinal tumors. Pathology, 194 (1), 705–711. https://doi.org/10.1016/S0344-0338(98)80130-1 .
Kirschner, A. N., Omerovic, J., Popov, B., Longnecker, R., & Jardetzky, T. S. (2006). Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. Journal of Virology, 80 (19), 9444–9454. https://doi.org/10.1128/JVI.00572-06 .
Xiao, J., Palefsky, J. M., Herrera, R., Berline, J., & Tugizov, S. M. (2008). The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology., 370 (2), 430–442. https://doi.org/10.1016/j.virol.2007.09.012 .
Vogelaar, I. P., van der Post, R. S., Bisseling, T. M., van Krieken, J. H. J., Ligtenberg, M. J., & Hoogerbrugge, N. (2012). Familial gastric cancer: detection of a hereditary cause helps to understand its etiology. Hereditary Cancer in Clinical Practice, 10 (1), 18. https://doi.org/10.1186/1897-4287-10-18 .
Josefson, D. (2001). Prophylactic gastrectomy seems to extend life of patients with a family history of stomach cancer. BMJ, 322 (7302), 1566 PMCID: PMC1173358.
Ferro, A., Rosato, V., Rota, M., Costa, A. R., Morais, S., Pelucchi, C., Johnson, K. C., Hu, J., Palli, D., Ferraroni, M., et al. (2019). Meat intake and risk of gastric cancer in the stomach cancer pooling (StoP) project. International Journal of Cancer . https://doi.org/10.1002/ijc.32707 .
Islami, F., Boffetta, P., Ren, J. S., Pedoeim, L., Khatib, D., & Kamangar, F. (2009). High-temperature beverages and foods and esophageal cancer risk—a systematic review. International Journal of Cancer, 125 (3), 491–524. https://doi.org/10.1002/ijc.24445 .
Hu, M. L., Yeh, K. T., Lin, P. M., Hsu, C. M., Hsiao, H. H., Liu, Y. C., Lin, H. Y., Lin, S. F., & Yang, M. Y. (2014). Deregulated expression of circadian rhythm clock genes in gastric cancer. BMC Gastroenterology, 14 , 67. https://doi.org/10.1186/1471-230x-14-67 .
Praud, D; Rota, M; Pelucchi, C; Bertuccio, P; Rosso, T; Galeone, C; Zhang, Z.F; Matsuo, K Ito, H; Hu, J; et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) project. European Journal of Cancer Prevention 2018; 27(2): 124–133. DOI: https://doi.org/10.1097/CEJ.0000000000000290 .
Amin, M. B., Greene, F. L., Edge, S. B., Compton, C. C., Gershenwald, J. E., Brookland, R. K., Meyer, L., Gress, D. M., Byrd, D. R., & Winchester, D. P. (2017). The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: a Cancer Journal for Clinicians, 67 (2), 93–99. https://doi.org/10.3322/caac.21388 .
Bollschweiler, E., Berlth, F., Baltin, C., Mönig, S., & Hölscher, A. H. (2014). Treatment of early gastric cancer in the Western world. World Journal of Gastroenterology, 20 (19), 5672–5678. https://doi.org/10.3748/wjg.v20.i19.5672 .
Wakahara, T., Ueno, N., Maeda, T., Kanemitsu, K., Yoshikawa, T., Tsuchida, S., & Toyokawa, A. (2018). Impact of gastric cancer surgery in elderly patients. Oncology, 94 (2), 79–84. https://doi.org/10.1159/000481404 .
Article PubMed Google Scholar
Cha, J. H., & Jang, J. S. (2020). Correlation between healing type of lesion and recurrence in gastric neoplastic lesions after endoscopic submucosal dissection. The Turkish Journal of Gastroenterology, 31 (1), 36–41. https://doi.org/10.5152/tjg.2020.18764 .
Sasako, M., Sakuramoto, S., Katai, H., Kinoshita, T., Furukawa, H., Yamaguchi, T., Nashimoto, A., Fujii, M., Nakajima, T., & Ohashi, Y. (2011). Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of Clinical Oncology, 29 (33), 4387–4393. https://doi.org/10.1200/JCO.2011.36.5908 .
Gastric Cancer. National Comprehensive Cancer Network (NCCN) Guidelines. Verison 1. (2020). March 19, 2020. In Available from https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf .
Google Scholar
Jayanathan, M., Erwin, R. P., Molacek, N., Fluck, M., Hunsinger, M., Wild, J., Arora, T. K., Shabahang, M. M., Franko, J., & Blansfield, J. A. (2019). MAGIC versus MacDonald treatment regimes for gastric cancer: Trends and predictors of multimodal therapy for gastric cancer using the National Cancer Database. American Journal of Surgery . https://doi.org/10.1016/j.amjsurg.2019.06.005 .
Schwartz, G.K.; Christman, K; Saltz, L; Casper, E; Quan, V; Bertino, J; Martin, D.S.; Colofiore, J; Kelsen, D. A phase I trial of modified, dose intensive FAMTX regimen (high dose 5-fluorouracil + doxorubicin + high dose methotrexate + leucovorin) with oral uridine rescue. ACS Journals. 1996. DOI: https://doi.org/10.1002/(SICI)1097-0142(19961101)79:9<1998::AID-CNCR21>3.0.CO;2-T .
Webb, A., Cunningham, D., Scarffe, J. H., Harper, P., Norman, A., Joffe, J. K., Hughes, M., Mansi, J., Findlay, M., et al. (1997). Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. Journal of Clinical Oncology, 15 (1), 261–267.
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F., Middleton, G., Daniel, F., Oates, J., & Norman, A. R. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England Journal of Medicine, 358 , 36–46.
Al-Batran, S. E., Homann, N., Pauligk, C., Goetze, T. O., Meiler, J., Kasper, S., Koop, H. G., Mayer, F., Haag, G. M., Luley, K., et al. (2019). Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, rescectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomized, phase 2/3 trial. Lancet., 393 (10184), 1948–1957. https://doi.org/10.1016/S0140-6736(18)32557-1 .
Masuishi, T., Kadowaki, S., Kondo, M., Komori, A., Sugiyama, K., Mitani, S., Honda, K., Narita, Y., Taniguchi, H., Ura, T., et al. (2017). FOLFOX as first-line therapy for gastric cancer with severe peritoneal metastasis. Anticancer Res, 37 (12), 7037–7042. https://doi.org/10.21873/anticanres.12174 .
Ahn, H. S., Jeong, S. H., Son, Y. G., Lee, H. J., Im, S. A., Bang, Y. J., Kim, H. H., & Yang, H. K. (2014). Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. The British Journal of Surgery, 101 (12), 1560–1565. https://doi.org/10.1002/bjs.9632 .
Al-Batran, S.-E., Hofheinz, R. D., Schmalenberg, H., Strumberg, D., Goekkurt, E., Angermeier, S., Zander, T., Potenberg, J., Koop, H. G., Pink, D., et al. (2020). Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): results of the phase II-portion—a multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. Journal of Clinical Oncology, 38 (15).
Hofheinz, R. D., Haag, G. M., Ettrich, T. J., Borchert, K., Kretzschmar, A., Teschendorf, C., Siegler, G. M., Ebert, M. P., Goekkurt, E., Welslau, M., et al. (2020). Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trail of the AIO. Journal of Clinical Oncology, 35 (18).
Bang Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Kim, Y.H; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. The Lancet (British edition). 2012;379(9813):315–321. DOI: https://doi.org/10.1016/S0140-6736(11)61873-4 .
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., Lordick, F., Ohtsu, A., Omuro, Y., Satoh, T., et al. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. The Lancet., 376 (9742), 687–697. https://doi.org/10.1016/S0140-6736(10)6121-X .
Abrahao-Machado, L. F. (2016). Scapulatempo-Neto; C. HER2 testing in gastric cancer: an update. World Journal of Gastroenterology, 22 (19), 4619–4625. https://doi.org/10.3748/wjg.v22.i19.4619 .
An Cutsem, E., Muro, K., Cunningham, D., Bodoky, G., Sobrero, A., Cascinu, S., Ajani, J., Al-Batran, S. E., Wainberg, Z. A., Wijayawardana, S. R., et al. (2020). Biomarker analyses of second-line Ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. European Journal of Cancer, 127 , 150–157. https://doi.org/10.1016/j.ejca.2019.10.026 .
FDA grands accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA . Accessed July 28, 2020.G.
Fuchs, C. S., Doi, T., Jang, R. W., Muro, K., Satoh, T., Machado, M., Sun, W., Jalal, S. I., Shah, M. A., Metges, J. P., et al. (2018). Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE- 059 trial. JAMA Oncology, 4 , e180013.
FDA approves pembrolizumab for adults and children with TMB-H solid tumors. (2020). FDA. Accessed July , 25.
Makiyama, A., Arimizu, K., Hirano, G., Makiyama, C., Matsushita, Y., Shirakawa, T., Ohmura, H., Komoda, M., Uchino, K., Inadomia, K., et al. (2018). Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer, 21 (3), 464–472. https://doi.org/10.1007/s10120-017-0759-9 .
Bando, H; Doi, T; Muro, K; Yasui, H; Nishina, T; Yamaquchi, K; Takahashi, S; Nomura, S; Kumo, H; Shitara, K; et al. A multicenter phase II study of TAS-102 monotherapy in patients with pretreated advanced gastric cancer (EPOC1201 https://doi.org/10.1016/j.ejca.2016.04.009 .
Hansson, L. E., Sparén, P., & Nyrén, O. (1999). Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Annals of Surgery, 230 (2), 162–169. https://doi.org/10.1097/00000658-199908000-00005 .
Ferguson, F. M., & Gray, N. S. (2018). Kinase inhibitors: the road ahead. Nature Reviews. Drug Discovery, 17 , 353–377.
Clinicaltrials.gov . A Phase II Trial of STI571 in the Treatment of Metastatic Gastric Cancer. Identifier: NCT00068380.
Bang, Y. J., Kang, Y. K., Kang, W. K., Boku, N., Chung, H. C., Chen, J. S., Doi, T., Sun, Y., Shen, L., Qin, S., et al. (2011). Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Investigational New Drugs, 29 (6), 1449–1458. https://doi.org/10.1007/s10637-010-9438-y .
Gotink, K.J.; Broxterman, H.J.; Labots, M; de Haas, R.R.; Dekker, H; Honeywell, R.J.; Rudek, M.A.; Beerepoot, L.V.; Musters, R.J; Jansen, G; et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clinical Cancer Research 2011 ; 17(23):7337–7346. DOI: https://doi.org/10.1158/1078-0432.CCR-11-1667 .
Guo, T; Hajdu, M; Agaram, N.P.; Shinoda, H; Veach, D; Clarkson, B.D.; Maki, R.G.; Singer, S; DeMatteo, R.P.; Besmer, P; et al. Mechanisms of sunitinib resistance in gastrointestinal stromal tumors harboring KITAY502-3ins mutation: an in vitro mutagenesis screen for drug-resistance. Clinical Cancer Research 2009; 15(22): 6862–6870. DOI: https://doi.org/10.1158/1078-0432.CCR-09-1315 .
Clinicaltrials.gov . Docetaxel with or without vandetanib in treating patients with metastatic stomach cancer. Identifier: NCT00683787.
Boland, P. M., Meyer, J. E., Berger, A. C., Cohen, S. J., Neuman, T., Cooper, H. S., Olszanski, A. J., Davey, M., Cheng, J. D., Lebenthal, A., et al. (2018). Induction therapy for locally advanced, resectable esophagogastric cancer: A phase I trial of vandetanib (ZD6474), paclitaxel, carboplatin, 5-fluorouracil and radiotherapy followed by resection. American Journal of Clinical Oncology, 40 (4), 393–398. https://doi.org/10.1097/COC.0000000000000171 .
Xie, L., Su, X., Zhang, L., Yin, X., Tang, L., Zhang, X., Xu, Y., Gao, Z., Liu, K., Zhou, M., et al. (2013). FGFR2 gene amplification in gastric cancer predicts sensitivity to the FGFR inhibitor AZD4547. Clinical Cancer Research, 19 (9), 2572–2583. https://doi.org/10.1158/1078-0432.CCR-12-3898 .
Mitani, S., & Kawakami, H. (2020). Emerging targeted therapies for HER2 positive gastric cancer that can overcome Trastuzumab resistance. Cancers (Basel) . , 12 (2), 400. https://doi.org/10.3390/cancers12020400 .
Article CAS PubMed Central Google Scholar
Bhat, K. M. R., & Setaluri, V. (2007). Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res, 13 (10). https://doi.org/10.1158/1078-043.CCR-06-3040 .
Huang, M., Ma, X., Shi, H., Hu, L., Fan, Z., Pang, L., Zhu, F., Yang, X., Xu, W., Liu, B., et al. (2017). FAM83D , a microtubule-associated protein, promotes tumor growth and progression of human gastric cancer. Oncotarget, 8 (43), 74479–74493. https://doi.org/10.18632/oncotarget.20157 .
Cochran, J. C., Gatial III, J. E., Kapoor, T. M., & Gilbert, S. P. (2005). Monastrol inhibition of the mitotic kinesin Eg5. JBC., 280 (13), 12658–12667. https://doi.org/10.1074/jbc.M413140200 .
Torgovnick, A., & Schumacher, B. (2015). DNA repair mechanisms in cancer development and therapy. Frontiers in Genetics, 6 , 157. https://doi.org/10.3389/fgene.2015.00157 .
Marin, J. J., Al-Abdulla, R., Lozano, E., Briz, O., Bujanda, L., Banales, J. M., & Macias, R. I. (2016). Mechanisms of resistance to chemotherapy in gastric cancer. Anti-Cancer Agents in Medicinal Chemistry, 16 (3), 318–334.
Meehan, R. S., & Chen, A. P. (2016). New treatment option for ovarian cancer: PARP inhibitors. Gynecologic Oncology Research and Practice., 7 (3).
Nijman, S. M. B. (2011). Synthetic lethality: general principles, utility and detection using genetic screens in human cells. FEBS Letters, 585 (1), 1–6. https://doi.org/10.1016/j.febslet.2010.11.024 .
Mo, M; Yang, J; Zhu, X; Zhu, J. Use of olaparib in patients with advanced gastric cancer. Oncology . 2018; 19(2): 75. DOIL https://doi.org/10.1016/S1470-2045(18)30023-8 .
Bang, Y.J.; Xu, R.H.; Chin, K; Lee, K.W.; Park, S.H.; Rha, S.Y.; Shen, L; Qin, S; Xu, N; Im, S.A; et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomized, placebo-controlled, phase 3 trial. Oncology. 2017; 18(12): 1637–1651. DOI: https://doi.org/10.1016/S1470-2045(17)30682-4 .
Cechini, M., LoRusso, P., Shyr, Y., Ivy, P., Sklar, J., Dooley, K., & Lacy, J. (2018). NCI 10066: A phase I-II study of Olaparib in combination with ramucirumab in metastatic gastric and gastroesophageal junction (GEJ) adenocarcinoma. Journal of Clinical Oncology, 36 (15).
Jang, J. Y., Kang, Y. J., Sung, B., Kim, M. J., Park, C., Kang, D., Moon, H. R., Chung, H. Y., & Kim, N. D. (2018). MHY440, a novel topoisomerase I inhibitor, induces cell cycle arrest and apoptosis via a ROS-dependent DNA damage signaling pathway in AGS human gastric Cancer cells. Molecules, 24 (1), E96. https://doi.org/10.3390/molecules24010096 .
Yi, H., Yan, X., Luo, Q., Yuan, L., Li, B., Pan, W., Zhang, L., Chen, H., Wang, J., Zhang, Y., et al. (2018). A novel small molecule inhibitor of MDM2-p53 (APG-115) enhances radiosensitivity of gastric adenocarcinoma. Journal of Experimental & Clinical Cancer Research, 37 (1), 97. https://doi.org/10.1186/s13046-018-0765-8 .
Wu, Q. N., Liao, Y. F., Lu, Y. X., Wang, Y., Lu, J. H., Zeng, Z. L., Huang, Q. T., Sheng, H., Yun, J. P., Xie, D., et al. (2018). Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Letters, 412 , 243–255. https://doi.org/10.1016/j.canlet.2017.10.007 .
Fok, J. H., Ramos-Montoya, A., Vazquez-Chantada, M., Wijnhoven, P. W. G., Follia, V., James, N., Farrington, P. M., Karmokar, A., Willis, S. E., Cairns, J., et al. (2019). AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nature Communications, 10 (5065).
Tamura, T., Ohira, M., Tanaka, H., Muquruma, K., Toyokawa, T., Kubo, N., Sakurai, K., Amano, R., Kimura, K., Shibutani, M., et al. (2015). Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric Cancer. Anticancer Research, 35 (10), 5369–5376.
CAS PubMed Google Scholar
B; Mondaca, S; Sanchez, C; Galindo, H; Nervi, B; Ibanez, C; et al. High proportion of potential candidates for immunotherapy in a Chilean cohort of gastric cancer patients: results of the FORCE1 Study. Cancers (Basel) . 2019 ; 11(9): E1275. DOI: https://doi.org/10.3390/cancers11091275 .
Lee, H. S., Chang, M. S., Yang, H. K., Lee, B. L., & Kim, W. H. (2004). Epstein-Barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein-Barr virus-negative carcinoma. Clinical Cancer Research, 10 , 1698–1705.
Fuchs, C.S.; Doi, T; Jang, R.W.; Muro, K; Satoh, T; Machado, M; Sun, W; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical keynote-059 trial. JAMA Oncology 2018; 4(5):e180013. DOI: https://doi.org/10.1001/jamaoncol.2018.0013 .
Xing, X., Guo, J., Ding, G., Li, B., Dong, B., Feng, Q., Li, S., Zhang, J., Ying, X., Cheng, X., et al. (2017). Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology, 7 (3), e1356114 DOI: 1080/2162402X.2017.1356144.
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., Chung, H. C., Chen, J. S., Muro, K., Kang, W. K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo controlled, phase 3 trial. The Lancet., 390 (10111), 2461–2471. https://doi.org/10.1016/S0140-6736(17)31827-5 .
Hara, H., Shoji, H., Takahari, D., Esaki, T., Machida, N., Nagashima, K., Aoki, K., Honda, K., Miyamoto, T., Boku, N., et al. (2019). Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study). Journal of Clinical Oncology, 37 (4).
Xu, Y. H., Li, Z. L., & Qiu, S. F. (2018). IFN-y induces gastric cancer cell proliferation and metastasis through upregulation of integrin B3-mediated NF-kB signaling. Translational Oncology, 11 (1), 182–192. https://doi.org/10.1016/j.tranon.2017.11.008 .
Ham, I. H., Oh, H. J., Jin, H., Bae, C. A., Jeon, S. M., Choi, K. S., Son, S. Y., Han, S. U., Brekken, R. A., Lee, D., & Hur, H. (2019). Targeting interleukin-6 as a stragety to overcome stroma-induced resistance to chemotherapy in gastric cancer. Molecular Cancer, 18 (68).
Zhang, Z., Zhang, J., Miao, L., Liu, K., Yang, S., Pan, C., & Jiao, B. (2012). Interleukin-11 promotes the progress of gastric carcinoma via abnormally expressed Versican. International Journal of Biological Sciences, 8 (3), 383–393. https://doi.org/10.7150/ijbs.3579 .
Wang, Y., Wu, H., Wu, Bian, Z., & Gao, Q. (2014). Interleukin 17A promotes gastric cancer invasiveness via NF-kB mediated matrix metalloproteinases 2 and 9 expression. PLoS One . https://doi.org/10.1371/journal.pone.0096678 .
Khawar, M. B., Abbasi, M. H., & Sheikh, N. (2016). IL-32: A novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators of Inflammation .
Lazar, D. C., Avram, M. F., Romosan, I., Cornianu, M., Taban, S., & Goldis, A. (2018). Prognostic significance of tumor immune microenvironment and immunotherapy: Novel insights and future perspectives in gastric cancer. World Journal of Gastroenterology, 24 (32), 3583–3616. https://doi.org/10.3748/wjg.v24.i32.3583 .
Zhan, X., Wang, B., Li, L., Li, J., Wang, H., Chen, L., Jiang, H., Wu, M., Xiao, J., Peng, X., et al. (2019). Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. Journal of Clinical Oncology, 37 (15).
Clinicaltrials.gov . Intraperitoneal infusion of EpCAM CAR-T cell in advanced gastric cancer with peritoneal metastasis. Identifier: NCT03563326.
Lu, J., Xu, Y., Wu, Y., Huang, X. Y., Xie, J. W., Wang, J. B., Lin, J. X., Zheng, C. H., Huang, A. M., & Huang, C. M. (2019). Tumor-inflitrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer, 19 (920).
Lee, S., & Margolin, K. (2013). Tumor-infiltrating lymphocytes in melanoma. Current Oncology Reports, 14 (5), 468–474. https://doi.org/10.1007/s11912-012-0257-5 .
Clinicaltrials.gov . Adoptive Transfer of Tumor Infiltrating Lymphocytes for Advanced Solid Cancers. Identifier: NCT03935893.
Lin, H. J. (2015). Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel), 7 (4), 2443–2458.
Zhang, Q; Peng, C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncology Letters 2018; 15(1): 691–698. DOI: https://doi.org/10.3892/ol.2017.7385 .
Sathe, A., Grimes, S. M., Lau, B. T., Chen, J., Suarez, C., Huang, R. J., Poultsides, G. A., & Ji, H. P. (2020). Single cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin Cancer Res . https://doi.org/10.1158/1078-0432.CCR-19-3231 .
Uppal, A., Dehal, A., Chang, S. C., Barrak, D., Naeini, Y., Jalas, J. R., & Bilchik, A. J. (2020). The immune microenvironment impacts survival in Western patients with gastric adenocarcinoma. Journal of Gastrointestinal Surgery, 24 (1), 28–38. https://doi.org/10.1007/s11605-019-04403-w .
Jiang, Y; Xie, J; Huang, W; Chen, H; Xi, S; Han, Z; Huang, L; Lin, T; Zhao, L.Y.; Hu, Y.F.; et al. Tumor immune microenvironment and chemosensitivity signature for predicting response to chemotherapy in gastric cancer. Cancer Immunol Res. 2019; 7(12): 2065–73. DOI: https://doi.org/10.1158/2326-6066 . CIR-19-0311.
Protti, L. F., Soto-Molinari, R., Calderon-Osorno, M., Mora, J., & Alpizar-Alpizar, W. (2019). Gastric cancer in the era of immune checkpoint blockade. Journal of Oncology . https://doi.org/10.1155/2019/107910 .
Kuroda, K., Yashiro, M., Sera, T., Yamamoto, Y., Kushitani, Y., Sugimoto, A., Kushiyama, S., Nishimura, S., Togano, S., Okuno, T., et al. (2019). The clinicopathological significance of thrombospondin-4 expression in the tumor microenvironment of gastric cancer. PLoS One, 14 (11), e0224727. https://doi.org/10.1371/journal.pone.0223727.
Wu, X., Tao, P., Zhou, Q., Li, J., Zhenjia, Y., Wang, X., Li, J., Li, C., Yan, M., Zhu, Z., et al. (2017). IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget, 8 (13), 20741–20750. https://doi.org/10.18632/oncotarget.15119 .
Shiga, K., Hara, M., Nagasaki, T., Sato, T., Takahashi, H., & Takeyama, H. (2015). Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel)., 7 (4), 2443–2458. https://doi.org/10.3390/cancers7040902 .
Yuan, L. W., Yamashita, H., & Seto, Y. (2016). Glucose metabolism in gastric cancer: The cutting edge. World Journal of Gastroenterology, 22 (6), 2046–2059. https://doi.org/10.3748/wjg.v22.i6.2046 .
Zhang, L., & Li, S. (2020). Lactic acid promotes macrophage polarization through MCT-HIFa signaling in gastric cancer. Experimental Cell Research , 111846. https://doi.org/10.1016/j.yexcr.2020.111846 .
Zhou, Q., Wu, X., Wang, X., Yu, Z., Pan, T., Li, Z., Chang, X., Jin, Z., Li, J., Zhu, Z., Liu, B., & Su, L. (2020). The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-a/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene, 39 (7), 1414–1428. https://doi.org/10.1038/s41388-019-1078-x .
Kemi, N., Eskuri, M., & Kauppila, J. H. (2019). Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: a systematic review and meta-analysis. Scientific Reports, 9 (1), 16018. https://doi.org/10.1038/s41598-019-52606-7 .
Liu, Y., Chen, X., Chen, X., Yang, X., Song, Q., & Wu, H. (2019). High SALM3 expression in tumor cells and fibroblasts is correlated with poor prognosis in gastric cancer patients. Disease Markers, 8282414 . https://doi.org/10.1155/2019/8282414 .
Jiang, C., Zhou, Y., Huang, Y., Wang, Y., Wang, W., & Kuai, X. (2019). Overexpression of ADAMTS-2 in tumor cells and stroma is predictive of poor clinical prognosis in gastric cancer. Human Pathology, 84 , 44–51. https://doi.org/10.1016/j.humpath.2018.08.030 .
Ding, X., Ji, J., Jiang, J., Cai, Q., Wang, C., Shi, M., Yu, Y., Zhu, Z., & Zhang, J. (2018). HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death & Disease, 9 (9), 867. https://doi.org/10.1038/s41419-018-0922-1 .
Yang, T., Chen, M., Yang, X., Zhang, X., Zhang, Z., Sun, Y., Xu, B., Hua, J., He, Z., & Song, Z. (2017). Down-regualtion of KLF-5 in cancer-associated fibroblasts inhibits gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biology & Therapy, 18 (10), 806–815. https://doi.org/10.1080/15384047.2017.1373219 .
Wang, X., Zhou, Q., Yu, Z., Wu, X., Chen, X., Li, J., Li, C., Yan, M., Zhu, Z., Liu, B., & Su, L. (2017). Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin B1-FAK signaling pathway. International Journal of Cancer, 141 (5), 998–1010. https://doi.org/10.1002/ijc.30801 .
Fujii, S., Fujihara, A., Natori, K., Abe, A., Kuboki, Y., Higuchi, Y., Aizawa, M., Kuwata, T., Kinoshita, T., Yasui, W., et al. (2015). TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Medicine, 4 (11), 1667–1678. https://doi.org/10.1002/cam4.515 .
Muz, B., de la Puente, P., Azab, F., & Azab, A. K. (2015). The role of hypoxia in cancer progression, angiogenesis, metastasis and resistance to therapy. Hypoxia (Auckl)., 3 , 83–92. https://doi.org/10.2147/HP.S93413 .
Sun, Z., Liu, C., Jiang, W. G., & Ye, L. (2020). Deregulated bone morphogenic proteins and their receptors are associated with disease progression of gastric cancer. Comput Struct Biotechnol., 18 , 177–188. https://doi.org/10.1016/j.csbj.2019.12.014 .
Sun, Z., Cai, S., Liu, C., Cui, Y., Ji, J., Jiang, W. G., & Ye, L. (2020). Increased expression of Gremlin1 promotes proliferation and epithelial mesenchymal transition in gastric cancer cells and correlates with poor prognosis of patients with gastric cancer. Cancer Genomics & Proteomics, 17 (1), 49–60. https://doi.org/10.21873/cgp.20167 .
Bae, W. J., Ahn, J. M., Byeon, H. E., Kim, S., & Lee, D. (2019). PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. Journal of Experimental & Clinical Cancer Research, 38 (1), 484. https://doi.org/10.1186/s13046-019-1469-4 .
Liu, R., Deng, X., Peng, Y., Feng, W., Xiong, R., Zou, Y., Lei, X., Zheng, X., Xie, Z., & Tang, G. (2020). Synthesis and biological evaluation of novel 5,6,7-trimethyloxy flavonoid salicylate derivates as potential anti-tumor agents. Bioorganic Chemistry, 96 , 103652. https://doi.org/10.1016/j.bioorg.2020.103652 .
Zhang, L., Liu, L., Zhan, S., Chen, L., Wang, Y., Zhang, Y., Du, J., Wu, Y., & Gu, L. (2018). Arsenic trioxide suppressed migration and angiogenesis by targeting FOXO3a in gastric cancer cells. International Journal of Molecular Sciences, 19 (12), E3739. https://doi.org/10.3390/ijms19123739 .
Lei, Z., Chai, N., Tian, M., Zhang, Y., Wang, G., Liu, J., Tian, Z., Yi, X., Chen, D., Li, X., et al. (2018). Novel peptide GX1 inhibits angiogenesis by specifically binding to transflutaminase-2 in the tumorous endothelial cells of gastric cancer. Cell Death & Disease, 9 (6), 579. https://doi.org/10.1038/s41419-018-0594-x .
Uhlik, M. T., Liu, J., Falcon, B. L., Iyer, S., Stewart, J., Celikkaya, H., O’Mahony, M., Sevinsky, C., Lowes, C., Douglass, L., et al. (2016). Stromal-based signatures for the classification of gastric cancer. Cancer Research, 76 (9). https://doi.org/10.1158/0008-5472.CAN-16-0022 .
Ismail, N. A., Ragab, S. H., ElBaky, A. A., Shoeib, A. R. S., Alhosary, Y., & Fekry, D. (2011). Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Archives of Medical Science, 7 (3), 501–507. https://doi.org/10.5114/aoms.2011/23418.
Bull, M. J., & Plummer, N. T. (2014). Part 1: the human gut microbiome in health and diseases. Interreg Mediterranean (Encinitas)., 13 (6), 17–22.
Cheng, H. Y., Ning, M. X., Chen, D. K., & Ma, W. T. (2019). Interactions between the gut microbiota and the host innate immune response against pathogens. Frontiers in Immunology, 10 , 607. https://doi.org/10.3389/fimmu.2019.00607 .
Oliphant, K., & Allen-Vercoe, E. (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome., 7 (91).
Abenavoli, L; Scarpellini, E; Colica, C; Boccuto, L; Salehi, B; Sharifi-rad J; Aiello, V; Romano, B; De Lorenzo, A; Izzo, A.A.; et al. Gut microbiota and obesity: a role for probiotics. Nutrients 2019;11(11):2690. DOI: https://doi.org/10.3390/nu11112690.
Galland, L. (2014). The gut microbiome and the brain. Journal of Medicinal Food, 17 (12), 1261–1272. https://doi.org/10.1089/jmf.2014.7000 .
Shin, A., Preidis, G. A., Shulman, R., & Kashyap, P. (2018). The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clinical Gastroenterology and Hepatology, 17 (2), 256–274. https://doi.org/10.10165/j.cgh.2018.08.054.
Harsch, I. A., & Konturek, P. C. (2018). The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: New insights into “old” diseases. Med Sci (Basel)., 6 (2), 32. https://doi.org/10.3390/medsci6020032 .
Gazerani, P. (2019). Probiotics for Parkinson’s disease. International Journal of Molecular Sciences, 20 (17), 4121. https://doi.org/10.3390/ijms20174121 .
Nagano, T., Otoshi, T., Hazama, D., Kiriu, T., Umezawa, K., Katsurada, N., & Nishimura, Y. (2019). Novel cancer therapy targeting microbiome. Oncotargets and Therapy, 12 , 3619–3624. https://doi.org/10.2147/OTT.S207546 .
Liu, X., Shao, L., Liu, X., Ji, F., Mei, Y., Cheng, Y., Liu, F., Yan, C., Li, L., & Lin, Z. (2019). Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine, 40 , 336–348. https://doi.org/10.1016/j.ebiom.2018.12.034 .
Hu, Y. L., Pang, W., Huang, Y., Zhang, Y., & Zhang, C. J. (2018). The gastric microbiome is perturbed in advanced gastric adenocarcinoma identified through shotgun metagenomics. Frontiers in Cellular and Infection Microbiology, 8 , 433. https://doi.org/10.3389/fcimb.2018.00433 .
Spanguolo, C., Russo, G. L., Orhan, I. E., Habtemariam, S., Dagila, M., Sureda, A., Nabavi, S. F., Devi, K. P., Loizzo, M. R., Tundis, R., et al. (2015). Genistein and cancer: current status, challenges, and future directions. Advances in Nutrition, 6 (4), 408–419. https://doi.org/10.3945/an.114.008052 .
Wang, Y., Liu, H., Peng, Y., Tong, L., Feng, L., & Ma, K. (2018). Characterization of the diethyl phthalate-degrading bacterium Sphingobium yanoikuyae SHJ. Data Breif, 20 , 1758–1763. https://doi.org/10.1016/j.dib.2018.09.033 .
Zhao, Q., Hu, H., Wang, W., Peng, H., & Zhang, X. (2015). Genome sequencing of Sphingobium yanoikuyae B1, a polycyclic aromatic hydrocarbon-degrading strain. Genome Announcements, 3 (1), e01522–e01514. https://doi.org/10.1128/genomeA.01522-14.
Terzi, H. A., Demiray, T., Koroglu, M., Cakmak, G., Ciftci, I. H., Ozbek, A., & Altindis, M. (2016). Intra-abdominal abscess and primary peritonitis caused by Streptococcus anginosus . , 9 (6), e33863. https://doi.org/10.5812/jjm.33863 .
Downes, J; Wade, W.G. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. International Journal of Systematic and Evolutionary Microbiology 2006; 56(Pt 4): 751–754. DOI: https://doi.org/10.1099/ijs.0.64041-0 .
Kim, K. S., Rowlinson, M. C., Bennion, R., Liu, C., Talan, D., Summanen, P., & Finegold, S. M. (2010). Characterization of Slackia exigua isolated from human wound infections, including abscesses of intestinal origin. Journal of Clinical Microbiology, 48 (4), 1070–1075. https://doi.org/10.1128/JCM.01576-09 .
Baghban, A., & Gupta, S. (2016). Parvimonas micra: a rare cause of native joint septic arthritis. Anaerobe., 39 , 26–27. https://doi.org/10.1016/j.anaerobe.2016.02.004 .
Khan, M. S., Ishaq, M., Hinson, M., Potugari, B., & Rehman, A. U. (2019). Parvimonas micra bacteremia in a patient with colonic carcinoma. Caspian Journal of Internal Medicine, 10 (4), 472–475. https://doi.org/10.22088/cjim.10.4.472.
Coker, O. O., Dai, Z., Nie, Y., Zhao, G., Cao, L., Nakatsu, G., Wu, W. K. K., Wong, S. H., Chen, Z., Sung, J. J. Y., & Yu, J. (2017). Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut.
Rousse, J. M., Bermond, D., Piemont, Y., Tournoud, C., Heller, R., Kehrli, P., Harlay, M. L., Monteil, H., & Jaulhac, B. (2002). Dialister pneumosintes associated with human brain abscesses. Journal of Clinical Microbiology, 40 (10), 3871–3873. https://doi.org/10.1128/JCM.40.10.3871-3873.2002 .
Contreras, A., Doan, N., Chen, C., Rusitanonta, T., Flynn, M. J., & Slots, J. (2001). The Importance of Dialister pneumosintes in human periodontitis. Oral Microbiology and Immunology . https://doi.org/10.1034/j.1399-302x.2000.150410.x .
Schulz, C., Schutte, K., Mayerle, J., & Malfertheiner, P. (2019). The role of the gastric bacterial microbiome in gastric cancer and beyond. Therapeutic Advances in Gastroenterology . https://doi.org/10.1177/1756284819894062 .
Liou, J. M., Lee, Y. C., El-Omar, E. M., & Wu, M. S. (2019). Efficacy and long-term safety of H. pylori eradication for gastric cancer prevention. Cancers (Basel), 11 (5), E593. https://doi.org/10.3390/cancers11050593 .
Romano, M., & Cuomo, A. (2004). Eradication of helicobacter pylori: a clinical update. MedGenMed, 6 (1), 19.
Hamilton-Miller, J. M. (2003). The role of probiotics in the treatment and prevention of helicobacter pylori infection. International Journal of Antimicrobial Agents, 22 (4), 360–366. https://doi.org/10.1016/s0924-8579(03)00153-5 .
Drago, L. (2019). Probiotics and colon cancer. Microorganisms, 7 (3), 66. https://doi.org/10.3390/microorganisms7030066 .
Quaresma, M., Damasceno, S., Monteiro, C., Lima, F., Mendes, T., Lima, M., Justino, P., Barbosa, A., Souza, M., Souza, E., et al. (2019). Probiotic mixture containing Lactobacillus spp. and Bifidobacterium spp. attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutrition and Cancer , 1–11. https://doi.org/10.1080/01635581.2019.167519.
Prisciandaro, L. D., Geier, M. S., Butler, R. N., Cummins, A. G., & Howarth, G. S. (2011). Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy- induced intestinal mucositis. Critical Reviews in Food Science and Nutrition, 51 (3), 239–247. https://doi.org/10.1080/10408390903551747 .
Cousin, F. J., Jouan-Lanhouet, S., Dimanche-Boitrel, M. T., Corcos, L., & Jan, G. (2012). Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric Cancer cells. PLoS One, 7 (3), e31892. https://doi.org/10.1371/journal.pone.0031892 .
Shimizu, N., Wakatsuki, T., Murakami, A., Yoshioka, H., Hamazoe, R., Kanayama, H., Maeta, M., & Koga, S. (1987). Carcinoembryonic antigen in gastric cancer patients. Oncology., 44 , 240–244. https://doi.org/10.1159/000226486 .
Tierman, J. P., Perry, S. L., Verghese, E. T., West, N. P., Yeluri, S., Jayne, D. G., & Hughes, T. A. (2013). Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Journal of Cancer, 108 (3), 662–667. https://doi.org/10.1038/bjc.2012.605 .
Pavai, S., & Yap, S. F. (2003). The clinical significance of elevated levels of serum CA 19-9. The Medical Journal of Malaysia, 58 (5), 667–672.
Wu, J., Li, G., Wang, Z., Yao, Y., Chen, R., Pu, X., & Wang, J. (2015). Circulating MicroRNA-21 is a potential diagnostic biomarker in gastric Cancer. Disease Markers, 435656 . https://doi.org/10.1155/2015/435656 .
Iwasaki, H., Shimura, T., Yamada, T., Okuda, Y., Natsume, M., Kitagawa, M., Horike, S., & Kataoka, H. (2019). A novel urinary microRNA biomarker panel for detecting gastric cancer. Journal of Gastroenterology, 54 , 1061–1069.
Sexton, R., Mahdi, Z., Chaudhury, R., Beydoun, R., Aboukameel, A., Khan, H. Y., Baloglu, E., Senapedis, W., Landesman, Y., Tesfaye, A., Kim, S., Philip, P. A., & Azmi, A. S. (2019). Targeting nuclear exporter protein XPO1/CRM1 in gastric cancer. International Journal of Molecular Sciences, 20 (19), E4826. https://doi.org/10.3390/ijms20194826 .
Shi, Y., Wang, Z., Zhu, X., Chen, L., Ma, Y., Wang, J., Yang, X., & Liu, Z. (2020). Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. International Journal of Clinical Oncology, 25 (1), 89–99. https://doi.org/10.1007/s10147-019-01532-9 .
Mei, J. W., Yang, Z. Y., Xiang, H. G., Bao, R., Ye, Y. Y., Ren, T., Wang, X. F., & Shu, Y. J. (2019). MicroRNA-1275 inhibits cell migration and invasion in gastric cancer by regulating vimentin and E-cadherin via JAZF1. BMC Cancer, 19 (1), 740. https://doi.org/10.1186/s12885-019-5929-1.
Novikova, I. V., Hennelly, S. P., & Sanbonmatsu, K. Y. (2012). Sizing up long non-coding RNAs do lncRNAs have secondary and tertiary structure? Bioarchitecture., 2 (6), 189–199. https://doi.org/10.4161/bioa.22592 .
Yao, X. M., Tang, J. H., Zhu, H., & Jing, Y. (2017). High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. European Review for Medical and Pharmacological Sciences, 21 , 5661–5667.
PubMed Google Scholar
Qi, D., Wang, Q., Wu, M., & Zhang, X. (2018). Comprehensive bioinformatics analysis of lncRNAs in gastric cancer. Oncology Letters , 1279–1291. https://doi.org/10.3892/ol.2018.9707 .
Fei, Z. H., Yu, X. J., Zhou, M., Su, H. F., Zheng, Z., & Xie, C. Y. (2016). Upregulated expression of long-noncoding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumour Biology, 37 (2), 1983–1993. https://doi.org/10.1007/s13277-015-3979-9 .
Lin, X., Xia, Y., Hu, D., Mao, Q., Yu, Z., Zhang, H., Li, C., Chen, G., Liu, F., Zhu, W., et al. (2019). Transcriptome-wide piRNA profiling in human gastric cancer. Oncol Rep, 41 (5), 3089–3099. https://doi.org/10.3892/or.2019.7073 .
Martinez, V. D., Enfield, K. S. S., Rowbotham, D. A., & Lam, W. L. (2015). An atlas of gastric PIWI-interacting RNA transcriptomes and their utility for identifying signatures of gastric cancer recurrence. Europe PMC., 19 (2), 660–665. https://doi.org/10.1007/s10120-015-0487-y .
Cabral, G. F., Azevedo dos Santos Pinheiro, J., Vidal, A. F., Santos, S., & Ribeiro-dos-Santos, A. (2020). piRNAs in gastric cancer: a new approach towards translational research. MDPI, 21 (6), 2126. https://doi.org/10.3390/ijms21062126 .
Kang, R., Zeh, H. J., Lotze, M. T., & Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death and Differentiation, 18 (4), 571–580. https://doi.org/10.1038/cdd.2010.191 .
Morris, D. H., Yip, C. K., Shi, Y., Chait, B. T., & Wang, Q. J. (2015). Beclin-1VSP34 complex architecture: understanding the nuts and bolts of therapeutic targets. Front Biol (Beijing)., 10 (5), 398–426. https://doi.org/10.1007/s11515-015-1374-y .
Galluzzi, L., Baehrecke, E. H., Ballabio, A., Boya, P., Bravo-San Pedro, J. M., Cecconi, F., Choi, A. M., Chu, C. T., Codogno, P., Colombo, M. I., et al. (2017). Molecular definitions of autophagy and related processes. The EMBO Journal, 36 (13), 1811–1836. https://doi.org/10.15252/embj.201796697 .
Fei, B., Ji, F., Chen, X., Liu, Z., Li, S., Mo, Z., & Fang, X. (2016). Expression and clinical significance of Beclin-1 in gastric cancer tissues of various clinical stages. Oncology Letters, 11 (3), 2271–2277. https://doi.org/10.3892/ol.2016.4183 .
Giatromanolaki, A., Koukourakis, M. I., Georgiou, I., Kouroupi, M., & Siviridis, E. (2018). LC3A, LC3B and Beclin-1 expression in gastric cancer. Anticancer Research, 38 (12), 6827–6833. https://doi.org/10.21873/anticanres.13056 .
Levy, J. M. M., Towers, C. G., & Thorburn, A. (2017). Targeting autophagy in cancer. Nature Reviews. Cancer, 17 (9), 528–542. https://doi.org/10.1038/nrc.2017.53 .
Wesselborg, S., & Stork, B. (2015). Autophagy signal transduction by ATG proteins: from hierarchies to networks. Cellular and Molecular Life Sciences, 72 , 4721–4757. https://doi.org/10.1007/s00018-015-2034-8 .
Suzuki, K; Noda, T; Ohsumi, Y. Interrelationships among ATG proteins during autophagy in Saccharomyces cerevisiae . 2004. https://doi.org/10.1002/yea.1152 .
Yu, L., Chen, Y., & Tooze, S. A. (2018). Autophagy pathway: cellular and molecular mechanisms. Autophagy., 14 (2), 207–215. https://doi.org/10.1080/15548627.2017.1378838 .
Cherra III, S. J., Kulich, S. M., Uechi, G., Balasubramani, M., Mountzouris, J., Day, B. W., & Chu, C. T. (2010). Regulation of the autophagy protein LC3 phosphorylation. Journal of Cellular Biochemistry, 190 (4), 553–539. https://doi.org/10.1083/jcb.201002108 .
Roach, P. J. (2011). AMPKà ULK1à Autophagy. Molecular and Cellular Biology, 31 (15), 3082–3084. https://doi.org/10.1128/MCB.05565-11 .
Backer, J. M. (2008). The regulation and function of class III PI3Ks: novel roles for Vps34. The Biochemical Journal, 410 (1), 1–17. https://doi.org/10.1042/BJ20071427 .
Chen, M. B., Ji, X. Z., Liu, Y. Y., Zeng, P., Xu, X. Y., Ma, R., Guo, Z. D., Lu, J. W., & Feng, J. F. (2017). Ulk1 over-expression in human gastric cancer is correlated with patients’ T classification and cancer relapse. Oncotarget., 8 (20), 33704–33712. https://doi.org/10.18632/Oncotarget.16734 .
Wang, X., Wu, W. K. K., Gao, J., Li, Z., Dong, B., Lin, X., Li, Y., Li, Y., Gong, J., Qi, C., Peng, Z., Yu, J., & Shen, L. (2019). Autophagy inhibition enhances PD-L1 expression in gastric cancer. Journal of Experimental & Clinical Cancer Research, 38 , 140. https://doi.org/10.1186/s13046-019-1148-5 .
Akin, D., Wang, S. K., Habibzadegah-Tari, P., Law, B., Ostrov, D., Li, M., Yin, X. M., Kim, J. S., Horenstein, N., & Dunn, W. A. (2014). A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy., 10 (11), 2021–2035. https://doi.org/10.4161/auto.32229 .
Kim, J. S., Bae, G. E., Kim, K. H., Lee, S. I., Chung, C., Lee, D., Lee, T. H., Kwon, I. S., & Yeo, M. K. (2019). Prognostic significance of LC3B and p62/SQSTM1 expression in gastric adenocarcinoma. Anticancer Research, 39 (12), 6711–6722. https://doi.org/10.21873/anticanres.13886 .
Sui, X., Chen, R., Wang, Z., Huang, Z., Kong, N., Zhang, M., Han, W., Lou, F., Yang, J., Zhang, Q., Wang, X., He, C., & Pan, H. (2013). Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death & Disease, 4 (10), 838. https://doi.org/10.1038/cddis.2013.350.
Jung, C. H., Ro, S. H., Cao, J., Otto, N. M., & Kim, D. H. (2010). mTOR regulation of autophagy. FEBS Letters, 584 (7), 1287–1295. https://doi.org/10.1016/j.febslet.2010.01.017 .
Laplante, M., & Sabatini, D. M. (2009). mTOR signaling at a glance. Journal of Cell Science, 122 , 3589–3594. https://doi.org/10.1242/jcs.051011 .
Jung, C. H., Jun, C. B., Ro, S. H., Kim, Y. M., Otto, N. M., Cao, J., Kundu, M., & Kim, D. H. (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular Biology of the Cell, 20 , 1992–2003. https://doi.org/10.1091/mbc.E08-12-1249 .
Ganley, I.G.; Lam du, H; Wang, J; Ding, X; Chen, S; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. Journal of Biological Chemistry . 2009; 284:12297–12305. DOI: https://doi.org/10.0174/jbc.M900573200 .
Tian, T., Li, X., & Zhang, J. (2019). mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. International Journal of Molecular Sciences, 20 (3), 755. https://doi.org/10.3390/ijms20030755 .
Galluzzi, L., Pietrocola, F., Bravo-San Pedro, J. M., Amaravadi, R. K., Baehrecke, E. H., Cecconi, F., Codogno, P., Debnath, J., Gewirtz, D. A., Karantza, V., et al. (2015). Autophagy in malignant transformation and cancer progression. The EMBO Journal, 34 , 856–880. https://doi.org/10.15252/embj.201490784 .
Amaravadi, R., Kimmelman, A. C., & White, E. (2016). Recent insights into the function of autophagy in cancer. Genes & Development, 30 , 1913–1930. https://doi.org/10.1101/gad.287524.116 .
Riquelme, I., Tapia, O., Espinoza, J. A., Leal, P., Buchegger, K., Sandoval, A., Bizama, C., Araya, J. C., Peek, R. M., & Roa, J. C. (2016). The gene expression status of the PI3K/AKT/mTOR pathway in gastric Cancer tissues and cell lines. Pathology Oncology Research, 22 , 797–805. https://doi.org/10.1007/s12253-016-0066-5 .
Byeon, S. J., Han, N., Choi, J., Kim, M. A., & Kim, W. H. (2014). Prognostic implication of TSC1 and mTOR expression in gastric carcinoma. Journal of Surgical Oncology, 109 , 812–817. https://doi.org/10.1002/jso.23585 .
Fukamachi, H., Kim, S. K., Koh, J., Lee, H. S., Sasaki, Y., Yamashita, K., Nishikawaji, T., Shimada, S., Akiyama, Y., Byeon, S. J., et al. (2019). A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. Journal of Experimental & Clinical Cancer Research, 38 (127).
Greenfield, L. K., & Jones, N. L. (2013). Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends in Microbiology, 21 (11), 602–612. https://doi.org/10.1016/j.tim.2013.09.004 .
Ohtsu, A; Ajani, J.A.; Bai, Y.X.; Bang, Y.J.; Chung, H.C.; Pan, H.M.; Sahmoud, T; Shen, L; Yeh, K.H.; Chin, K; et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double blind, phase III GRANITE-1 study. Gastrointestinal Cancer . 2013; 31: 3935–3943. DOIL https://doi.org/10.1200/JCO.2012.48.3552 .
Rosich, L., Xargay-Torrent, S., Lopez-Guerra, M., Campo, E., Colomer, D., & Roue, G. (2012). Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Cancer Therapy: Preclinical., 18 (19). https://doi.org/10.1158/1078-0432.CCR-12-0351 .
Wang, W., Lin, L., Zhou, Y., Ye, Q., Yang, X., Jiang, J., Ye, Z., Gao, F., Tan, X., Zhang, G., et al. (2019). Hydroxychloroquine enhances the antitumor effects of BC001 in gastric cancer. International Journal of Oncology, 55 (2), 405–414. https://doi.org/10.3892/ijo.2019.4824.
Rong, L., Li, Z., Leng, X., Li, H., Ma, Y., Chen, Y., & Song, F. (2020). Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomedicine & Pharmacotherapy, 122 , 109726.
Liu, J., Zhang, Y., Qu, J., Xu, L., Hou, K., Zhang, J., Qu, X., & Liu, Y. (2011). B-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer, 11 (183).
Sun, X., Zhang, X., Zhai, H., Zhang, D., & Ma, S. (2019). Chicoric acid (CA) induces autophagy in gastric cancer through promoting endoplasmic reticulum (ER) stress regulated by AMPK. Biomedicine & Pharmacotherapy, 118 , 109144. https://doi.org/10.1016/j.biopha.2019.109144 .
Zhang, Y., Liu, S., Feng, Q., Huang, X., Wang, X., Peng, Y., Zhao, Z., & Liu, Z. (2018). Perialdehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. Journal of Cellular Biochemistry . https://doi.org/10.1002/jcb.27491 .
Zhi, X., Li, B., Li, Z., Zhang, J., Yu, J., Zhang, L., & Xu, Z. (2019). Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. International Journal of Oncology, 54 (5), 1625–1638. https://doi.org/10.3892/ijo.2019.4753 .
Ji, C. H., & Kwon, Y. T. (2017). Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Biology, Medicine. https://doi.org/10.14348/molcells.2017.0115 .
Mrakovcic, M., Kleinheinz, J., & Frohlich, L. F. (2017). Histone deacetylase inhibitor-induced autophagy in tumor cells: implications for p53. International Journal of Molecular Sciences, 18 (9), 1883. https://doi.org/10.3390/ijms18091883 .
Peracchio, C., Alabiso, O., Valente, G., & Isidoro, C. (2012). Involvement of autophagy in ovarian cancer: a working hypothesis. Journal of Ovarian Research, 5 , 22. https://doi.org/10.1186/1757-2215-5-22 .
Zhou, J., Liao, W., Yang, J., Ma, K., Li, X., Wang, Y., Wang, D., Wang, L., Zhang, Y., Yin, Y., Zhao, Y., & Zhu, W. G. (2012). FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy, 8 (12), 1712–1723. https://doi.org/10.46161/auto.21830 .
Avivar-Valderas, A., Salas, E., Bobrovnikova-Marjon, E., Diehl, J. A., Nagi, C., Debnath, J., & Aguirre-Ghiso, J. A. (2011). PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and Cellular Biology, 31 (17), 3616–3629. https://doi.org/10.1128/MCB.05164-11 .
Chen, Y. F., Liu, H., Luo, X. J., Zhao, Z., Zou, Z. Y., Li, J., Lin, X. J., & Liang, Y. (2017). The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Critical Reviews in Oncology/Hematology, 112 , 21–30. https://doi.org/10.1016/j.critevonc.2017.02.004.
Li, Z. Y., Yang, Y., Ming, M., & Liu, B. (2011). Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochemical and Biophysical Research Communications, 414 (1), 5–8. https://doi.org/10.1016/j.bbrc.2011.09.046 .
Zou, P., Xia, Y., Chen, T., Zhang, J., Wang, Z., Chen, W., Chen, M., Kanchana, K., Yang, S., & Liang, G. (2015). Selective killing of gastric cancer cells by small molecule targeting ROS-mediated ER stress activation. Molecular Carcinogenesis . https://doi.org/10.1002/mc.22351 .
Wang, T., Gao, J., Yu, J., & Shen, L. (2013). Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenographs. Chinese Journal of Cancer Research, 25 (5), 505–513. https://doi.org/10.3978/j.issn.1000-9604.2013.08.14 .
Li, S.J.; Sun, S.J.; Gao, J; Sun, F.B. Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cells. Oncology Letters 2016; 12(6): 5059–5067. DOI: https://doi.org/10.3892/ol.2016.5367 .
Aviles-Jimenez, F., Vazquez-Jimenez, F., Medrano-Guzman, R., Mantilla, A., & Torres, J. (2014). Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Scientific Reports, 4 , 4202. https://doi.org/10.1038/srep04202 .
Hu, Y. L., Pang, W., Huang, Y., Zhang, Y., & Zhang, C. J. (2018). The gastric Microbioe is perturbed in advanced gastric adenocarcinoma identified through shotgun metagenomics. Frontiers., 8 , 433. https://doi.org/10.3389/fcimb.2018.00433 .
Liu, X., Nie, W., Liang, J., & Li, Y. (2017). Interaction of Helicobacter pylori with other microbiota species in the development of gastric cancer. Clinical Microbiology.
Dias, Jacome, E; Libanio, D; Borges-Canha, M; Galaghar, A; Pimentel-Nunes, P. Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria—a systematic review. Revistia Espanola de Enfermedades Digestivas. 2016. DOI: https://doi.org/10.17235/reed.2016.4261/2016 .
Salazar, C. R., Sun, J., Li, Y., Francois, F., Corby, P., Perez-Perez, G., Dasanayake, A., Pei, Z., & Chen, Y. (2013). Association between selected Oral pathogens and gastric precancerous lesions. PLoS One, 8 (1), e51604. https://doi.org/10.1371/journal.pone.0051604 .
Hsieh, Y. Y., Tung, S. Y., Pan, H. Y., Yen, C. W., Xu, H. W., Lin, Y. J., Deng, Y. F., Hsu, W. T., Wu, C. S., & Li, C. (2018). Increased abundance of Clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Scientific Reports, 8 (158).
Roberts, P. J., Dickinson, R. J., Whitehead, A., Laughton, C. R., & Foweraker, J. E. (2002). The culture of lactobacilli species in gastric carcinoma. Journal of Clinical Pathology, 55 , 477–480.
Weng, M. T., Chiu, Y. T., Wei, P. Y., Chiang, C. W., & Fang, H. L. (2019). Wei, S.C. microbiota and gastrointestinal cancer. Journal of the Formosan Medical Association, 118 (1), S32–S41. https://doi.org/10.1016/j.fma.2019.01.002.
Wang, L. L., Yu, X. J., Zhan, S. H., Jia, S. J., Tian, Z. B., & Dong, Q. J. (2014). Participation of microbiota in the development of gastric cancer. World Journal of Gastroenterology, 20 (17), 4948–4952. https://doi.org/10.3748/wjg.v20.i17.4948 .
Zhao, Y., Gao, X., Guo, J., Yu, D., Xiao, Y., Wang, H., & Li, Y. (2019). Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter., 24 , e12567. https://doi.org/10.1111/hel.12567 .
Zhang, L., Fu, L., Zhang, S., Zhang, J., Zhao, Y., Zheng, Y., He, G., Yang, S., Ouyang, L., & Liu, B. (2017). Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chemical Science, 8 (4), 2687–2601. https://doi.org/10.1039/c6sc05368h .
Fu, X., Huang, Z., Hong, L., Lu, J. H., Feng, D., Yin, X. M., & Li, M. (2019). Targeting ATG4 in cancer therapy. Cancers., 11 (5), 649. https://doi.org/10.3390/cancers11050649 .
Xie, J., Wang, X., & Proud, C. G. (2016). mTOR inhibitors in cancer therapy. F1000Res, 5 , F1000 Faculty Rev-2078. https://doi.org/10.12688/f1000research.9207.1 .
Rebecca, V. W., Nicastri, M. C., Fennelly, C., Chude, C. L., Barber-Rotenberg, J. S., Ronghe, A., McAfee, Q., McLaughlin, N. P., Zhang, G., Goldman, A. R., et al. (2018). PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. AACR Journals. Published . https://doi.org/10.1158/2159-8290.CD-18-0706 .
Zhou, J., Li, G., Zheng, Y., Shen, H. M., Hu, X., Ming, Q. L., Huang, S., Li, P., & Gao, N. (2015). A novel autophagy/mitophagy inhibitor Liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy., 11 (8), 1259–1279. https://doi.org/10.1080/15548627.2015.1056970 .
Mahameed, M., Wilhelm, T., Darawshi, O., Obiedat, A., Tommy, W. S., Chintha, C., Schubert, T., Samali, A., Chevet, E., et al. (2019). The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death & Disease, 10 (300).
Axten, J. M., Romeril, S. P., Shu, A., Ralph, J., Medina, J. R., Feng, Y., Li, W. H. H., Grant, S. W., Heerding, D. A., et al. (2013). Discovery of GSK2656157: An optimized PERK inhibitor selected for preclinical development. ACS Medicinal Chemistry Letters . https://doi.org/10.1021/ml400228e .
Li, J., Tu, H. J., Li, J., Dai, G., Dai, Y. C., Wu, Q., Shi, Q. Z., Cao, Q., & Li, Z. J. (2007). N-acetyl cysteine inhibits human signet ring cell gastric cancer cell line (SJ-89) cell growth by inducing apoptosis and DNA synthesis arrest. European Journal of Gastroenterology & Hepatology, 19 (9), 769–774. https://doi.org/10.1097/MEG.0b013e3292202bda.
Klenke, S., Akdeli, N., Stelmach, P., Heukamp, L., Schulte, J. H., & Bachmann, H. S. (2019). The small molecule Bcl-2/mcl-1 inhibitor TW-37 shows single-agent cytotoxicity in neuroblastoma cell lines. BMC Cancer, 19 (1), 243. https://doi.org/10.1186/s12885-019-5439-1 .
Chen, X., Mao, G., Chen, H., Liu, S., Wang, S., Li, X., Ye, Y., Wu, H., & Liu, J. (2018). TW37 enhances the pro-apoptosis and anti-migratory ability of gefitinib in non-small cell lung cancer. Molecular and Cellular Biology (Noisy-le-grand)., 64 (4), 6–10.
Krasniqi, E., Pellicori, S., & Formica, V. (2015). Emerging role of S-1 in gastric cancer. Indian Journal of Medical and Paediatric Oncology, 36 (4), 219–228. https://doi.org/10.4103/0971-5851.171542 .
Manero, F., Gautier, F., Gallenne, T., Cauquil, N., Gree, D., Catron, P. F., Geneste, O., Gree, R., Vallette, F. M., & Juin, P. (2006). The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Research, 66 (5), 2757–2764.
Chang, J., Wang, Y., Shao, L., Laberge, R. M., Demaria, M., Campisi, J., Janakiraman, K., Sharpless, N. E., Ding, S., Feng, W., et al. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22 (1), 78–83.
Zhang, H., Zhong, X., Zhang, X., Shang, D., Zhou, Y. I., & Zhang, C. (2016). Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Experimental and Therapeutic Medicine, 11 (2), 669–673.
Download references
Work in the lab of ASA is supported by NIH R37CA215427 and SKY Foundation Inc.
Author information
Authors and affiliations.
Department of Oncology, Karmanos Cancer Institute, Wayne State University School of Medicine, 4100 John R, HWCRC 732, Detroit, MI, 48201, USA
Rachel E. Sexton, Mohammed Najeeb Al Hallak, Maria Diab & Asfar S. Azmi
You can also search for this author in PubMed Google Scholar
Contributions
RS, MNH, MD, and ASA: concept design, literature search, writing, and editing the manuscript.
Corresponding author
Correspondence to Asfar S. Azmi .
Ethics declarations
Conflict of interests.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Sexton, R.E., Al Hallak, M.N., Diab, M. et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 39 , 1179–1203 (2020). https://doi.org/10.1007/s10555-020-09925-3
Download citation
Received : 24 June 2020
Accepted : 12 August 2020
Published : 07 September 2020
Issue Date : December 2020
DOI : https://doi.org/10.1007/s10555-020-09925-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gastric cancer
- Gastric adenocarcinoma
- Non coding RNAs
- Novel targets
- Find a journal
- Publish with us
- Track your research
ORIGINAL RESEARCH article
Role and mechanism of ncapd3 in promoting malignant behaviors in gastric cancer.

- 1 Fujian Medical University Union Hospital, Fuzhou, Fujian Province, China
- 2 The Second Hospital of Shandong University, Jinan, Shandong Province, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Background: Gastric cancer (GC) is one of the major malignancies threatening human lives and health. Non-SMC condensin II complex subunit D3 (NCAPD3) plays a crucial role in the occurrence of many diseases. However, its role in GC remains unexplored.The Cancer Genome Atlas (TCGA) database, clinical samples, and cell lines were used to analyze NCAPD3 expression in GC. NCAPD3 was overexpressed and inhibited by lentiviral vectors and the CRISPR/Cas9 system, respectively. The biological functions of NCAPD3 were investigated in vitro and in vivo. Gene microarray, Gene set enrichment analysis (GSEA) and ingenuity pathway analysis (IPA) were performed to establish the potential mechanisms.Results: NCAPD3 was highly expressed in GC and was associated with a poor prognosis.NCAPD3 upregulation significantly promoted the malignant biological behaviors of gastric cancer cell, while NCAPD3 inhibition exerted a opposite effect. NCAPD3 loss can directly inhibit CCND1 and ESR1 expression to downregulate the expression of downstream targets CDK6 and IRS1 and inhibit the proliferation of gastric cancer cells. Moreover, NCAPD3 loss activates IRF7 and DDIT3 to regulate apoptosis in gastric cancer cells.Our study revealed that NCAPD3 silencing attenuates malignant phenotypes of GC and that it is a potential target for GC treatment.
Keywords: 0000-0001-8813-6751 (Su-Yun Zhang), 0000-0001-8216-4524 (Qiong Luo), 0009-0004-0783-3635 (Li-Rong Xiao), 0000-0002-9634-7003 (Fan Yang), 0000-0003-3596-4339 (Jian Zhu), 0000-0003-3367-6261 (Xiang-Qi Chen), 0000-0003-4226-2159 (Sheng Yang) Gastric cancer, NCAPD3, Proliferation, Apoptosis, Molecular mechanism
Received: 19 Nov 2023; Accepted: 30 Mar 2024.
Copyright: © 2024 Zhang, Luo, Xiao, Yang, Zhu, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Sheng Yang, Fujian Medical University Union Hospital, Fuzhou, 350001, Fujian Province, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Patient Care & Health Information
- Diseases & Conditions
- Stomach cancer
- What is stomach cancer? A Mayo Clinic expert explains
Learn more about stomach cancer from oncologist Mohamad (Bassam) Sonbol, M.D.
I'm Dr. Bassam Sonbol, an oncologist at Mayo Clinic. In this video we'll cover the basics of stomach cancer: What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. Stomach cancer, also called gastric cancer, can happen in any part of the stomach. However, in the U.S., most stomach cancers occur in the gastroesophageal junction, which is where the esophagus - the tube that carries chewed up food - meets the stomach. There are several different types of stomach cancers, but most are curable if detected at an early stage. What once was the leading cause of cancer death is now well down on the list thanks to the advancement in technology and scientific research. In fact, new cases of stomach cancers have dropped by about 1.5% every year for the last 10 years.
Stomach cancer more commonly affects older people. The average age of those diagnosed with stomach cancer is 68. Around 60% of cases occur in patients older than 65, and there is a slightly higher lifetime risk of stomach cancer in men. However, it can affect anyone. Stomach cancer tends to develop slowly over time, usually over many years. What happens is small changes occur in the DNA of the stomach cells, telling them to over multiply and then they accumulate, forming abnormal growth called tumors. There are several known risk factors that could increase your risk of developing stomach cancer, for instance, smoking doubles your risk of stomach cancer, family history of stomach cancer, infection with H. pylori, long-term stomach inflammation, gastroesophageal reflux disease, or stomach polyps. Eating a diet high in salty and smoked foods or low in fruits and vegetables can be also a risk. And there is some correlation between higher weight and risk, as well.
Stomach cancer can present itself in several different ways, such as difficulty swallowing, feeling bloated after eating, feeling full after only eating a small amount of food, heartburn, indigestion, nausea, stomach pain, unintentional weight loss, and vomiting. If you have any signs and symptoms that worry you, make an appointment with your doctor. Your doctor may investigate the more common causes of these symptoms first or refer you to a specialist, like a gastroenterologist or an oncologist, like me.
To determine if you have stomach cancer, your doctor may start with an upper endoscopy, where a tiny camera is passed through the throat and into the stomach. If your doctor finds something suspicious, they remove some tissue for a biopsy, where the cells gets sent to a lab for further analysis. Your doctor may also run some imaging tests, like CT scan or a special x-ray called a barium swallow. Identifying the extent of the cancer helps your doctor determine the best treatment. To determine the stage, they will run more tests, like blood tests, endoscopic ultrasound, CT scan, or a PET scan. In some cases, your doctor may recommend laparoscopic surgery, where the doctor inserts a special camera directly into the abdomen.
Creating a treatment plan for stomach cancer is a collaborative effort between doctors from different specialties. Our goal is to make the best treatment plan for your overall health and personal well-being. There are five main treatment options for stomach cancer: Surgery to remove all of the cancerous tissue and probably some of the healthy tissue around it. Chemotherapy, which uses drugs that journey throughout the body, destroying any cancer cells in its path. Radiation therapy, which uses high-powered beams of energy to target cancer cells. Targeted drug therapy, focusing on blocking specific weaknesses present within cancer cells. And immunotherapy, a drug treatment that helps your immune system recognize which cells are dangerous and attack them.
Finding out you have cancer can be really overwhelming and difficult. It can help to find spaces where other people understand what you're going through. Try connecting with cancer survivors online or in your community. Learning about your condition can help you make confident decisions about your care. If you'd like to learn more about stomach cancer, watch our other related videos or visit mayoclinic.org. We wish you well.
Gastroesophageal junction and stomach
The stomach is a muscular sac in the middle of the upper abdomen that helps break down and digest food. Food you eat passes down your esophagus, through the gastroesophageal junction and into the stomach.
Gastroesophageal junction cancer
Cancer of the gastroesophageal junction develops in the area where the esophagus joins the top part of the stomach.
Stomach cancer most commonly begins in the cells that line the inside of the stomach.
Stomach cancer, which is also called gastric cancer, is a growth of cells that starts in the stomach. The stomach is in the upper middle part of the belly, just below the ribs. The stomach helps to break down and digest food.
Stomach cancer can happen in any part of the stomach. In most of the world, stomach cancers happen in the main part of the stomach. This part is called the stomach body.
In the United States, stomach cancer is more likely to start by the gastroesophageal junction. This is the part where the long tube that carries food you swallow meets the stomach. The tube that carries food to the stomach is called the esophagus.
Where the cancer starts in the stomach is one factor health care providers think about when making a treatment plan. Other factors might include the cancer's stage and the type of cells involved. Treatment often includes surgery to remove the stomach cancer. Other treatments may be used before and after surgery.
Stomach cancer treatment is most likely to be successful if the cancer is only in the stomach. The prognosis for people with small stomach cancers is quite good. Many can expect to be cured. Most stomach cancers are found when the disease is advanced and a cure is less likely. Stomach cancer that grows through the stomach wall or spreads to other parts of the body is harder to cure.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- A Book: Mayo Clinic on Digestive Health
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of stomach cancer may include:
- Trouble swallowing
- Feeling bloated after eating
- Feeling full after eating small amounts of food
- Not feeling hungry when you would expect to be hungry
- Indigestion
- Losing weight without trying
- Feeling very tired
- Stools that look black
Stomach cancer doesn't always cause symptoms in its early stages. When they happen, symptoms might include indigestion and pain in the upper part of the belly. Symptoms might not happen until the cancer is advanced. Later stages of stomach cancer might cause symptoms such as feeling very tired, losing weight without trying, vomiting blood and having black stools.
Stomach cancer that spreads to other parts of the body is called metastatic stomach cancer. It causes symptoms specific to where it spreads. For example, when cancer spreads to the lymph nodes it might cause lumps you can feel through the skin. Cancer that spreads to the liver might cause yellowing of the skin and whites of the eyes. If cancer spreads within the belly, it might cause fluid to fill the belly. The belly might look swollen.
When to see a doctor
If you have signs and symptoms that worry you, make an appointment with your health care provider. Many conditions can cause symptoms that are like the ones caused by stomach cancer. Your provider might test for those other causes first before testing for stomach cancer.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
It's not clear what causes stomach cancer. Experts believe most stomach cancers start when something hurts the inside lining of the stomach. Examples include having an infection in the stomach, having long-standing acid reflux and eating a lot of salty foods. Not everyone with these risk factors gets stomach cancer, though. So more research is needed to find out exactly what causes it.
Stomach cancer begins when something hurts cells in the inner lining of the stomach. It causes the cells to develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. The changes tell the cells to multiply quickly. The cells can go on living when healthy cells would die as part of their natural lifecycle. This causes a lot of extra cells in the stomach. The cells can form a mass called a tumor.
Cancer cells in the stomach can invade and destroy healthy body tissue. They might start to grow deeper into the wall of the stomach. In time, cancer cells can break away and spread to other parts of the body. When cancer cells spread to another part of the body it's called metastasis.
Types of stomach cancer
The type of stomach cancer you have is based on the type of cell where your cancer began. Examples of stomach cancer types include:
- Adenocarcinoma. Adenocarcinoma stomach cancer starts in cells that produce mucus. This is the most common type of stomach cancer. Nearly all cancers that start in the stomach are adenocarcinoma stomach cancers.
- Gastrointestinal stromal tumors (GIST). GIST starts in special nerve cells that are found in the wall of the stomach and other digestive organs. GIST is a type of soft tissue sarcoma.
- Carcinoid tumors. Carcinoid tumors are cancers that start in the neuroendocrine cells. Neuroendocrine cells are found in many places in the body. They do some nerve cell functions and some of the work of cells that make hormones. Carcinoid tumors are a type of neuroendocrine tumor.
- Lymphoma. Lymphoma is a cancer that starts in immune system cells. The body's immune system fights germs. Lymphoma can sometimes start in the stomach if the body sends immune system cells to the stomach. This might happen if the body is trying to fight off an infection. Most lymphomas that start in the stomach are a type of non-Hodgkin's lymphoma.
Risk factors
Factors that increase the risk of stomach cancer include:
- Ongoing problems with stomach acid backing up into the esophagus, which is called gastroesophageal reflux disease
- A diet high in salty and smoked foods
- A diet low in fruits and vegetables
- Infection in the stomach caused by a germ called Helicobacter pylori
- Swelling and irritation of the inside of the stomach, which is called gastritis
- Growths of noncancerous cells in the stomach, called polyps
- Family history of stomach cancer
- Family history of genetic syndromes that increase the risk of stomach cancer and other cancers, such as hereditary diffuse gastric cancer, Lynch syndrome, juvenile polyposis syndrome, Peutz-Jeghers syndrome and familial adenomatous polyposis
To lower the risk of stomach cancer, you can:
- Eat plenty of fruits and vegetables. Try to include fruits and vegetables in your diet each day. Choose a variety of colorful fruits and vegetables.
- Reduce the amount of salty and smoked foods you eat. Protect your stomach by limiting these foods.
- Stop smoking. If you smoke, quit. If you don't smoke, don't start. Smoking increases your risk of stomach cancer and many other types of cancer. Quitting smoking can be very hard, so ask your health care provider for help.
- Tell your health care provider if stomach cancer runs in your family. People with a strong family history of stomach cancer might have stomach cancer screening. Screening tests can detect stomach cancer before it causes symptoms.
Stomach cancer care at Mayo Clinic
Living with stomach cancer?
Connect with others like you for support and answers to your questions in the Proton Beam Therapy support group on Mayo Clinic Connect, a patient community.
Proton Beam Therapy Discussions

47 Replies Mon, Mar 25, 2024

54 Replies Tue, Mar 26, 2024

4 Replies Sun, Feb 25, 2024
- AskMayoExpert. Gastric cancer (adult). Mayo Clinic; 2020.
- Gastric cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434. Accessed July 22, 2022.
- Niederhuber JE, et al., eds. Cancer of the stomach. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2022.
- Gastric cancer treatment (PDQ). National Cancer Institute. https://www.cancer.gov/types/stomach/patient/stomach-treatment-pdq. Accessed July 22, 2022.
- Gastric (stomach) cancer prevention (PDQ). National Cancer Institute. https://www.cancer.gov/types/stomach/patient/stomach-prevention-pdq. Accessed July 22, 2022.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed July 22, 2022.
- Odze RD, et al., eds. Epithelial neoplasms of the stomach. In: Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. 4th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Aug. 5, 2022.
- Mansfield PF. Clinical features, diagnosis and staging of gastric cancer. https://www.uptodate.com/contents/search. Accessed Aug. 5, 2022.
- Andreas A, et al., eds. The stomach. In: Grainger & Allison's Diagnostic Radiology: A Textbook of Medical Imaging. 7th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Aug. 5, 2022.
- Xia JY, et al. Advances in screening and detection of gastric cancer. Journal of Surgical Oncology. 2022; doi:10.1002/jso.26844.
- Best hospitals for gastroenterology and GI surgery. U.S. News & World Report. https://health.usnews.com/best-hospitals/rankings/gastroenterology-and-gi-surgery. Accessed Aug. 2, 2022.
- Best hospitals for cancer. U.S. News & World Report. https://health.usnews.com/best-hospitals/rankings/cancer. Accessed Sept. 9, 2022.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Feb. 12, 2020.
- Stomach cancer FAQs
Associated Procedures
- Barium enema
- Chemotherapy
- Endoscopic ultrasound
- Needle biopsy
- Palliative care
- Positron emission tomography scan
- Radiation therapy
- Upper endoscopy
News from Mayo Clinic
- Mayo Clinic Minute: Stomach cancer concerns Nov. 10, 2023, 05:00 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Jacksonville, Florida, and Mayo Clinic in Phoenix/Scottsdale, Arizona, have been recognized among the top Cancer hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.


What Life Is Like After A Stomach Cancer Diagnosis
F or people who don't have a loved one dealing with chronic disease or have never experienced it themselves, the realities of living with something like stomach cancer can seem unfathomable. But when you've been through a diagnosis yourself, you know about how the disease can change your life overnight.
The world's interest in stomach cancer, also known as gastric cancer, would've probably been piqued when Toby Keith's cause of death was explained. When cells in any part of your stomach begin to grow abnormally, tumors develop, and although the cancer typically grows from the inner lining of your stomach, it can also originate in the gastroesophageal junction, which is what's commonly seen in the U.S. The incidence of gastric cancer is declining in the U.S. but it remains the fifth most common type of cancer globally (per World Cancer Research Fund International ).
Being over 60 years of age, being male, and being native to East Asia, Eastern Europe, and South and Central America are considered risk factors. Obesity, smoking, and alcohol use are risk factors, too. Those who've had prior stomach surgery and those who eat a diet heavy in salt and processed meat and poor in fruits and vegetables are also more at risk.
One of the main ways in which a stomach cancer diagnosis changes your life has to do with how the disease makes you feel. Fatigue, fullness, heartburn, bloating, gassiness, indigestion, nausea, vomiting, trouble swallowing, stomach pain, tar-like poop, vomiting blood, and weight loss are all symptoms. How stomach cancer is treated can impact your quality of life too.
Read more: Health Symptoms That Are Serious Red Flags
Treatment And Nutrition After Stomach Cancer Diagnosis
Early detection can greatly direct treatment plans and thereby your quality of life. While stage 1 stomach cancer might only include surgery to remove the tumor, stages 2, 3, and 4 will look a lot different. Chemotherapy, radiation, targeted therapy, and immunotherapy can be part of the treatment plan. Using anticancer drugs like fluorouracil and cisplatin to kill cancer cells via chemotherapy can have immediate or delayed effects, depending on the medication, combination of treatments, and the person's general health. Loss of appetite, nausea, vomiting, diarrhea, fatigue, bleeding, sore throat, hair loss, skin issues, and tingling in hands and feet are considered side effects of chemotherapy and radiation.
With targeted therapy (using drugs to single out specific molecules such as proteins in the cancer cells), the side effects can be similar, with the addition of stomach pain, muscle and joint pain, headaches, and heart issues. Immunotherapy (using your own immune system to fight the tumor) can make someone feel weak, tired, and short of breath. Fever, cough, nausea, itching, skin issues, muscle and joint pain, loss of appetite, and irregular bowel movements can be part of the side effects, too.
How you consume food and what you can consume could also look different, particularly after stomach surgery. You may need to eat smaller meals more frequently rather than three large meals. You might experience dumping syndrome, characterized by nausea, diarrhea, sweating, and flushing after eating. This can happen when all or part of your stomach is removed. Using feeding tubes to get in nutrition, taking nutritional supplements, and working with a dietician can all become a part of your life.
Stomach Cancer And Mental Health
One of the topics that isn't addressed enough in stomach cancer-related data is how the disease can affect your mental health. Apart from the shock, confusion, despair, rage, and fear that come with the initial diagnosis, anxiety can become an everyday companion. Even if the cancer was removed, it is not uncommon to live with a fear of recurrence.
While some people living with gastric cancer turn to outlets like journaling and yoga to cope with the disease and all that comes with it, others have found speaking with their oncologist, therapist, social worker, or clergy member about concerns to be helpful. Having a supportive network of friends and family around you can also greatly influence your quality of life after a stomach cancer diagnosis, whether the prognosis is hopeful or not. Training your thoughts to remain mindful, pulling your attention to the present, and trying to enjoy the little joys and process the sorrows of the day can all be helpful ways to not let your fears cause undue stress.
Dealing with healthcare providers, especially when new information is being given to you about your diagnosis, can be frightening. Making a list of concerns before your visit can help. Finding and connecting with people going through the same thing you are can also be helpful for some. And if you're reading this article on behalf of a loved one, it doesn't hurt to know the sneaky signs of stomach cancer you shouldn't ignore . Knowledge about the disease can go a long way in detecting it early and having a better quality of life.
If you or someone you know needs help with mental health, please contact the Crisis Text Line by texting HOME to 741741, call the National Alliance on Mental Illness helpline at 1-800-950-NAMI (6264), or visit the National Institute of Mental Health website .
Read the original article on Health Digest .

- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- 25 year trends in...
25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis
Linked editorial.
Cancer trends in the UK
- Related content
- Peer review
- Jon Shelton , head of cancer intelligence 1 ,
- Ewa Zotow , visiting lecturer (statistics) 2 ,
- Lesley Smith , senior research fellow 3 ,
- Shane A Johnson , senior data and research analyst 1 ,
- Catherine S Thomson , service manager (cancer and adult screening) 4 ,
- Amar Ahmad , principal statistician 1 ,
- Lars Murdock , data analysis and research manager 1 ,
- Diana Nagarwalla , data analysis and research manager 1 ,
- David Forman , visiting professor of epidemiology 5
- 1 Cancer Research UK, London, UK
- 2 University College London, London, UK
- 3 Leeds Institute of Clinical Trials Research, University of Leeds, Leeds, UK
- 4 Public Health Scotland, Edinburgh, UK
- 5 Faculty of Medicine and Health, University of Leeds, Leeds, UK
- Correspondence to: J Shelton jon.shelton{at}cancer.org.uk
- Accepted 19 January 2024
Objective To examine and interpret trends in UK cancer incidence and mortality for all cancers combined and for the most common cancer sites in adults aged 35-69 years.
Design Retrospective secondary data analysis.
Data sources Cancer registration data, cancer mortality and national population data from the Office for National Statistics, Public Health Wales, Public Health Scotland, Northern Ireland Cancer Registry, NHS England, and the General Register Office for Northern Ireland.
Setting 23 cancer sites were included in the analysis in the UK.
Participants Men and women aged 35-69 years diagnosed with or who died from cancer between 1993 to 2018.
Main outcome measures Change in cancer incidence and mortality age standardised rates over time.
Results The number of cancer cases in this age range rose by 57% for men (from 55 014 cases registered in 1993 to 86 297 in 2018) and by 48% for women (60 187 to 88 970) with age standardised rates showing average annual increases of 0.8% in both sexes. The increase in incidence was predominantly driven by increases in prostate (male) and breast (female) cancers. Without these two sites, all cancer trends in age standardised incidence rates were relatively stable. Trends for a small number of less common cancers showed concerning increases in incidence rates, for example, in melanoma skin, liver, oral, and kidney cancers. The number of cancer deaths decreased over the 25 year period, by 20% in men (from 32 878 to 26 322) and 17% in women (28 516 to 23 719); age standardised mortality rates reduced for all cancers combined by 37% in men (−2.0% per year) and 33% in women (−1.6% per year). The largest decreases in mortality were noted for stomach, mesothelioma, and bladder cancers in men and stomach and cervical cancers and non-Hodgkin lymphoma in women. Most incidence and mortality changes were statistically significant even when the size of change was relatively small.
Conclusions Cancer mortality had a substantial reduction during the past 25 years in both men and women aged 35-69 years. This decline is likely a reflection of the successes in cancer prevention (eg, smoking prevention policies and cessation programmes), earlier detection (eg, screening programmes) and improved diagnostic tests, and more effective treatment. By contrast, increased prevalence of non-smoking risk factors are the likely cause of the observed increased incidence for a small number of specific cancers. This analysis also provides a benchmark for the following decade, which will include the impact of covid-19 on cancer incidence and outcomes.
Introduction
The availability of comprehensive cancer registration data across the UK since 1993 makes comparison of cancer incidence and mortality trends over 25 years possible. We examined UK trends in cancer incidence and mortality for men and women, aged 35-69 years, for all cancers combined and for the most common sites (or site groups) of cancer between 1993 and 2018.
This study focuses on the 35-69 years age group because cancer trend data are more reliable and easier to interpret in this age range. 1 Diagnostic accuracy is better in this age range than in older patients who have a greater proportion of clinical and uncertain diagnoses, as evidenced by the relatively low proportion of microscopically verified tumours, 2 especially in the earlier part of the period analysed. By the age of 35 years, the pattern of cancer broadly represents the usual adult profiles because specific cancers that are observed in childhood, adolescence, and young people would not impact on the overall pattern. Trends in the 35-69 years age group are also reflective of causal factors in the more recent and medium term past rather than in the longer term and, therefore, will be more indicative of future patterns of cancer in the older populations.
This time period has also seen the introduction of three population screening programmes across the UK, which have affected trends by diagnosing some cancers at an earlier stage, preventing cancers, but also had the potential for diagnosing some cancers that would not have otherwise caused harm to the individual, particularly breast cancer. 3 4 Cervical smear tests have been used since the 1960s and the national screening programme was introduced in 1988, with over 85% coverage of the target population (women and people with a cervix aged 25-64 years) in the UK by 1994. 5 The breast screening programme was introduced in 1988 and covered all UK countries by the mid-1990s, with women aged 50-70 years being invited. 6 The bowel screening programme was introduced from 2006 and took a number of years to reach full roll-out. Currently, people aged 60-74 across England, Wales, and Northern Ireland, and 50-74 for Scotland are eligible. Prostate specific antigen testing is not part of the national screening programme. Anyone older than 50 years with a prostate can request a prostate specific antigen test from their family doctor (general practitioner).
The past 25 years have seen differing trends in cancer risk factors, with the two most important risk factors displaying trends in opposing directions. In one direction, smoking prevalence is reducing due to introductions of tax rises on tobacco products, further advertising bans, and smokefree policies, including education and encouraging quitting, and, in the other direction, the proportion of the population classified as overweight or obese is increasing, of which diet and exercise contribute to, as well as being independent risk factors for cancer. 7
Cancer registration data are currently collected by four national registries in the UK. These organisations collect detailed information on newly diagnosed primary tumours, referred to as registrations. Prior to 2013, cancer registrations in England were collected by eight regional registries and compiled by the Office for National Statistics, 8 with these regional registries producing complete population coverage for England since 1971. 9 Cancer Research UK aggregate these data from the UK registries, with incidence, mortality, and corresponding national population data provided by the Office for National Statistics, Public Health Wales, 10 Public Health Scotland, 11 the Northern Ireland Cancer Registry, 12 NHS England, 13 and the General Register Office for Northern Ireland. 14 Coding of cancer registrations is consistent between countries of the UK, using internationally accepted codes from the International Classification of Diseases 10th revision (ICD-10) and collaboration through the UK and Ireland Association of Cancer Registries. 15
Cancer sites (for single sites) or site groups (with multiple sites, such as oral) included in these analyses were selected as the most common causes of cancer incidence or death. These cancer sites are: all cancers combined (excluding non-melanoma skin cancer for incidence) (C00-C97, excluding C44); bladder (C67); bowel (C18-C20); brain and central nervous system (C70-C72, C75.1-C75.3, D32-D33, D35.2-D35.4, D42-D43, D44.3-D44.5); breast (women only) (C50); cervix (C53); Hodgkin lymphoma (C81); kidney (C64-C66, C68); larynx (C32); leukaemia (C91-C95); liver (C22); lung (C33-C34); melanoma skin(C43); mesothelioma (C45); myeloma (C90); non-Hodgkin lymphoma (C82-C86); oesophagus (C15); lip, oral cavity, and pharynx (oral) (C00-C06, C09-C10, C12-C14); ovary (C56-C57.4); pancreas (C25); prostate (C61); stomach (C16); testis (C62); and uterus (C54-C55). In addition, sex specific all cancer groups are also presented without breast and prostate cancers to inspect the overall trends in the absence of the most common cancer site for each sex. Sex is reported as recorded by the cancer registries at the time of registration. Mesothelioma was a new specific code introduced in ICD-10 and no reliable mortality data are available for this site before 2001, hence, we have not included this type of cancer prior to then. Non-malignant brain and central nervous system codes (ICD-10 D codes) are included despite their benign nature because they can cause mortality due to their location in the cranial cavity. The codes included for the brain and central nervous system have been chosen following clinical engagement and discussion with cancer registries across the UK. Non-melanoma skin cancer is excluded for incidence data because of the lack of completeness in the recording of these cancers and therefore unreliability of the data; this process is standard practice among UK cancer registries. 16 A proportion of non-melanoma skin cancer cases can be diagnosed and treated within primary care and have not consistently been captured within cancer registration data. 17
To overcome yearly variation for sites with low numbers of cases, we calculated three-year rolling average age standardised rates per 100 000 population. 18 These rates were based on the European standard population 2013 for men and women separately for each cancer site or site group for both incidence and mortality, restricted to the 35-69 years age group. 19
The estimated annual percentage change is commonly computed using a generalised linear regression model with Gaussian or Poisson link function. 18 20 In this analysis, a generalised linear model was performed with quasi-Poisson link function as overdispersion is very common when modelling rates and count data. 21 The outcome was the age standardised cancer (incidence or mortality) rate per 100 000 and the independent variable was the period variable, which was defined as the three year period for each data point, starting from 1993-95 and ending with 2016-18. Estimated annual percentage change was estimated as (exp (β^−1)' 100, where β^ is the estimated slope of the period variable, with corresponding 95% confidence interval, which is derived from the fitted quasi-Poisson regression model. 22 The determination of trends was based on the following criteria: firstly, an increasing trend was identified when the estimated annual percentage change value and its 95% confidence interval were greater than zero. This value suggests a statistically significant increase in the age standardised rate over time. Secondly, a decreasing trend was indicated when both the estimated annual percentage change value and its 95% confidence interval were less than zero, signifying a statistically significant decline in the age standardised rate over the period considered. Finally, in cases where these conditions were not met, the age standardised rate was concluded to have remained relatively stable. This designation means that no significant change in the age standardised rate over the period examined was noted. These criteria ensure a thorough and precise interpretation of the estimated annual percentage change values and their corresponding trends. These analyses were carried out for each sex and site or site group separately. Statistical analysis was performed using R version 4.0.2. 23
Patient and public involvement
This work uses aggregated and non-identifiable routine data that have been provided by patients and collected by the health services of the UK as part of their care and support. Given the aggregated nature of the data, attempts to identify or involve any of the patients whose data are included is not possible nor permitted. Although patients and the public were not involved in the design and conduct of this research, the aim of this research is to provide an assessment of trends in cancer incidence and mortality and the impacts of treatment and policy changes to improve outcomes for cancer patients across the UK. Dissemination to the public will include a press release and a summary published online, written using layman’s terms, and a webinar to discuss the results.
Table 1 and table 2 show the percentage of all newly diagnosed cancer cases and deaths by age group in 1993 and 2018. For male registrations, around 43% of all registrations were in the 35-69 years age group in 1993 and 2018, while for female registrations, between 47% and 48% of all registrations were in this age group in 1993 and 2018, respectively. For mortality, around 40% of male cancer deaths occurred in the 35-69 years age group in 1993 and this value was lower at 30% in 2018. For female cancer deaths, a slightly smaller reduction was noted, from 38% in the 35-69 years age group in 1993 to 31% in 2018.
Number of newly diagnosed cancer cases (% of total) in the UK for all cancers, excluding non-melanoma skin cancer, (ICD-10 C00-C97 excluding C44) by sex and age group in 1993 and 2018
- View inline
Number of deaths (% of total) in the UK for all cancers, (ICD-10 C00-C97) by sex and age group in 1993 and 2018
Figure 1 shows the number of newly diagnosed cancer cases and deaths in the 35-69 years age group between 1993 and 2018 by sex. Across the UK, of cancer registrations in 2018, 83% were from England, and 5.1% from Wales, 9.2% from Scotland, and 2.7% from Northern Ireland; for deaths in 2018, 81.4%, 5.3%, 10.4%, and 2.9% were from England, Wales, Scotland, and Northern Ireland, respectively. These proportions remained relatively stable over the study period. For men, the number of cancer registrations increased by 57% from 55 014 cases registered in 1993 to 86 297 cases registered in 2018, while for women, cases increased by 48% from 60 187 in 1993 to 88 970 in 2018. The rate of increase in the number of cases of cancer was more marked between 2003 and 2013 for both sexes than in other time periods in the study.

Number of newly diagnosed cancer cases and deaths in the UK for all cancers, excluding non-melanoma skin cancer for incidence (International Classification of Diseases (10th revision) codes C00-C97 (excluding C44 for incidence)), men and women, 35-69 years, 1993 to 2018. An interactive version of this graphic is available at https://bit.ly/4acPDjP
- Download figure
- Open in new tab
- Download powerpoint
The number of cancer deaths in men and women aged 35-69 years decreased: by 20% in men from 32 878 in 1993 to 26 322 deaths in 2018 and by 17% in women from 28 516 in 1993 to 23 719 deaths in 2018. The main decrease in the number of deaths per year occurred before the year 2000 ( fig 1 ) with a decrease of 14% in males and 11% in females between 1993 and 2000. Since 2000, the number of deaths each year in both men and women has remained fairly constant ( fig 1 ).
Table 3 , table 4 , figure 2 and figure 3 , and figure 4 and figure 5 show the trends over time in both incidence and mortality rates by sex and cancer site or site group. The tables only include specific age standardised incidence and mortality rates for the first (1993-95) and last (2016-18) period to give an indication of the change over the 25 year period. The trends in incidence and mortality age standardised rates for all years are shown in the figures. Figure 6 and figure 7 show the age adjusted average annual percentage change in the rates. Between 1993-95 and 2016-18, the age standardised incidence rate for all cancers (excluding non-melanoma skin cancer) increased slightly in men and women with age adjusted annual increases of 0.8% for both sexes. The trends in prostate and breast cancer, as the two largest cancer sites in men and women, respectively, substantially contribute to the overall all sites trends for cancer incidence. Figure 3 shows the trends for each sex without the largest cancer site. In contrast to the male age standardised incidence rate for all cancers, which showed a general increase, the incidence trend for men for all cancers excluding non-melanoma skin and prostate cancer, showed a decrease before 2000, but very little change in the following period. For women, an increase in age standardised incidence rates for all cancers excluding non-melanoma skin and breast cancer is still observed but the rate of increase is lower, at 0.7% per annum on average, over the 25 year period. Over the same period reductions in age standardised mortality for all cancers, including non-melanoma skin cancer, were −2.0% per year in men and −1.6% in women. Exclusion of prostate cancer from the mortality trends for men had a negligible effect on the average annual percentage change. For women, the exclusion of breast cancer from mortality trends led to a smaller decrease in mortality of −1.3% per annum.
Age standardised* incidence and mortality rates in 1993-95 and 2016-18 and percentage change by cancer type, men aged 35-69 years, UK
Age standardised* incidence and mortality rates in 1993-95 and 2016-18 and percentage change by cancer type, women aged 35-69 years, UK

European 2013 population age standardised incidence and mortality rates in the UK for all cancers, 19 excluding non-melanoma skin cancer for incidence (International Classification of Diseases (10th revision) codes C00-C97 excluding C44 for incidence), men and women, 35-69 years, 1993-95 to 2016-18. An interactive version of this graphic is available at https://bit.ly/4a484aE

European 2013 population age standardised incidence and mortality rates in the UK for all cancers in men and women aged 35-69 years during 1993-95 to 2016-18, 19 excluding non-melanoma skin cancer for incidence, and breast cancer in women and prostate cancer in men were excluded for incidence and mortality (International Classification of Diseases (10th revision) codes C00-C97 excluding C44 for incidence, C50, C61). An interactive version of this graphic is available at https://bit.ly/3vakQoX

European 2013 age standardised incidence and mortality rates by year, 19 in the UK, for men and women aged 35-69 years from 1993-95 to 2016-18, by cancer site. An interactive version of this graphic is available at https://bit.ly/49a6ovn

Relative European 2013 age standardised incidence and mortality rates by year, 19 in the UK, for men and women aged 35-69 years from 1993-95 to 2016-18 (the reference year is 1993-95=100), by cancer site. CNS=central nervous system. An interactive version of this graphic is available at https://bit.ly/3PiKGOk

Average annual percentage change in incidence and mortality rates, in the UK, for men aged 35-69 years from 1993-95 to 2016-18 by cancer site. An interactive version of this graphic is available at https://bit.ly/3wMR6yU

Average annual percentage change in incidence and mortality rates, in the UK, for women aged 35-69 years, from 1993-95 to 2016-18, by cancer site. An interactive version of this graphic is available at https://bit.ly/3v0QdT7
Incidence rates varied over time across the different cancer sites and site groups. The largest average annual percentage increases over time for cancer incidence rates for men aged 35-69 years were for cancers of the liver (4.7%), prostate (4.2%), and melanoma skin cancer (4.2%). Increases of 1% or more per annum were also seen for oral cancer (3.4%), kidney cancer (2.7%), myeloma (1.6%), Hodgkin lymphoma (1.5%), testicular cancer (1.3%), non-Hodgkin lymphoma (1.0%), and leukaemia (1.0%). The largest annual decreases over the two decades were seen for stomach (−4.2%), bladder (−4.1%), and lung cancers (−2.1%), with decreases of more than 1% per annum also observed for mesothelioma (−1.9% from 2001 onwards) and laryngeal cancer (−1.5%).
For women, the largest average annual percentage increases in incidence rates were noted for liver (3.9%), melanoma skin (3.5%), and oral (3.3%) cancers with increases in incidence of more than 1% per annum also observed for kidney (2.9%), uterus (1.9%), brain and central nervous system cancers (1.8%), Hodgkin lymphoma (1.6%), myeloma (1.1%), and non-Hodgkin lymphoma (1.0%). The largest annual decreases were reported for bladder (−3.6%) and stomach (−3.1%) cancers while the only other site showing a decrease of more than 1% per annum was cervical cancer (−1.3%). Although breast cancer represents the largest individual cancer site for women and therefore plays a large part in all cancer trends, the average annual increase was only 0.9%. All the incidence changes mentioned, for both men and women, and most incidence changes shown in table 3 and table 4 and in figure 6 and figure 7 were statistically significant (P<0.05) even when the size of change was relatively small.
Mortality rates mainly decreased over time in both sexes. For men, the cancer sites that showed average annual percentage reductions in mortality rates of more than 1% per annum were stomach (−5.1%), mesothelioma (–4.2% from 2001), bladder (–3.2%), lung (–3.1%), non-Hodgkin lymphoma (–2.9%), testis (–2.8%), Hodgkin lymphoma (–2.6%), bowel (–2.5%), larynx (–2.5%), prostate (–1.8%), myeloma (–1.7%), and leukaemia (–1.6%). Only liver (3.0%) and oral (1.1%) cancers showed an average annual increase in mortality of 1% or more with melanoma skin cancer (0.3%) the only other site showing an increase. For women, the cancer sites with average annual decreases in mortality per year of 1% or more were stomach (–4.2%), cervix (–3.6%), non-Hodgkin lymphoma (–3.2%), breast (–2.8%), Hodgkin lymphoma (–2.8%), ovary (–2.8%), myeloma (–2.3%), bowel (–2.2%), leukaemia (–2.1%), larynx (–2.0%), mesothelioma (–2.0% since 2001), bladder (–1.6%), oesophagus (–1.2%), and kidney (1.0%). As with men, liver (2.7%) and oral (1.2%) cancers showed average annual increases of more than 1%, in addition to uterine cancer (1.1%). For both men and women, the mortality changes mentioned previously and most mortality changes shown in table 3 and table 4 and in figure 6 and figure 7 were statistically significant (P<0.05), even when the size of change was relatively small.
Principal findings
The most striking finding in this analysis of UK cancer trends among the 35-69 years age group is the substantial decline in cancer mortality rates observed in both sexes (37% decline in men and 33% decline in women) across the period examined. A decrease in mortality was reported across nearly all the specific types of cancer examined (23 in total), with only liver, oral, and uterine cancers showing an increase together with melanoma skin cancer in men and pancreatic cancer in women, both showing small increases. By contrast, the incidence trends in this age group showed varying patterns with some sites increasing, some decreasing and some remaining relatively constant. Over all sites, a modest increase was noted in cancer incidence rates of around 0.8% per annum in both sexes, amounting to an increase of 15% in men and 16% in women over the 25 year time frame.
The increase in prostate cancer incidence over this period, especially in the 35-69 years age group considered here, is very likely to be a direct result of the uptake of prostate specific antigen testing, which results in the detection of early stage disease and, to an unknown extent, indolent disease that may otherwise never have been regarded as clinically significant. 24 25 The results do, however, affect people diagnosed and represent a large increase in workload for clinical staff. The fact that the overall mortality trends for men show no difference whether prostate cancer is included or excluded in the analysis indicates that the incidence increase for this cancer has largely been of non-fatal disease. That the specific mortality rates for prostate cancer showed an appreciable rate of decline during this time (–1.8% per annum) also indicates improved clinical treatment of the disease or an increase in the proportion of men diagnosed with a favourable prognosis, or both. 24 26 However, the increase in prostate cancer incidence still results in thousands of men each year dealing with the concerns of a cancer diagnosis and the impact this may have on their lives.
Breast cancer comprehensively dominated incidence and mortality trends in female cancer. Even though the average annual incidence increase of breast cancer over this period (0.9%) was modest in comparison to the prostate cancer increase in men (4.2%), breast cancer incidence rates remained substantially higher than those for any other cancer site in either sex. Inspection of figure 4 shows that breast cancer incidence rates (age standardised) increased at a faster rate until around 2003-05 (from 194.7 in 1993-95 to 229.9 in 2003-05), a slower rate from then until 2013-15 (240.8) but have levelled off in the most recent years analysed (238.0 in 2016-18). These changes in the incidence trend likely reflect a reduced effect of the initial incidence increases brought about by mammography screening in the UK introduced from the late 1980s or a possible effect of a decline in usage of hormone replacement treatment. 27 28 However, the effect of hormone replacement treatment on breast cancer risk is small in comparison to other risk factors, 7 and trends in this treatment has varied over time, such as changes in preferred formulations, doses, and treatment durations, 29 30 31 which may impact breast cancer risk levels. 32 33 As has been reported elsewhere, 34 35 36 mortality for breast cancer has declined substantially despite the incidence increase, which is indicative of improvements in early detection (including through screening 37 ) and improved treatment.
The other two major sites of cancer in men apart from prostate cancer, namely lung and bowel cancers, showed substantial reductions in mortality. These results are likely from primary prevention (historical reduction in smoking rates) 38 39 40 41 for lung cancer and earlier detection (including screening) and improved treatment for bowel cancer. 42 43 44 While lung cancer incidence substantially decreased, the incidence rates of bowel cancer remained unchanged. However, closer inspection of the bowel cancer incidence trends over the full period shows an increase from the point the bowel screening programme was first introduced from 2006 in the UK. This rate, however, has now decreased back to the observed level prior to the introduction of the screening programme. As others have shown, the introduction of bowel screening leads to an initial short-term increase in cancer incidence due to detection of as-yet undiagnosed cancer cases, followed by a decrease because of removal of adenomas. 42 45 46 Therefore, bowel cancer incidence trends can reasonably be assumed to decrease further over the coming years, unless other preventable risk factors for bowel cancer affect the trend.
Similarly, lung and bowel were the other two major cancer sites for women (alongside breast cancer), and both showed reductions in mortality. The decline in lung cancer mortality was, however, not as extensive as that for men (–0.5% compared with –3.1% per annum) likely reflecting the different demographic pattern in smoking rates that led to peak smoking prevalence in women occurring around 30 years later than men, albeit at around half the peak prevalence observed in men. 40 47 Smoking prevalence in women has always been lower than in men. 39 48 The lung cancer incidence trends showed a significant increase in women of 0.8% per annum as opposed to the –2.1% per annum decrease in men. That the incidence rate in 2016-18 was still higher in men than in women again is almost certainly a reflection of historical differences in smoking patterns. 39 49 50 Bowel cancer incidence in women followed a similar pattern to men and is equally reflective of the introduction of the bowel screening programme. Bowel cancer mortality in women has declined at a similar rate to men (–2.2% compared with –2.5% per annum), indicative of the same improvements in early detection and improved treatment.
These reductions in mortality across the most common cancers in both sexes are likely a representation of considerable success in cancer prevention, diagnosis, and treatment. Further improvements are likely to be realised from the continued reduction in smoking prevalence, of which smoking prevention policies continue to contribute, 51 alongside the recent move to faecal immunochemical testing in the bowel screening programme adopted throughout the UK during 2019. 52 The recommended rollout of targeted lung screening is expected to further help with the earlier diagnosis of lung cancer where surgery is a viable treatment option and outcomes are vastly improved. 53 54
Although four major sites influenced the overall pattern of cancer incidence and mortality, increases in rates among some of the less common sites do raise concerns. Four cancers showed substantial increases in incidence (more than 2% per annum) in both sexes: liver, melanoma skin, oral, and kidney cancers. All have strong associations with established risk factors: alcohol consumption, smoking, and HPV for oral cancer; 7 55 56 overweight and obesity, smoking, alcohol, and hepatitis B and C for liver cancer; 7 57 58 ultraviolet light for melanoma; 59 60 and obesity and smoking for kidney cancer. 61 62 63 Increases in liver cancer incidence and mortality for both men and women are very concerning, with nearly one in two attributable to modifiable risk factors. 7 With high prevalence of overweight and obesity and diabetes in the general population, other studies expect the rates to remain high. 64 For oral and kidney cancer, despite the association with smoking, incidence rates have not followed the decrease seen for lung cancer incidence in men. This is likely to be due to the smaller proportion of cases attributable to smoking in these two sites. Whilst smoking accounts for around 17% of oral cancers, over one in three are attributed to alcohol consumption. 7 For kidney cancer, smoking is attributable to 13% of cases whereas obesity causes around 25%, however, increasing trends in kidney mortality are shown for this age group and period. 7 Therefore, the increasing incidence trends could potentially have been worse, especially in men, if the reduction in smoking prevalence had not occurred. The increased incidence of melanoma skin cancer is likely to be caused by the increased sunlight and ultraviolet exposure caused by the availability of cheaper air travel to countries with a warmer climate and insufficient regulation of tanning beds until 2010. 65 66
In women, uterine cancer incidence increased by 1.9% per annum; although, this increase was predominantly seen over the period 1993-2007 and since then incidence trends have increased at a slower rate. One of the main risk factors for uterine cancer is the use of oestrogen-based hormone replacement therapy, 67 68 and since around 2000, use has substantially declined. 27 Around a third of uterine cancers in the UK are also attributed to overweight and obesity, but the increase in incidence is also likely to be caused by a decrease in the number of women undergoing hysterectomies for menorrhagia, in favour of endometrial ablation. 69
Other cancers that showed increases in incidence were cancers of the pancreas, brain, and central nervous system, together with Hodgkin and non-Hodgkin lymphoma, myeloma, and leukaemia in both sexes, and oesophageal and testicular cancers in men. With the exception of pancreatic cancer, which only decreased in women, all these cancers also showed a reduction in mortality in both sexes, indicating improving treatment or earlier detection, or both. Generally, the causes of these cancers are not well understood although obesity is associated with the adenocarcinoma histological subtype of oesophageal cancer, 70 especially in men, 7 while a combination of smoking and alcohol is implicated in the squamous cell carcinoma subtype. 71 The considerable male excess in oesophageal adenocarcinoma in comparison with squamous cell carcinoma rates, 72 possibly underlined by the higher incidence of gastroesophageal reflux disease in men 73 and the protective effect of oestrogen, 74 75 may explain the differing trends now observed between men and women.
Several cancer sites showed decreases in both incidence and mortality rates over the time period, notably stomach, larynx, and bladder cancer in both sexes, as well as cervical and ovarian cancers in women and mesothelioma in men. The changes in stomach cancer rates were of a similar magnitude and represented the largest percentage mortality decline in both sexes. This decline can probably be attributed to a combination of a reduction in the prevalence of Helicobacter pylori infection and an increase over time in fruit and vegetable consumption reducing the dependency on preserved foods. 76 77 Challenges in coding of stomach and oesophageal cancer before 2000 may also have had a role in shaping these trends. Laryngeal cancer is associated with tobacco use and alcohol consumption as well as occupational exposures, 56 78 79 and the decline in rates is most likely to be related to the decrease in smoking prevalence as well as decreases in occupational exposure. 80 The refinement of understanding pathology for bladder cancer during this period, in which previously diagnosed malignant disease is now categorised as benign, 81 is likely to have resulted in an artificial decline in incidence rates. 82 83 This artefact should not, however, have affected the decline in mortality rates given the benign nature of these tumours that do not cause death. 81 This decline in mortality, although not as marked as that for incidence, remained appreciable. The changes in cervical cancer rates, which showed the largest percentage mortality decline amongst gynaecological cancers, are almost certainly attributed to the success of the cytological screening programme during the whole of the time period considered. 84 85 With the introduction of the HPV vaccination programme for girls in 2008 86 and the subsequent expansion to boys in 2019, 87 rates of cervical cancer are expected to fall substantially over the following decades as the first cohort of vaccinated women reaches the peak age for cervical cancer incidence (aged 30-34 years). A reduction has already been shown for women aged 20-24. 88 The absolute incidence rates of mesothelioma in women were small in magnitude in 1993-95 (0.8 per 100 000 per annum) and remained similar over time (0.7 per 100 000 per annum in 2016-18). The incidence rates of mesothelioma in men were considerably greater, especially in 1993-95 (around 6.3 per 100 000 per annum), due largely to occupational asbestos exposure, 89 but a significant decrease was noted over time (to 3.6 per 100 000 per annum in 2016-18) resulting from both the decline in asbestos exposure and the decline in heavy industries, such as coal mining. Mortality decreased substantially in both sexes over the period for which data are available (2001-03 to 2016-18).
The conclusions that can be drawn from this analysis are, overall, positive and reassuring. Within the 35-69 year age group, cancer mortality rates have shown a substantial overall decline during the last quarter of a century in both men and women. The most probable causes are a combination of changes in the underlying risk of disease for some cancers (notably lung and stomach), in increased levels of early detection (notably breast 37 and cervix 90 ) and improved treatment (notably breast and bowel) for others. The specific circumstances leading to the increased incidence of breast cancer, of which risk factors are complex, need to be better understood and controlled. Similar results have been shown for incidence within Great Britain and mortality in the UK for some cancer sites. 91 Speculated overdiagnosis, where tumours are detected that would not have caused the patient any harm during their lifetimes, has been thought to increase rates for breast and prostate cancers in particular, of which prostate is especially affected by the widespread use of prostate specific antigen testing. 4 92 However, given the decreases in mortality across the wide set of cancer sites analysed here, improvements in early diagnosis, treatment, or both are having a positive effect for most cancer patients, although cancer mortality in this age group still needs reducing.
After accounting for the major two sites in men and women, the increase in overall incidence rates disappeared in men while it remained significant in women. This difference between sexes is due to a decrease in cancers with substantially higher initial incidence rates in men, such as lung, stomach, and bladder, resulting in a higher overall impact on male incidence, combined with an increase in incidence in uterine cancer, one of the most common cancers in women.
Strengths and limitations
This study benefits from high quality cancer registry data collected by all four cancer registries in each country across the UK, which allows for the inspection of a wide range of cancer sites over 25 years. ICD-10 coding changes have been minimal, only affecting trends in cancer incidence for bladder and ovarian cancers and cancer mortality for mesothelioma, whereas challenges in coding stomach and oesophageal cancer may have affected trends for these sites. Changes in registration practice may well have had a small effect on certain cancer sites. By focusing only on the 35-69 age range, we present a clear and reliable comparative picture of cancer incidence across 25 years within the UK, which provides a reliable indicator regarding future cancer incidence trends. Understanding cancer in older people and changes in the trends of different cancers is also of interest, but subject to a different study given the increasing life expectancy over this period, impact of comorbidities, and differing interaction with health services in this age group.
Limitations include the absence of staging data to substantiate any improvements in earlier diagnosis. Due to the number of sites analysed, we also have not broken down sites by histological type, which could be beneficial in certain sites to understand the trends within cancer sites—eg, small cell and non-small cell lung cancer or oestrogen receptor-positive and oestrogen receptor-negative breast cancer. In focusing on the age group selected, we are excluding older ages where rates of cancer are higher. Although this exclusion reduces the number of cases included, providing a smaller cohort for each year, the age group selected provides a more reliable comparator for future trends given the accuracy of incidence recording and also focuses on the cancers that lead to a larger number of years of life lost. The age range included in this study has been well defined; however, other studies are indicating potentially different trends worldwide in young adults with potential increases in risk factors such as dietary risk factors playing a role. 93 94 The data captured across the UK registries provides a basis for further understanding to see whether different trends are observed across younger age groups and whether the causes of this can be determined. Additionally, although we have included a broad range of cancer sites, cancers that have not been included in this study could well be showing different trends, such as a more recent increase in thyroid cancer in the UK. 95
This study also provides a baseline covering a 25 year period uninterrupted by covid-19. Trends in cancer incidence and mortality beyond these years will be affected and therefore understanding the causes of trends will be more complicated. Having a 25 year baseline provides the observed trend for which expected cases can be assessed against observed. This benchmark will present a comparison for the following decade as the presentation, diagnosis, and treatment of cancer have been hugely affected by rules and regulations affecting public and health service staff. Mortality trends will also be impacted with decision making regarding coding of deaths with covid-19 likely to be the underlying cause of death for people with cancer if that has directly led to the patient dying, rather than their cancer.
This study focuses on the overall sex specific trends for cancer incidence and mortality in the specified age group to observe and understand trends over the 25 year period across the entire UK. Further breakdowns have not been possible. Paucity of numbers for less common cancers precluded separate analyses for the individual UK nations while data limitations precluded analyses by other demographic characteristics, for example, ethnic group and deprivation. The main obstacle to analysing data by ethnic group is the completeness of recordings in hospitals. In England, completeness improved substantially in 2012, but prior to this, the proportion of cases with unknown ethnic group renders results over time to be incomparable. In other UK countries, completeness of ethnic group recording is still not good enough to conduct country-wide cancer incidence or mortality analyses by ethnicity. For deprivation, the measures currently available are derived within each UK nation, and a specific validated UK-wide deprivation measure does not yet exist. Given the obvious importance of looking at variation in UK trends within ethnic groups and deprivation categories, such analyses represent a priority for further research and highlights the importance of data collection across all UK nations.
Conclusions
Overall, these results substantiate the view that in this age group there is no generalised increase in cancer incidence, while there is a substantial decrease in cancer mortality in the UK over the 25 year study period. Specific concerns about individual cancer sites identified were raised, of which the most important numerically, apart from the increases in breast and prostate cancer incidence, was the need to accelerate the decrease in female lung cancer. After which, concerns about oral cancer, liver cancer, kidney cancer, uterine cancer, and melanoma skin cancer present the most pressing issues. There are also several cancer sites that showed decreases in both incidence and mortality, notably, stomach, larynx, bladder, and cervical.
What is already known on this topic
No recent studies have investigated cancer incidence and mortality rates over such a long time frame within the 35-69 year age group in the UK
Short term trends for specific cancer sites are related to known risk factors, screening programmes, and improved treatment
Trends in the 35-69 years age group can be indicative of future patterns of cancer in older people
What this study adds
Decreased rates of many cancers, including lung and laryngeal, is positive, and likely to be driven by the decrease in smoking prevalence across the UK
An increase in rates of other cancer sites, including uterine and kidney, was noted, which may be a result of the increasing prevalence of overweight/obesity and other risk factors
Organised population screening programmes have led to an increase in cancer incidence but also look to have contributed to a reduction in cancer mortality across the UK
Ethics statements
Ethical approval.
Ethics approval for this work was not required as the study used publicly available data.
Data availability statement
Data sharing may be possible for additional analyses. All code used for analyses in this paper are also available from the Cancer Research UK website and GitHub. Information on how to access the data used in this analysis are available from the Cancer Research UK website.
Acknowledgments
This work uses data that has been provided by patients and collected by the health services as part of their care and support. The data is collated, maintained, and quality assured by NHS England, Public Health Wales, Public Health Scotland, and the Northern Ireland Cancer Registry.
Contributors: All authors participated in study conception and design, and/or the analysis and interpretation of results. Conception and design: DF, LS, and CT. Analysis and interpretation: all authors. Writing manuscript: all authors. Supervision and guarantor: JS and DF. All authors critically reviewed drafts of the manuscript, read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The manuscript’s guarantor (DF) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related and public communities: study results will be disseminated to the public and health professionals by a press release written using layman’s terms; findings will also be shared through mass media communications and social media postings. A webinar produced alongside a patient advocacy group is also planned to accompany the publication of this study, a recording of which will be made available on the Cancer Research UK website. Since the study analyses cancer registry data collected during routine care, and provided in aggregated form, we are unable to specifically disseminate results to study participants beyond the usual channels of publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Queen’s University Belfast, Northern Ireland Cancer Registry
- van Seijen M ,
- Thompson AM ,
- PRECISION team
- Independent UK Panel on Breast Cancer Screening
- Kitchener H ,
- ↵ Cancer Research UK. Screening for cancer. https://www.cancerresearchuk.org/about-cancer/screening
- Office for National Statistics
- Public Health Wales
- Public Health Scotland
- NHS Digital
- NI Direct Government Services
- United Kingdom and Ireland Association of Cancer Registries
- UK Health Security Agency
- National Cancer Intelligence Network
- Benhamou E ,
- Laversanne M ,
- Gardner W ,
- Mulvey EP ,
- Hankey BF ,
- Kosary CL ,
- R Core Team
- Pashayan N ,
- Pharoah P ,
- Martin RM ,
- Donovan JL ,
- Turner EL ,
- CAP Trial Group
- Neuberger MM ,
- Djulbegovic M ,
- Johnson A ,
- Bromley SE ,
- de Vries CS ,
- Burkard T ,
- Alsugeir D ,
- Adesuyan M ,
- Vinogradova Y ,
- Coupland C ,
- Hippisley-Cox J
- Collaborative Group on Hormonal Factors in Breast Cancer
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)
- Coleman DA ,
- Swerdlow AJ ,
- Youlden DR ,
- Cancer Research UK
- ↵ International Agency for Research on Cancer, World Health Organization. Tobacco Smoke and Involuntary Smoking. 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol. 83. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Tobacco-Smoke-And-Involuntary-Smoking-2004
- McClements PL ,
- Madurasinghe V ,
- Thomson CS ,
- Ait Ouakrim D ,
- Niikura R ,
- Brenner H ,
- Schrotz-King P ,
- Holleczek B ,
- Katalinic A ,
- Hoffmeister M
- Lieberman DA ,
- McFarland B ,
- American Cancer Society Colorectal Cancer Advisory Group ,
- US Multi-Society Task Force ,
- American College of Radiology Colon Cancer Committee
- Silcocks P ,
- Whitley E ,
- Malvezzi M ,
- Bertuccio P ,
- Lortet-Tieulent J ,
- Renteria E ,
- UK National Screening Committee
- Royal College of Physicians
- McLaughlin JK ,
- Hashibe M ,
- Brennan P ,
- Chuang SC ,
- Armstrong GL ,
- Farrington LA ,
- Hutin YJF ,
- Miyamura T ,
- Gilchrest BA ,
- Geller AC ,
- Berwick M ,
- van der Hel OL ,
- McMillan GP ,
- Boffetta P ,
- Zeegers MPA ,
- van Den Brandt PA
- Driver RJ ,
- HCC-UK/BASL/NCRAS Partnership Steering Group
- Doherty VR ,
- Brewster DH ,
- UK government
- Gebretsadik T ,
- Kerlikowske K ,
- Ernster V ,
- Million Women Study Collaborators
- Mukhopadhaya N ,
- Manyonda IT
- Lagergren J
- Rubenstein JH
- Czanner G ,
- Chandanos E ,
- Roberts SE ,
- Morrison-Rees S ,
- Samuel DG ,
- Williams JG
- Rothenbacher D ,
- Talamini R ,
- Bosetti C ,
- La Vecchia C ,
- Menvielle G ,
- Goldberg P ,
- National Institute for Health and Care Excellence
- Vaccarella S ,
- Plummer M ,
- Franceschi S ,
- University of Oxford
- Castanon A ,
- Windridge P ,
- Hodgson JT ,
- Matthews FE ,
- Castañón A ,
- O’Sullivan JW ,
- Nicholson BD
- Bjurlin MA ,
- Nicholson J ,
- Sasamoto N ,
- Share full article
Advertisement
Supported by
More Young People Than Ever Will Get Colorectal Cancer This Year
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why.

By Knvul Sheikh
Marisa Peters had been experiencing symptoms for years: blood on her toilet paper after going to the bathroom, changes in her stool and difficulty controlling the urge to poop. But she was in her 30s, healthy and physically active. She did not have any abdominal pain, and doctors dismissed the symptoms as hemorrhoids, or normal postpartum changes after the birth of her first son. When Ms. Peters finally visited a gastroenterologist in 2021, after having her third child and experiencing worsening bleeding from her rectum along with changes in her stool consistency, an urgent colonoscopy confirmed that she had colorectal cancer.
It had been four or five years since her symptoms had first emerged. Yet “I did not expect that cancer was going to be what they found,” Ms. Peters said.
A report published by the American Cancer Society in January suggests that rates of colorectal cancer are rising rapidly among people in their 20s, 30s and 40s — even as incidence is declining in people over the age of 65.
“It’s unfortunately becoming a bigger problem every year,” said Dr. Michael Cecchini, a co-director of the colorectal program in the Center for Gastrointestinal Cancers and a medical oncologist at Yale Cancer Center. He added that early-onset colorectal cancers have been increasing by about 2 percent per year since the mid-1990s. This increase has moved colorectal cancer up to being the top cause of cancer deaths in men under the age of 50 and the second-leading cause of cancer deaths in women under 50 in the United States.
In fact, experts are noticing a rise in early-onset colorectal cancers around the world — a trend that they are racing to explain.
Why is colorectal cancer increasing among young people?
Colon and rectal cancers share many similarities and are typically lumped into one category, called colorectal cancer. Studies, however, show that the increase in diagnoses is mainly driven by a rise in rectal cancers and cancers found in the left, or distal, side of the colon, near the rectum. “That maybe provides an important clue for understanding what might be going on,” said Caitlin Murphy, an associate professor and cancer researcher at UTHealth Houston.
Colorectal cancers in younger people also tend to be more aggressive, and they are often found at a more advanced stage, Dr. Murphy said. But most people affected by early-onset colorectal cancer are too young to be recommended for routine cancer screenings, which have helped decrease rates in adults over 50. In 2021, the U.S. Preventive Services Task Force reduced the recommended age for starting colorectal cancer screening by just five years — from 50 to 45 .
A vast majority of colorectal cancer diagnoses are still made in people 50 and older. The American Cancer Society predicted last year that roughly 153,000 new diagnoses would be made in the U.S. in 2023, of which 19,550 would be in people younger than 50. But millennials born around 1990 now have twice the risk of colon cancer compared with people born around the 1950s, while millennials’ risk for rectal cancer is about four times higher than that of older age groups, according to a study published in the Journal of the National Cancer Institute . That means diagnoses are likely to “continue going up as these higher-risk generations age,” Dr. Murphy said.
When cancer is found at a younger-than-usual age, doctors usually suspect that genetic mutations may be to blame. And some molecular studies suggest that tumors in early-onset colorectal cancers do have different mutations driving the cancer compared with tumors in older adults. Another piece of evidence that there is a genetic component: It is clear that having a first-degree relative who had colorectal cancer — or even a precancerous polyp — can increase your risk, Dr. Cecchini said. But genetic changes do not explain the full picture, he said.
Some research has linked lifestyle and dietary changes to increased rates of colorectal cancer in both young people and older adults. Recent generations have consumed more red meat , ultraprocessed foods and sugary beverages , and have been known to binge drink more frequently; between 1992 and 1998, cigarette smoking also increased before declining again, while physical activity has continuously declined for decades. All of these factors — along with the rise in obesity rates since the 1980s — are associated with cancer risk. But once again, none of them fully account for the increase in early-onset colorectal cancer.
“For a lot of these risk factors, like smoking, you have to be exposed for long periods of time before the cancer develops,” said Dr. Andrea Cercek, a co-director of the Center for Young Onset Colorectal and Gastrointestinal Cancers at Memorial Sloan Kettering Cancer Center. And many patients in their 20s and 30s do not even fit in these risk groups, she said. “Many of our patients are athletes,” she said. “Many of them were never heavy, not even in childhood.”
Experts are beginning to investigate if there are other environmental drivers of early-onset cancer. For instance, some small studies have hinted at the idea that people who develop colorectal cancer at an early age have an imbalance of “good” and “bad” bacteria in their gut. Researchers are not only looking at antibiotic use, which can alter the gut microbiome, but also nonsteroidal anti-inflammatory drugs that are used as painkillers, proton pump inhibitors that are used to counter stomach acid issues and several psychiatric medications that may be absorbed through the intestinal lining and have increased in use in recent decades, Dr. Cercek said.
Some experts believe exposure to toxic chemicals in the environment may also be to blame. “There’s patterns of environmental exposures by geography, by race, by sex, by all the things that we know colorectal cancer rates also differ by,” Dr. Murphy said.
For instance, for many years, the rates of colorectal cancer diagnoses were highest among non-Hispanic Black people, but research shows that these cancers increased more among non-Hispanic white people in the 1990s and early 2000s, Dr. Murphy said. Now, both groups have fairly similar rates of cancer. “Does this mean that white people are now being exposed to something that Black people have been exposed to for many, many years? We just don’t know yet,” Dr. Murphy said.
There are also geographic disparities in the increase in cancer, with experts seeing more cases emerge in cities and towns along the Mississippi River, in Southeastern states and in Appalachia, which may be explained by occupational exposures to trace elements like arsenic, chromium, and nickel , which are often used in coal production, chemical plants and other industries in those regions. So-called forever chemicals like per- and polyfluoroalkyl substances, better known as PFAS , have been linked to other cancers and could also be driving some of the increase in early-onset colorectal cancer.
“I don’t think there’s going to be one smoking gun that explains everything,” Dr. Murphy said. “It’s a whole bunch of things.”
What can you do to identify and reduce your risk?
After Ms. Peters was diagnosed and started chemotherapy, radiation, and reconstructive surgery, she encouraged her younger sister and brother to get screened immediately. “Because now they had a family history of the disease,” she said.
The Colon Cancer Coalition has developed a script you can use to bring up colon and rectal cancer questions in conversations with relatives, which may help you determine whether you should be screened 10 to 15 years earlier than the current recommended age.
If you are not in contact with your immediate family or are unaware of their medical history, it is important to know the symptoms of colorectal cancer, such as unexplained abdominal pain, changes in your stool and rectal bleeding. If you have any of these symptoms, talk to a doctor and get tested to rule out cancer.
After her experience being dismissed by doctors, Ms. Peters founded an organization called Be Seen to raise awareness of symptoms and encourage people to pledge to be screened .
Colonoscopies remain the gold standard for screening because they allow medical experts to not only see where tumors are, but also to remove them in the same procedure. There are now several different ways patients can prepare their bowels — including liquid laxatives, pills and powders — that are not as uncomfortable as options that were available to previous generations. “I can promise you that doing a one-day cleanse to prep for a colonoscopy is far better than having poop coming out of your stomach into a bag,” Ms. Peters said. “Thankfully, it was temporary for me, but it’s not for many people.”
There is also a home test that can detect 92 percent of colorectal cancers through DNA in your stool, though it is less sensitive at picking up precancerous polyps and cannot be used to remove any tissue, Dr. Cercek said. A blood test that is on the horizon may further increase the number of people willing to get screened.
Even though the trend in early-onset colorectal cancers is concerning, “what I take away from it is that the time to intervene is even earlier,” Dr. Murphy said. “And certainly what is happening now is going to affect the health of generations many, many years from now.”
Knvul Sheikh is a Times reporter covering chronic and infectious diseases and other aspects of personal health. More about Knvul Sheikh
The Fight Against Cancer
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
Early detection is a powerful weapon in preventing deaths from colon cancer, but many patients are reluctant to undergo colonoscopies or conduct at-home fecal tests. Doctors see potential in another screening method .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot.
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.
No single food can prevent cancer on its own, but experts say that there are some that may help you build the best defense .
The F.D.A. has proposed banning the use of formaldehyde in chemical hair straighteners , which have been linked to increased cancer risk, particularly among Black women .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies
Julita machlowska.
1 Center for Medical Genomics OMICRON, Jagiellonian University Medical College, 31-034 Kraków, Poland; [email protected]
2 Department of Human Anatomy, Medical University of Lublin, 20-090 Lublin, Poland; [email protected] (J.B.); [email protected] (R.M.)
Monika Sitarz
3 Department of Conservative Dentistry with Endodontics, Medical University of Lublin, 20-090 Lublin, Poland; moc.liamg@zratiskm
Ryszard Maciejewski
Robert sitarz.
4 Department of Surgery, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-090 Lublin, Poland
Gastric cancer (GC) is one of the most common malignancies worldwide and it is the fourth leading cause of cancer-related death. GC is a multifactorial disease, where both environmental and genetic factors can have an impact on its occurrence and development. The incidence rate of GC rises progressively with age; the median age at diagnosis is 70 years. However, approximately 10% of gastric carcinomas are detected at the age of 45 or younger. Early-onset gastric cancer is a good model to study genetic alterations related to the carcinogenesis process, as young patients are less exposed to environmental carcinogens. Carcinogenesis is a multistage disease process specified by the progressive development of mutations and epigenetic alterations in the expression of various genes, which are responsible for the occurrence of the disease.
1. Introduction
The cancer development process is caused by both genetic and environmental factor influences. Around 50% of cancer incidents might be provoked by environmental agents, mostly dietary habits and social behavior. The development and progression of tumors is a multiannual and multistage process. Cancer usually occurs after 20–30 years of exposure to damaging carcinogenic agents. The possibilities of modern medicine allow for better recognition of most cancers, in their advanced stages, where among 50% of cases radical resection enables recovery.
Gastric cancer (GC) is a multifactorial disease, where many factors can influence its development, both environmental and genetic [ 1 ]. Current statistics display GC as the fourth leading cause of cancer deaths worldwide, where the rate of median survival is less than 12 months for the advanced stage [ 2 ]. Gastric carcinoma as a malignancy of a high aggressiveness with its heterogenous nature, and still constitutes a global health problem [ 3 ]. That is why alternative prevention, considered as a proper diet, early diagnosis and follow-up proper treatments, leads to the reduction of recorded incidents [ 4 ]. GC is rather rare and is not prevalent in the young population (under 45 years of age), where no more than 10% of patients are suffering from disease development [ 5 , 6 , 7 , 8 , 9 ].
The most popular classification of GC is the Lauren classification. According to this division, two subtypes of GC are displayed: intestinal and diffuse [ 10 ]. They present different characteristics, including clinical features, genetics, morphology, epidemiology and expansion properties. This division also has an impact on surgical decisions, regarding the range of stomach resections. The intestinal subtype encompasses tubular and glandular elements, with multiple degrees of differentiation. The diffuse subtype displays poorly cohesive single cells without gland formation [ 11 , 12 ]. Additionally, GC with signet ring cells is relatively prevalent, being classified as a “diffuse type” according to the Lauren classification [ 10 ]. Currently, signet ring cell carcinoma is described as a weakly cohesive type of cancer, consisting mostly of tumor cells with prominent cytoplasmic mucin and an eccentrically placed crescent-shaped nucleus [ 13 ]. Regarding the age at the diagnosis, GC is divided into early-onset gastric carcinoma (45 years or younger) and conventional GC (older than 45) [ 9 , 14 ].
Describing the pattern of signatures for GC development might be an important approach for better recognition of treatment strategies. To reveal these signatures, it is of great importance to find future appropriate therapies in personalized medicine.
2. Epidemiology and Risk Factors for Gastric Carcinoma Development
2.1. incidence, mortality and geographical variability.
Every year, around 990,000 people are diagnosed with GC worldwide, of whom approximately 738,000 die [ 15 ]. GC is the fourth most common incident cancer and the second most common cause of cancer death [ 16 ].
GC incidence is different concerning sex and geographical variability. Men are two to three times more susceptible than women [ 15 ]. The incidence displays huge geographical diversity. It is noted that more than 50% of new incidents come up in developing countries. Areas with the highest probability for GC development encompass regions like Central and South America, Eastern Europe and East Asia (China and Japan). The low-risk regions include Australia and New Zealand, Southern Asia, North and East Africa and North America [ 17 ]. The five-year survival rate is mildly good only in Japan. In Europe, the ratio fluctuates between 10–30% [ 18 ]. The increased five-year survival rate is probably due to early diagnosis using the endoscopic examination method, which allows for the early detection and resection of cancer.
2.2. Trends
GC incidence rates have decreased in the last few decades in most parts of the world [ 19 ]. The decline in sporadic intestinal types of GC is observed, and the occurrence of the diffuse type GC has increased [ 20 , 21 ]. The rate of proximal GC is higher in comparison to the distal one. This trend might be explained by the increased standards of hygiene, better food conservation, a high intake of fresh fruits and vegetables and Helicobacter pylori eradication [ 22 ].
2.3. Risk Factors
Several factors have been noted to have a significant impact on the increased risk of developing GC, like family history, diet, alcohol consumption, smoking, Helicobacter pylori and Epstein–Barr virus ( EBV ) infections, which are summarized in Figure 1 .

Risk factors for GC development.
A family history of GC is also one of the most crucial risk factors [ 23 ]. However, GCs are mostly sporadic, around 10% display a familial aggregation [ 24 ]. Inherited GCs with a Mendelian inheritance pattern encompass less than 3% of all gastric carcinomas [ 25 ]. Hereditary diffuse gastric cancer (HDGC) is the most recognizable familial GC, which is caused by cadherin 1 gene ( CDH1) alterations. The risk of gastric carcinoma in patients with a family history is around three-fold higher than among individuals without such a history [ 26 ]. The number of available studies on GC incidence and family history is rather low, the family history of individuals undergoing health check-ups has been noted for around 11% [ 27 ]. The ratio of GC with a family history is greater in Asian regions than in Europe and North America, however the frequency of HDGC, in comparison to the incidence of familial gastric carcinogenesis in Asia, is rather low [ 28 ]. Therefore, environmental agents, more than genetic alterations, can affect the development of familial GC in countries with an increased incidence of the disease.
The correlation between dietary factors and the risk of GC development has been broadly studied. The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) summarized that fruit and vegetables are protectors against GC development, whereas broiled and charbroiled animal meats, salt-preserved foods and smoked foods probably enhance GC progression [ 29 ]. Food carcinogens might interact with gastric epithelial cells and provoke changes in genes and their expression. Interestingly, a high intake of sodium chloride was described as devastating the gastric mucosa, promoting cell death and regenerative cell proliferation in animal models [ 30 ]. The dietary or endogenous role of N -nitroso compounds has been displayed to significantly increase gastrointestinal cancer risk, mostly among non-cardia GCs [ 31 ].
Among a variety of habits which play a role in GC development, the impact of smoking and alcohol intake has been considered. Studies show that smokers display around an 80% increase in the risk for GC development among non-drinkers. Additionally, heavy drinkers show a higher risk of GC; in a group of smokers, the risk of GC is estimated to be 80% [ 32 ]. In the European prospective nutrition cohort study, 444 cases of GCs were examined; heavy alcohol intake at the baseline was positively correlated with GC risk, whereas a decreased intake was not [ 33 ]. Intestinal non-cardia carcinoma was accompanied by heavy alcohol consumption. The dependence between alcohol intake and the risk of GC development was studied in a Korean population showing the ALDH2 genotype [ 34 ]. Among a group of patients with ALDH2 *1/*2 carriers, current/ex-drinkers displayed a higher probability for cancer development in comparison to the group of never/rare drinkers. The study showed the association for alcohol consumption and GC development among a group of patients with ALDH2 polymorphisms and the ALDH2 *1/*2 genotype.
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that has been described as a class I carcinogen of GC development by the World Health Organization since 1994 [ 35 ]. The effect of H. pylori on the oncogenesis process has been described by two main mechanisms: an indirect inflammatory reaction to H. pylori infection on the gastric mucosa and a direct epigenetic outcome of H. pylori on gastric epithelial cells [ 36 ]. Several virulence factors of H. pylori , like CagA or VacA, are noted to increase the risk of GC development [ 37 ]. H. pylori with cagA and vacA relate to a higher risk of developing both intense tissue responses and premalignant and malignant lesions in the distal stomach [ 38 ]. Multiple epidemiological studies have shown that H. pylori infection is one of the risk factors of GC development. Besides, H. pylori infection impairs the gastric tissue microenvironment, promoting epithelial–mesenchymal transition (EMT) and further GC progression [ 39 , 40 ].
Apart from H. pylori infection, the second factor associated with GC development is the Epstein–Barr virus ( EBV ). EBV is a ubiquitous infectious factor. The EBV genome subsists in the tumor cells and transforming EBV proteins are expressed among them [ 41 ]. About 10% of GCs have been described to be EBV -positive, but there is not enough evidence for a distinct etiological role of EBV in GC development [ 42 ]. EBV -positive gastric carcinomas differ due to patients’ characteristics, like sex, age or anatomic subsite, and decrease with age among males [ 43 ].
3. Gastric Cancer Classification
3.1. classification systems in gastric cancer.
In 1965 the Lauren classification of GC was established, and nowadays it is the most frequently used, compared to other available GC classifications [ 10 ]. According to the Lauren division, two histological subtypes of GC can be distinguished—intestinal and diffuse; later the indeterminate type was also included to characterize infrequent histology. Signet ring cell carcinoma is assigned to the diffuse subtype. Multiple studies have shown that the intestinal type is the most common, the second is diffuse and ending with the indeterminate type [ 10 ]. Intestinal carcinoma is characterized by visible glands and cohesion between tumor cells. The diffuse subtype encompasses poorly cohesive cells, diffusely infiltrating the gastric wall with little or no gland formation. The cells are usually small and round, also with a signet ring cell formation. There is evidence that the intestinal subtype is associated with intestinal metaplasia of the gastric mucosa and the occurrence of H. pylori infection. Some studies also revealed that the incidence of the diffuse GC subtype is higher among females and younger patients, and that this type of GC originates from the normal gastric mucosa [ 44 ].
The World Health Organization (WHO) classification issued in 2010 is perceived to be the most detailed among all classification systems. The WHO classification, apart from stomach adenocarcinomas, also describes other types of gastric tumors with decreased attendance [ 45 ]. The gastric adenocarcinoma type includes multiple subgroups, like tubular, mucinous, papillary and mixed carcinoma, which are similar to the indeterminate type according to the Lauren classification system. The poorly cohesive carcinoma type contains the signet ring cell carcinoma. The remainder of the classified gastric adenocarcinomas are described as uncommon, mainly because of their low clinical importance. Following the WHO classification, the most common GC subtype is tubular adenocarcinoma, then the papillary and mucinous types. The signet ring cell carcinoma encompasses around 10% of GCs and is described by the occurrence of signet ring cells in over 50% of the tumor [ 44 , 45 , 46 , 47 ].
GC development onsets are present in Figure 2 , where the percentage of each carcinoma is displayed.

Onsets of gastric cancer development.
3.2. Conventional Gastric Cancer
Gastric carcinomas that appear intermittently mostly occur among the older population, at over 45 years of age, and are so-called “conventional gastric cancers”. The genetic factors that cause cancer development are less important in this type of cancer, where environmental agents are prevalent [ 48 ]. Patients are diagnosed between 60 and 80 years of age. These gastric carcinomas affect mostly men, who are two times more likely to develop them than women [ 49 , 50 ].
3.3. Early-Onset Gastric Cancer
Early-onset gastric cancer (EOGC) is described as a GC occurring at the age of 45 years or younger. Around 10% of GCs are categorized as EOGCs, however rates differ between 2.7% and 15%, depending on the performed cohort studies [ 14 ]. In the young population, diffuse lesions are more frequent and they are related to the background of the histologically “normal” gastric mucosa. Young patients are less exposed to environmental carcinogens, therefore, an EOGC is a good model to study genetic alterations in the gastric carcinogenesis process [ 51 ]. H. pylori infection is important for the development of tumors in EOGC patients, however, there is no statistically significant difference in the distribution of IL1 β polymorphisms between young and old patients [ 9 ]. EBV infection is observed to be importantly decreased or absent in EOGCs [ 52 ]. It is postulated that around 10% of EOGCs have a positive family history [ 53 ]. It has been revealed that the early-onset type has different clinicopathological characteristics compared to the conventional subtype, which suggests that they display separate models within gastric carcinogenesis, and molecular patterns support this [ 54 ].
3.4. Gastric Stump Cancer
Gastric stump cancer (GSC) is described as a carcinoma in the gastric remnant after partial gastric resection, usually due to peptic ulcer disease (PUD). The incidence of GSC varies from 1% to 8% [ 55 ]. The major pathogenesis of GSC is biliary pancreatic reflux, provoking chronic inflammation of the mucosa, followed by atrophic gastritis, intestinal metaplasia and dysplasia. Other possible causes are achlorhydria and bacteria overgrowth, and H. pylori seem to be the main agent included in the etiopathogenesis of the GSC [ 56 ]. The surveillance of these patients with endoscopy and biopsies might allow for the early diagnosis of these patients, however, the benefit to cost ratio is still to be considered. Viste et al. (1986), made a comparison of GSC patients with other GC patients and discovered relevant differences in gender, age, staging, resectability rates and operative procedures, however, the postoperative mortality and survival rates were approximate [ 57 ].
3.5. Hereditary Diffuse Gastric Cancer
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant susceptibility for diffuse GC, a weakly differentiated adenocarcinoma that penetrates into the stomach wall leading to the thickening of the wall, usually without producing an explicit mass. The median age of HDGC onset is around 38 years, with a range of 14–69 years [ 58 ]. HDGC should be considered for screening with several important symptoms, like two or more documented cases of diffuse GC in first- or second-degree relatives, with at least one diagnosed before the age of 50, or three or more cases of documented diffuse gastric cancer in first/second-degree relatives, independent of the age of onset [ 59 ]. When clinical characteristics and family history are insufficient, the identification of a heterozygous germline CDH1 pathogenic variant using screening with available genetic tests checks out the diagnosis and enables for family research [ 60 , 61 ].
Among CDH1 mutation-negative patients within HDGC families, there were displayed candidate mutations within genes of high and moderate penetrance, like: BRCA2 , STK11 , ATM , SDHB , PRSS1 , MSR1 , CTNNA1 and PALB2 [ 62 ]. Therefore, in HDGC families, with no detected alterations in the CDH1 gene, the clinical importance of other tumor suppressor genes, like CTNNA1 , should be considered. CTNNA1 is concerned in intercellular adhesion and is a questionable tumor suppressor gene for HDGC. The group discovered a novel variant (N1287fs) in the BRCA2 gene, which is the first report of the occurrence of a truncating BRCA2 variant among HDGC families. That is why it is important to consider HDGC syndrome as associated to CDH1 mutations and closely related genes, then consider the clinical criteria of families with heterogeneous susceptibility profiles.
4. Genomic Characteristics of Gastric Cancer Development
Many studies on the molecular biomarkers of GC have been broadly investigated to reveal the wide spectrum of recognition patterns in this field. The main signatures for GC disease development encompass the modules of HER2 expression, factors that regulate apoptosis, cell cycle regulators, factors that influence cell membrane properties, multidrug resistance proteins and microsatellite instability [ 63 ], which are presented in Table 1 .
Molecular biomarkers in gastric cancer development.
Abbreviations: HER2 —tyrosine kinase-type cell surface receptor, p53 —tumor protein p53, PD-1 —cell surface receptor programmed death-1 and its ligand ( PDL1 ), p73 —tumor protein p73, mdm2 —murine double minute gene 2, Bcl-2 —B-cell lymphoma 2, pRb —retinoblastoma protein, CCND1— cyclin D1 gene, p16 —cyclin dependent kinase inhibitor 2A, p27 Kip1 —cyclin-dependent kinase inhibitor 1B, MUC —mucin, MRP2 —multidrug resistance-associated protein 2, MDR1 —multidrug resistance 1 gene, GST-P —glutathione S-transferases Pi, MSI —microsatellite instability, PIK3CA— phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, EGFR – epidermal growth factor receptor, ERBB3— Erb-B2 receptor tyrosine kinase 3 , ERBB2— Erb-B2 receptor tyrosine kinase 2.
Possible Biomarkers of Gastric Cancer
Carbohydrate antigen 19-9 (CA 19-9) is the serum tumor marker most commonly used in cases of pancreatic cancer diagnosis or therapy monitoring. Physiologically, the serum concentration of CA 19-9 is small (less than 37 U/mL), being overexpressed in inflammatory conditions (e.g., pancreatitis) or other gastrointestinal diseases (esophageal, gastric or biliary cancers) [ 85 ]. The utility of CA 19-9 as a diagnostic biomarker of GC is slightly controversial and the results of the studies usually remain contradictory. Feng et al. reported that increased levels of CA 19-9 are associated with female gender and the presence of lymph node metastasis [ 86 ]. CA 19-9 might be associated with the tumor depth, tumor stage and lymph node metastasis in GC patients [ 87 , 88 ]. Besides, serum CA 19-9 levels are more diagnostically important than CEA regarding the estimation of the tumor size [ 89 ]. Serum levels of CA 19-9 are higher in GC patients compared to those with gastric benign diseases [ 90 ]. Increased CA 19-9 concentrations can also constitute a marker of an early recurrence after curative gastrectomy for GC, as well as of possible peritoneal dissemination [ 91 , 92 ]. Increased serum CA 19-9 and CA 72-4 levels are associated with an increased mortality rate among GC patients [ 93 ]. Song et al. reported that increased CA 19-9 levels are primarily observed in cases of stage III/IV group GC relative to the I/II group [ 94 ]. Usually, single tumor markers are not sufficiently sensitive and specific, therefore, the combined detection of several markers is inherent. In cases of GC, serum CA 19-9, carcinoembryonic antigen (CEA), carbohydrate antigen 72-4 (CA 72-4) and carbohydrate antigen 15-3 (CA 15-3) are important during an early GC diagnosis and therapy monitoring [ 95 , 96 ].
5. Prevention and Treatment Strategies
5.1. prevention strategies for gastric cancer.
The two main primary prevention activities for gastric carcinoma at a population level could encompass a better diet habit and a lowering of the occurrence of H. pylori infection, the major cause of GC. The secondary prevention strategy is early detection using available resources, mainly the endoscopic method, as a gold standard.
5.2. Improvement in Diet
Prevention through dietary intervention might be possible through a higher intake of fresh fruit and vegetables and the restricted consumption of salt and salt-preserved food. Lifestyle modifications, including a higher level of physical activities and smoking limitation, could also reduce the risk of getting the disease. Fruit and vegetables are rich sources of folate, carotenoids, vitamin C and phytochemicals, which might have a protective role in the carcinogenesis process [ 97 ]. In the European Prospective Investigation into Cancer and Nutrition, 330 GC patients, both men and women, were examined [ 98 ]. A preventive role of vegetable consumption was displayed, mostly for the intestinal type of GC. Citrus fruit intake could play a role in protection against gastric cardia cancer. A subsequent report by the International Agency for Research on Cancer (IARC) described that the increased consumption of fruit “probably”, and higher intake of vegetables “possibly”, reduces the risk of GCs [ 99 ].
5.3. Helicobacter pylori Eradication
The prevention of GC development through H. pylori eradication is another approach. The explanation that the bacterium is a disease-causing factor allowed some authors, by 2005, to call for different programs to eradicate the infection among the population, as a way to limit the disease development [ 100 ]. A meta-analysis conducted by Ford et al. (2014) provides limited, moderate-quality proof that H. pylori eradication causes a reduction in the incidence of GC in healthy, asymptomatic, infected Asian individuals, however, these results cannot necessarily be extrapolated to various populations [ 101 ]. In the Shandong Intervention Trial, after two weeks of antibiotic dosing for H. pylori, the prevalence of precancerous gastric lesions decreased, while 7.3 years of oral supplementation with garlic extract, selenium and vitamins C and E did not [ 102 ]. In the prospective trial performed by Choi et al. 2014, the eradication of H. pylori after the endoscopic resection of GCs did not lower the incidence of metachronous gastric carcinoma [ 103 ]. Fukase et al. (2008) checked the prophylactic effect of H. pylori eradication on the development of metachronous gastric carcinoma after the endoscopic resection of early GC [ 104 ]. The study confirmed that the prophylactic eradication of H. pylori after the endoscopic resection of early GC should be used to prevent the development of metachronous gastric carcinoma. Although the randomized trials showed that H. pylori treatment might decrease GC incidence by 30–40%, there are still significant restrictions to the displayed data [ 104 ].
5.4. Early Detection Importance
The early detection of GC requires financial and population support, as well as available health services. Several tests are recommended and were used in various countries for GC screening. In Japan, mass screening for gastric carcinoma with a photofluorography method was started in 1960. Currently, over 6 million people are examined each year. The sensitivity and specificity of photofluorography are 70–90% and 80–90%, respectively. The five-year survival rate is 15–30% better among screen-detected cases than in symptom-diagnosed patients [ 105 ]. Additionally, endoscopic examination for gastric carcinoma has a higher sensitivity than the radiographic method [ 106 ]. The sensitivity of the endoscopic method in the population study was higher or the disclosure of distant or regional GC than for localized GC [ 106 ]. Upper gastrointestinal endoscopy has been established as the gold standard for the diagnosis of gastric carcinoma [ 107 ]. It is also performed for the minimally invasive treatment of early GC by endoscopic mucosal resection and endoscopic submucosal dissection. Matsumoto et al. (2013) performed the evaluation of the efficacy of radiographic and endoscopic examination for GC patients and suggested that both screening methods can allow for the avoidance of gastric carcinoma development [ 108 ]. Hamashima et al. (2013) investigated the evaluation of the reduction of mortality for GC patients by endoscopic examination. The results showed a 30% reduction in GC mortality using endoscopic screening in comparison to a control, the non-examined group, within 36 months before the date of diagnosis of GC [ 109 ].
5.5. Treatment Strategies for Gastric Cancer: Surgical Resection
Surgery plays a crucial role as a strategy in the treatment of GC [ 110 ]. The best time for surgery is when a tumor is mostly sensitive to the chemotherapy. The development of two new methods, endoscopic resection and minimally invasive access, have had an important impact on the treatment strategies revolution in the last few decades [ 111 ] Nevertheless, vertical and horizontal margin invasion and the chance of nodal implication should also to be taken under serious consideration to prevent real oncological lapses. The standard treatments are directed to the endoscopic mucosal resection, or, even better, endoscopic submucosal dissection (ESD) for differentiated types of gastric adenocarcinoma without ulcerative findings [ 112 ]. Both endoscopic mucosal resection and ESD provide favorable long-term outcomes. Laparoscopic surgery of GCs, as a minimally invasive method, was originally limited to treat distal-sided early GCs, with no necessity for complete gastrectomy or extended lymphadenectomy [ 113 ]. Both laparoscopic and robotic-assisted gastrectomies are considered to provide positive clinical outcomes, equivalent to those in cases of open surgeries. Furthermore, compared to open surgeries, minimally invasive techniques have even lower rates of postoperative complications, such as incisional hernias or bowel obstructions [ 114 , 115 , 116 ]. Limited surgical approaches—pylorus-preserving gastrectomy, proximal gastrectomy and local resection—significantly reduce the resection area of the stomach, as well as the extent of nodal dissection [ 117 ]. Conversion therapy in GC is an application of either chemotherapy or radiotherapy followed by surgical treatment in cases of originally unresectable or marginally resectable GCs, the application of which might be of great importance, especially in cases of stage IV GCs. [ 118 ]. Comprehensive surgical resection with lymphadenectomy D2 still constitutes the major treatment strategy aimed at cure for GC. The continuation of chemotherapy is usually crucial after the resection, preventing adverse events. Several reconstruction methods, such as Billroth I gastroduodenostomy, Billroth II gastrojejunostomy, casual/uncut Roux-en-Y gastrojejunostomy and jejunal interposition are often employed after the subtotal gastrectomy [ 119 ].
5.6. Adjuvant Chemotherapy
In the last few decades, multiple phase III trials have been undertaken to consider the potential of adjuvant chemotherapy versus surgery, however, no consistent outcomes have been observed [ 120 , 121 , 122 , 123 ]. The observations might be explicated by several important factors, like the huge heterogeneity of the study cohort, a low number of performed series, various levels of surgical precision and dissimilar chemotherapy regimens. A meta-analysis study, performed by the GASTRIC group in 2010, showed that postoperative adjuvant chemotherapy based on fluorouracil regimens significantly reduces the mortality rate of GC patients in comparison to surgery alone [ 124 ]. Adjuvant chemotherapy was correlated with a statistically important benefit in terms of overall survival and disease-free survival. There was no distinct heterogeneity for overall survival across randomized clinical trials. Five-year overall survival increased from 49.6% to 55.3% with chemotherapy. An application of oral fluoropyrimidine might also be effective in cases of advanced GCs [ 125 , 126 ]. Likewise, other phase III trials, including the CLASSIC or the ACTS-GC, proved that postoperative adjuvant therapy following D2 gastrectomy is a highly effective treatment strategy [ 127 , 128 ]. An activity of pembrolizumab in the neoadjuvant setting provides a rationale for its application in combination with chemotherapy in patients with resectable GCs [ 129 ]. The systematic review and meta-analysis performed by Yan et al. (2007) was undertaken to check the efficiency and safety of adjuvant intraperitoneal chemotherapy for patients with locally advanced resectable GC [ 130 ]. The study displayed that hyperthermic intraoperative intraperitoneal chemotherapy (HIIC), with or without early postoperative intraperitoneal chemotherapy (EPIC) after the resection of advanced gastric primary cancer, is assigned to increase the overall survival rate. Unfortunately, higher risks of intra-abdominal abscess and neutropenia are also displayed. Adjuvant XELOX might be a valid approach in curable gastric carcinomas among Asian patients. Nowadays, it is clear that adjuvant chemotherapy brings a survival benefit in radically resected GC for stage ≥ T2 or N+ [ 131 , 132 ]. Neoadjuvant chemotherapy followed by surgery is also highly recommended in cases of limited metastatic GCs [ 133 ]. What is also crucial while applying neoadjuvant chemotherapy is the genotype of the GC, which might additionally constitute a prognostic or predictive factor of the clinical outcome.
5.7. Neo-Adjuvant Chemotherapy
The importance of neoadjuvant chemotherapy in GC, gastroesophageal junction and lower esophageal adenocarcinoma has been highlighted over the past few decades. In the first Dutch randomized controlled trial of neoadjuvant chemotherapy, patients with proven adenocarcinoma of the stomach were randomized to obtain four series of chemotherapy with 5-fluorouracil, doxorubicin and methotrexate (FAMTX) prior to surgery or to undergo surgery alone. With a median follow-up of 83 months, the median survival after randomization was 18 months in the FAMTX group, versus 30 months in the surgery alone group [ 134 ]. In European regions, perioperative chemotherapy has been advertised based on the MAGIC [ 135 ] and FFCD9703 [ 136 ] randomized trials. In the first trial, Cunningham et al. (2006) investigated tests with epirubicin, cisplatin and infused fluorouracil (ECF) on patients’ survival with incurable locally advanced or metastatic gastric adenocarcinomas. Among a group of patients with operable gastric or lower esophageal adenocarcinomas, a perioperative regimen of ECF caused a lowering in tumor size, stage and importantly benefited progression-free and overall survival [ 135 ]. Boige et al. (2007) used the combination of 5-Fluorouracil (5FU) in a continuous infusion and cisplatin (FP) as one of the important approaches for advanced adenocarcinoma of the stomach and lower esophagus (ASLE). Preoperative chemotherapy using 5-fluorouracil/cisplatin improved the disease-free and overall survival of patients with ASLE [ 136 ]. Radiation therapy uses high-energy rays or particles to kill cancer cells. It is sometimes applied to treat stomach cancer. In the majority of cases, radiation therapy is given with chemotherapy (chemoradiation). Both neo-adjuvant chemoradiation therapy and neo-adjuvant chemotherapy significantly improve the clinical outcomes of patients with resectable GC with a similar efficiency [ 137 ].
5.8. Targeted Therapy
The major therapeutic options, based on the molecular characteristics of the gastric tumor, are ramucirumab and trastuzumab (targeting VEGFR2 and HER2 , respectively) [ 138 ]. Gastric cancer often displays heterogeneity of the HER2 genotype and phenotype, which might be partly accountable for testing inaccuracy. Phase II trials studied trastuzumab plus chemotherapy (cisplatin, capecitabine) versus chemotherapy alone in HER2+ advanced gastric patients and underlined that trastuzumab is the most appropriate therapeutic approach for strongly HER2+ patients [ 139 , 140 ]. Other studies suggested that lapatinib, as a single targeted therapy, is weakly effective against gastric cancer, which might be explained by the contribution of antibody-dependent cell-mediated cytotoxicity (ADCC), which is lacking in the small molecule therapeutic approach [ 141 ]. Pertuzumab is another HER2 monoclonal antibody that interacts with HER 2 heterodimerization with different members of the EGFR family [ 142 ].
The epidermal growth factor receptor (EGFR) is amplified in approximately 5% of gastric cancers, specified by poor prognosis. Experiments have displayed a positive correlation between EGFR overexpression and cetuximab response [ 143 ]. A phase II trial assessing cetuximab plus oxaliplatin/leucovorin/5-fluorouracil displayed a dependence between a higher EGFR copy number and overall survival [ 144 ].
VEGF / VEGFR2 -dependent signaling is significant in tumor angiogenesis. It has been noted that among GC cases, VEGF status and serum levels correlated with advanced stage and poor prognosis [ 145 ]. The role of ramucirumab, a VEGFR-2 mAb, was evaluated in the REGARD study, as a second line therapy after disease progression on a first line chemotherapy regimen, among cases with unresectable, advanced gastroesophageal tumors [ 146 ]. A phase III study (RAINBOW) tested this antibody, in combination with paclitaxel, as a second line treatment among cases with metastatic GC who progressed after a first line chemotherapy [ 147 ]. Overall survival was importantly increased in the paclitaxel plus ramucirumab group in comparison to the placebo.
The fibroblast growth factor 2 receptor tyrosine kinase ( FGFR-2 ) is overexpressed among approximately 10% of gastric tumors and its amplification is related to lymphatic invasion and poor prognosis [ 148 ]. Clinical trials in which patients picked for FGFR2 amplification are treated with inhibitors, such as dovitinib or AZD4547, are ongoing [ 149 ]. The activation of the PI3K / AKT / mTOR pathway is often among GC tumors. A phase III clinical study investigated the mTOR inhibitor (everolimus) in patients with advanced gastric cancer, and the results showed no improvement in the overall survival [ 150 ]. Additionally, a phase II study of MK-2206, an inhibitor of AKT , displayed no positive results [ 151 ].
5.9. Imaging Strategies
Gastric cancer requires multimodal staging approaches, in which computed tomography (CT) is the first staging modality, mostly because of its broad availability and proper accuracy [ 152 ]. This method is very often used to assess local tumor invasion. It allows for poor soft tissue contrast; the intravenous contrast material and exposure to radiation is needed. Computed tomography for overall T-staging displayed a diagnostic accuracy between around 77% and 89% [ 153 ]. CT is frequently applied to image the occurrence of lymph node metastases among GC patients. The sensitivity was assessed as being between 63–92% and the specificity between 50–88%, according to a systematic review covering 10 studies [ 154 ]. The method of choice for M-staging is a CT of the abdomen and pelvis [ 155 ]. The sensitivity for the imaging of M1 disease using CT is approximately between 14–59%, and the specificity is between 93–100% [ 156 ].
Magnetic resonance imaging (MRI) is an auspicious method for depicting various gastric wall layers and the differentiation of tumor tissue from fibrosis [ 157 ]. The accuracy for the proper evaluation of the T-stage is between 64–88% [ 158 ]. MRI in T-staging was compared with CT, and the accuracy was rather higher for MRI, however, this difference was only proven to be statistically significant in two studies: 73% for MRI versus 67% for helical CT [ 159 ] and 81% for MRI versus 73% for spiral CT [ 160 ]. The precision of MRI for the correct distinction between node-negative and node-positive cases with GC varied between 65% and 100%, sensitivities and specificities ranged between 72–100%, 20–100%, 69–100% and 40–100%, respectively [ 161 ]. MRI is broadly applied to the diagnosis of liver metastases, as well as displaying capability for the diagnosis of peritoneal seeding [ 162 ]. The treatment response evaluation and the detection of lymph node metastases could take advantage of imaging biomarkers derived from functional MRI in the future [ 163 ].
Positron emission tomography (PET) imaging is not the best option for the evaluation of the T-stage. The resolution of PET is limited by the volume averaging of the metabolic signal, with prominent uptake averaged across several millimeters [ 164 ]. PET might be a very good method to detect anatomically small and metabolically active focuses of metastatic disease. The comparatively poor spatial resolution of PET causes the decreased productivity of differentiation compartment I and II nodes from the primary tumor itself [ 165 ]. PET is probably the most useful for the detection of distant areas of solid organ metastases. Kinkel et al. (2002) performed a metanalysis and underlined PET as the most sensitive noninvasive imaging strategy in this field [ 166 ]. PET may be a useful tool to prefigure answers to preoperative chemotherapy in GC cases.
6. Conclusions
In this review, we described GC characteristics, considering the epidemiology, risk factors, classification and molecular and genomic markers, as well as treatment strategies. We characterize the incidence of GC, which is variable when taking into account the geographical and sex variability. We displayed that the decline in sporadic intestinal types of GC is present, whereas the diffuse type prevalence is increased, and the proximal GC prevalence is higher than for the distal one. Several risk factors with an important impact on developing GC are mentioned, including family history, diet, alcohol consumption and smoking, as well as Helicobacter pylori and Epstein–Barr virus infection. The two main classifications of GC are described: Lauren, which is the most commonly used, and WHO, which is perceived to be the most detailed among all of the pathohistological classification systems. The signatures, which are described, are based on the current literature and research performed on this topic, which encompass: the module of HER2 expression, factors that regulate apoptosis, cell cycle regulators, factors that influence cell membrane properties, multidrug resistance proteins and microsatellite instability. We highlighted the two main primary prevention strategies for gastric carcinoma, which are better diet habits and a lowering of the occurrence of H. pylori infection, and the secondary prevention approach, which is early detection using the endoscopic method as a gold standard. Different treatment strategies are also displayed, including surgical resection, adjuvant and neo-adjuvant chemotherapy, radiation therapy, hyperthermic intraperitoneal chemotherapy (HIPEC) and pressurized intraperitoneal aerosol chemotherapy (PIPAC).
Abbreviations
Author contributions.
The authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Unlocking mRNA’s cancer-fighting potential
Press contact :.

Previous image Next image
What if training your immune system to attack cancer cells was as easy as training it to fight Covid-19? Many people believe the technology behind some Covid-19 vaccines, messenger RNA, holds great promise for stimulating immune responses to cancer.
But using messenger RNA, or mRNA, to get the immune system to mount a prolonged and aggressive attack on cancer cells — while leaving healthy cells alone — has been a major challenge.
The MIT spinout Strand Therapeutics is attempting to solve that problem with an advanced class of mRNA molecules that are designed to sense what type of cells they encounter in the body and to express therapeutic proteins only once they have entered diseased cells.
“It’s about finding ways to deal with the signal-to-noise ratio, the signal being expression in the target tissue and the noise being expression in the non-target tissue,” Strand CEO Jacob Becraft PhD ’19 explains. “Our technology amplifies the signal to express more proteins for longer while at the same time effectively eliminating the mRNA’s off-target expression.”
Strand is set to begin its first clinical trial in April, which is testing a self-replicating mRNA molecule’s ability to express immune signals directly from a tumor, triggering the immune system to attack and kill the tumor cells directly. It’s also being tested as a possible improvement for existing treatments to a number of solid tumors.
As they work to commercialize its early innovations, Strand’s team is continuing to add capabilities to what it calls its “programmable medicines,” improving mRNA molecules’ ability to sense their environment and generate potent, targeted responses where they’re needed most.
“Self-replicating mRNA was the first thing that we pioneered when we were at MIT and in the first couple years at Strand,” Becraft says. “Now we’ve also moved into approaches like circular mRNAs, which allow each molecule of mRNA to express more of a protein for longer, potentially for weeks at a time. And the bigger our cell-type specific datasets become, the better we are at differentiating cell types, which makes these molecules so targeted we can have a higher level of safety at higher doses and create stronger treatments.”
Making mRNA smarter
Becraft got his first taste of MIT as an undergraduate at the University of Illinois when he secured a summer internship in the lab of MIT Institute Professor Bob Langer.
“That’s where I learned how lab research could be translated into spinout companies,” Becraft recalls.
The experience left enough of an impression on Becraft that he returned to MIT the next fall to earn his PhD, where he worked in the Synthetic Biology Center under professor of bioengineering and electrical engineering and computer science Ron Weiss. During that time, he collaborated with postdoc Tasuku Kitada to create genetic “switches” that could control protein expression in cells.
Becraft and Kitada realized their research could be the foundation of a company around 2017 and started spending time in the Martin Trust Center for MIT Entrepreneurship. They also received support from MIT Sandbox and eventually worked with the Technology Licensing Office to establish Strand’s early intellectual property.
“We started by asking, where is the highest unmet need that also allows us to prove out the thesis of this technology? And where will this approach have therapeutic relevance that is a quantum leap forward from what anyone else is doing?” Becraft says. “The first place we looked was oncology.”
People have been working on cancer immunotherapy, which turns a patient’s immune system against cancer cells, for decades. Scientists in the field have developed drugs that produce some remarkable results in patients with aggressive, late-stage cancers. But most next-generation cancer immunotherapies are based on recombinant (lab-made) proteins that are difficult to deliver to specific targets in the body and don’t remain active for long enough to consistently create a durable response.
More recently, companies like Moderna, whose founders also include MIT alumni , have pioneered the use of mRNAs to create proteins in cells. But to date, those mRNA molecules have not been able to change behavior based on the type of cells they enter, and don’t last for very long in the body.
“If you’re trying to engage the immune system with a tumor cell, the mRNA needs to be expressing from the tumor cell itself, and it needs to be expressing over a long period of time,” Becraft says. “Those challenges are hard to overcome with the first generation of mRNA technologies.”
Strand has developed what it calls the world’s first mRNA programming language that allows the company to specify the tissues its mRNAs express proteins in.
“We built a database that says, ‘Here are all of the different cells that the mRNA could be delivered to, and here are all of their microRNA signatures,’ and then we use computational tools and machine learning to differentiate the cells,” Becraft explains. “For instance, I need to make sure that the messenger RNA turns off when it's in the liver cell, and I need to make sure that it turns on when it's in a tumor cell or a T-cell.”
Strand also uses techniques like mRNA self-replication to create more durable protein expression and immune responses.
“The first versions of mRNA therapeutics, like the Covid-19 vaccines, just recapitulate how our body’s natural mRNAs work,” Becraft explains. “Natural mRNAs last for a few days, maybe less, and they express a single protein. They have no context-dependent actions. That means wherever the mRNA is delivered, it’s only going to express a molecule for a short period of time. That’s perfect for a vaccine, but it’s much more limiting when you want to create a protein that’s actually engaging in a biological process, like activating an immune response against a tumor that could take many days or weeks.”
Technology with broad potential
Strand’s first clinical trial is targeting solid tumors like melanoma and triple-negative breast cancer. The company is also actively developing mRNA therapies that could be used to treat blood cancers.
“We’ll be expanding into new areas as we continue to de-risk the translation of the science and create new technologies,” Becraft says.
Strand plans to partner with large pharmaceutical companies as well as investors to continue developing drugs. Further down the line, the founders believe future versions of its mRNA therapies could be used to treat a broad range of diseases.
“Our thesis is: amplified expression in specific, programmed target cells for long periods of time,” Becraft says. “That approach can be utilized for [immunotherapies like] CAR T-cell therapy, both in oncology and autoimmune conditions. There are also many diseases that require cell-type specific delivery and expression of proteins in treatment, everything from kidney disease to types of liver disease. We can envision our technology being used for all of that.”
Share this news article on:
Related links.
- Strand Therapeutics
- Department of Biological Engineering
Related Topics
- Biological engineering
- Bioengineering and biotechnology
- Drug development
- Tissue engineering
- Synthetic biology
- Innovation and Entrepreneurship (I&E)
Related Articles
Synthetic biology circuits can respond within seconds
MIT launches Center for Multi-Cellular Engineered Living Systems
This RNA-based technique could make gene therapy more effective
Previous item Next item
More MIT News
Does technology help or hurt employment?
Read full story →
Most work is new work, long-term study of U.S. census data shows
A first-ever complete map for elastic strain engineering

Shining a light on oil fields to make them more sustainable

“Life is short, so aim high”

MIT launches Working Group on Generative AI and the Work of the Future
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 23 March 2023
Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention
- Aaron P. Thrift 1 , 2 ,
- Theresa Nguyen Wenker ORCID: orcid.org/0000-0002-4021-9774 3 , 4 &
- Hashem B. El-Serag ORCID: orcid.org/0000-0001-5964-7579 3 , 4
Nature Reviews Clinical Oncology volume 20 , pages 338–349 ( 2023 ) Cite this article
5684 Accesses
67 Citations
16 Altmetric
Metrics details
- Cancer screening
- Epidemiology
- Gastrointestinal diseases
- Risk factors
Gastric cancer remains a major cause of cancer-related mortality worldwide. The temporal trends for this malignancy, however, are dynamic, and reports from the past decade indicate important declines in some regions and demographic groups, as well as a few notable exceptions in which gastric cancer rates are either stable or increasing. Two main anatomical subtypes of gastric cancer exist, non-cardia and cardia, with different temporal trends and risk factors (such as obesity and reflux for cardia gastric cancer and Helicobacter pylori infection for non-cardia gastric cancer). Shifts in the distribution of anatomical locations have been detected in several high-incidence regions. H. pylori is an important aetiological factor for gastric cancer; importantly, the anticipated long-term findings from studies examining the effect of H. pylori eradication on the risk of (re)developing gastric cancer have emerged in the past few years. In this Review, we highlight the latest trends in incidence and mortality using an evidence-based approach. We make the best possible inferences, including clinical and public health inference, on the basis of the quality of the evidence available, and highlight burning questions as well as gaps in knowledge and public health practice that need to be addressed to reduce gastric cancer burden worldwide.
Globally, gastric cancer remains the fifth most common malignant cancer and the fourth leading cause of cancer-related mortality. Despite declining incidence rates, the global burden of this malignancy is expected to have a 62% increase by 2040.
Overall, gastric cancer incidence rates have been decreasing over the past 5 decades in the USA, although the incidence of non-cardia gastric cancer among adults aged <50 years and that of advanced-stage gastric cancer in Hispanic individuals are both increasing.
Worldwide, Helicobacter pylori infection accounts for almost 90% of distal gastric cancers; other well-established risk factors include excess body fat, cigarette smoking and diets high in salt and processed meats.
Other possible risk factors for gastric cancer include Epstein–Barr virus infection, autoimmune gastritis and Ménétrier disease, and possible protective factors include high vegetable intake and treatment with nonsteroidal anti-inflammatory drugs and statins.
A small proportion of all gastric cancers are diagnosed in patients not infected with H. pylori ; other components of the gastric microbiome might have a role in the development of these cancers.
Population-based screening and surveillance programmes and H. pylori eradication hold promise for reducing gastric cancer-related mortality.
The knowledge on risk factors needs to be translated into actionable diagnostic algorithms for public health and clinical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
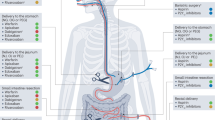
Optimizing antithrombotic therapy in patients with coexisting cardiovascular and gastrointestinal disease
Azita H. Talasaz, Parham Sadeghipour, … Behnood Bikdeli

Is early-onset cancer an emerging global epidemic? Current evidence and future implications
Tomotaka Ugai, Naoko Sasamoto, … Shuji Ogino

Hepatocellular carcinoma
Josep M. Llovet, Robin Kate Kelley, … Richard S. Finn
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 , 209–249 (2021).
Article PubMed Google Scholar
Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 64 , 31–49 (1965).
Article CAS PubMed Google Scholar
Hansford, S. et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 1 , 23–32 (2015).
Correa, P. et al. A model for gastric cancer epidemiology. Lancet 2 , 58–60 (1975).
Correa, P. Gastric cancer: overview. Gastroenterol. Clin. North Am. 42 , 211–217 (2013).
Article PubMed PubMed Central Google Scholar
Fock, K. M. Review article: the epidemiology and prevention of gastric cancer. Aliment. Pharmacol. Ther. 40 , 250–260 (2014).
Arnold, M. et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 69 , 823–829 (2020).
Lin, Y. et al. Global patterns and trends in gastric cancer incidence rates (1988–2012) and predictions to 2030. Gastroenterology 161 , 116–127.e8 (2021).
Morgan, E. et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine 47 , 101404 (2022).
Hooi, J. K. Y. et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153 , 420–429 (2017).
Ferlay J, et al. Global Cancer Observatory: Cancer Today (International Agency for Research on Cancer, 2020).
Thrift, A. P. & El-Serag, H. B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 18 , 534–542 (2020).
Wang, Z. et al. Incidence of gastric cancer in the USA during 1999 to 2013: a 50-state analysis. Int. J. Epidemiol. 47 , 966–975 (2018).
Thrift, A. P. & Nguyen, T. H. Gastric cancer epidemiology. Gastrointest. Endosc. Clin. N. Am. 31 , 425–439 (2021).
Anderson, W. F. et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 303 , 1723–1728 (2010).
Article CAS PubMed PubMed Central Google Scholar
SEER. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database: Incidence – SEER Research Data, 8 Registries, Nov 2021 Sub (1975–2019) – Linked To County Attributes – Time Dependent (1990–2019) Income/Rurality, 1969–2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission (2022).
Wang, Z., El-Serag, H. B. & Thrift, A. P. Increasing incidence of advanced non-cardia gastric cancers among younger Hispanics in the USA. Dig. Dis. Sci. 66 , 1669–1672 (2021).
SEER. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database: Incidence - SEER Research Data, 17 Registries, Nov 2021 Sub (2000–2019) – Linked To County Attributes – Time Dependent (1990–2019) Income/Rurality, 1969–2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission (2022).
IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Schistosomes, Liver Flukes and Helicobacter pylori (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans vol. 61) (IARC, 1994).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans vol. 100B) (IARC, 2012).
Gonzalez, C. A. et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann. Oncol. 23 , 1320–1324 (2012).
Lochhead, P. & El-Omar, E. M. Helicobacter pylori infection and gastric cancer. Best Pract. Res. Clin. Gastroenterol. 21 , 281–297 (2007).
Plummer, M. et al. Global burden of gastric cancer attributable to Helicobacter pylori . Int. J. Cancer 136 , 487–490 (2015).
Parsonnet, J. et al. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J. Natl. Cancer Inst. 83 , 640–643 (1991).
Hansson, L. R. et al. Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology 109 , 885–888 (1995).
Zamani, M. et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 47 , 868–876 (2018).
Peleteiro, B. et al. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig. Dis. Sci. 59 , 1698–1709 (2014).
Lu, Y. et al. Prevalence of Helicobacter pylori in non-cardia gastric cancer in China: a systematic review and meta-analysis. Front. Oncol. 12 , 850389 (2022).
Ricci, C., Holton, J. & Vaira, D. Diagnosis of Helicobacter pylori : invasive and non-invasive tests. Best Pract. Res. Clin. Gastroenterol. 21 , 299–313 (2007).
McNulty, C. et al. Test and treat for dyspepsia–but which test? BMJ 330 , 105–106 (2005).
Vaira, D. & Vakil, N. Blood, urine, stool, breath, money, and Helicobacter pylori . Gut 48 , 287–289 (2001).
Simán, J. H. et al. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand. J. Gastroenterol. 42 , 933–940 (2007).
Simán, J. H. et al. Association between Helicobacter pylori and gastric carcinoma in the city of Malmö, Sweden. A prospective study. Scand. J. Gastroenterol. 32 , 1215–1221 (1997).
Mitchell, H. et al. Immunoblotting using multiple antigens is essential to demonstrate the true risk of Helicobacter pylori infection for gastric cancer. Aliment. Pharmacol. Ther. 28 , 903–910 (2008).
CAS PubMed Google Scholar
Morais, S. et al. “True” Helicobacter pylori infection and non-cardia gastric cancer: a pooled analysis within the Stomach cancer Pooling (StoP) Project. Helicobacter 27 , e12883 (2022).
Pormohammad, A. et al. Global estimate of gastric cancer in Helicobacter pylori -infected population: a systematic review and meta-analysis. J. Cell Physiol. 234 , 1208–1218 (2019).
Testoni, P. A. et al. Gastric cancer in chronic atrophic gastritis. Associated gastric ulcer adds no further risk. J. Clin. Gastroenterol. 9 , 298–302 (1987).
Uemura, N. et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345 , 784–789 (2001).
Suzuki, G. et al. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori . Cancer Epidemiol. Biomark. Prev. 16 , 1224–1228 (2007).
Article CAS Google Scholar
Higashi, H. et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 99 , 14428–14433 (2002).
Hayashi, T. et al. Differential mechanisms for SHP2 binding and activation are exploited by geographically distinct Helicobacter pylori CagA oncoproteins. Cell Rep. 20 , 2876–2890 (2017).
Tatemichi, M. et al. Ethnic difference in serology of Helicobacter pylori CagA between Japanese and non-Japanese Brazilians for non-cardia gastric cancer. Cancer Sci. 94 , 64–69 (2003).
El Hafa, F. et al. Association between Helicobacter pylori antibodies determined by multiplex serology and gastric cancer risk: a meta-analysis. Helicobacter 27 , e12881 (2022).
Kpoghomou, M. A. et al. Association of Helicobacter pylori babA2 gene and gastric cancer risk: a meta-analysis. BMC Cancer 20 , 465 (2020).
Ma, J. et al. Associations between cytokine gene polymorphisms and susceptibility to Helicobacter pylori infection and Helicobacter pylori related gastric cancer, peptic ulcer disease: a meta-analysis. PLoS ONE 12 , e0176463 (2017).
van der Kaaij, R. T. et al. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur. J. Cancer 130 , 23–31 (2020).
Wachtel, M. S. et al. Different regression equations relate age to the incidence of Lauren types 1 and 2 stomach cancer in the SEER database: these equations are unaffected by sex or race. BMC Cancer 6 , 65 (2006).
Yao, Q., Qi, X. & Xie, S. H. Sex difference in the incidence of cardia and non-cardia gastric cancer in the United States, 1992–2014. BMC Gastroenterol. 20 , 418 (2020).
Karimi, P. et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 23 , 700–713 (2014).
Article Google Scholar
IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Tobacco Smoke and Involuntary Smoking (IARC Monographs on the Evaluation of Carcinogenic Risk to Humans vol. 83) (IARC, 2004).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans vol. 94) (IARC, 2010).
Ladeiras-Lopes, R. et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 19 , 689–701 (2008).
Praud, D. et al. Cigarette smoking and gastric cancer in the Stomach cancer Pooling (StoP) Project. Eur. J. Cancer Prev. 27 , 124–133 (2018).
Sasazuki, S., Sasaki, S. & Tsugane, S. Cigarette smoking, alcohol consumption and subsequent gastric cancer risk by subsite and histologic type. Int. J. Cancer 101 , 560–566 (2002).
Pelucchi, C. et al. The Stomach cancer Pooling (StoP) Project: study design and presentation. Eur. J. Cancer Prev. 24 , 16–23 (2015).
Li, W. Y. et al. Smoking status and subsequent gastric cancer risk in men compared with women: a meta-analysis of prospective observational studies. BMC Cancer 19 , 377 (2019).
Sadjadi, A. et al. Neglected role of hookah and opium in gastric carcinogenesis: a cohort study on risk factors and attributable fractions. Int. J. Cancer 134 , 181–188 (2014).
Chen, Y. et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol. Biomark. Prev. 22 , 1395–1408 (2013).
Yang, P. et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur. J. Cancer 45 , 2867–2873 (2009).
Bae, J. M. Body mass index and risk of gastric cancer in Asian adults: a meta-epidemiological meta-analysis of population-based cohort studies. Cancer Res. Treat. 52 , 369–373 (2020).
Jang, J. et al. Association between body mass index and risk of gastric cancer by anatomical and histological subtypes in over 500,000 East and Southeast Asian cohort participants. Cancer Epidemiol. Biomark. Prev. 31 , 1727–1734 (2022).
Zheng, J. et al. Haemoglobin A1c and serum glucose levels and risk of gastric cancer: a systematic review and meta-analysis. Br. J. Cancer 126 , 1100–1107 (2022).
Dabo, B. et al. The association between diabetes and gastric cancer: results from the Stomach cancer Pooling Project consortium. Eur. J. Cancer Prev. 31 , 260–269 (2022).
Zhao, Z., Yin, Z. & Zhao, Q. Red and processed meat consumption and gastric cancer risk: a systematic review and meta-analysis. Oncotarget 8 , 30563–30575 (2017).
Kim, S. R. et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose - response meta-analysis. Nutrients 11 , 826 (2019).
Harrison, L. E. et al. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case–control study in the U.S. Cancer 80 , 1021–1028 (1997).
Tricker, A. R. & Preussmann, R. Carcinogenic N -nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 259 , 277–289 (1991).
Song, P., Wu, L. & Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7 , 9872–9895 (2015).
Fox, J. G. et al. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 59 , 4823–4828 (1999).
D’Elia, L. et al. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin. Nutr. 31 , 489–498 (2012).
Peleteiro, B. et al. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br. J. Cancer 104 , 198–207 (2011).
Firestone, M. J. et al. Asian American dietary sources of sodium and salt behaviors compared with other racial/ethnic groups, NHANES, 2011–2012. Ethn. Dis. 27 , 241–248 (2017).
Morais, S. et al. Salt intake and gastric cancer: a pooled analysis within the Stomach cancer Pooling (StoP) Project. Cancer Causes Control 33 , 779–791 (2022).
Toyoda, T. et al. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol. Histopathol. 23 , 593–599 (2008).
Loh, J. T., Torres, V. J. & Cover, T. L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 67 , 4709–4715 (2007).
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective (AICR, 2007).
Fang, X. et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Cancer 51 , 2820–2832 (2015).
Kobayashi, M. et al. Vegetables, fruit and risk of gastric cancer in Japan: a 10-year follow-up of the JPHC Study Cohort I. Int. J. Cancer 102 , 39–44 (2002).
Rota, M. et al. Education and gastric cancer risk-an individual participant data meta-analysis in the StoP Pproject consortium. Int. J. Cancer 146 , 671–681 (2020).
Alicandro, G. et al. The mediating role of combined lifestyle factors on the relationship between education and gastric cancer in the Stomach cancer Pooling (StoP) Project. Br. J. Cancer 146 , 855–681 (2022).
Miao, P. & Guan, L. Association of dietary cholesterol intake with risk of gastric cancer: a systematic review and meta-analysis of observational studies. Front. Nutr. 8 , 722450 (2021).
Guo, Y. et al. Dairy consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Nutr. Cancer 67 , 555–568 (2015).
Martimianaki, G. et al. Tea consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling (StoP) Project consortium. Br. J. Cancer 127 , 726–734 (2022).
Lazarević, K., Nagorni, A. & Jeremić, M. Carbohydrate intake, glycemic index, glycemic load and risk of gastric cancer. Cent. Eur. J. Public. Health 17 , 75–78 (2009).
Du, Y. et al. Chili consumption and risk of gastric cancer: a meta-analysis. Nutr. Cancer 73 , 45–54 (2021).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans vol. 100E) (IARC, 2012).
Na, H.-K. & Lee, J. Y. Molecular basis of alcohol-related gastric and colon cancer. Int. J. Mol. Sci. 18 , 1116 (2017).
Jelski, W. et al. Alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the sera of patients with gastric cancer. Dig. Dis. Sci. 53 , 2101–2105 (2008).
Deng, W. et al. Alcohol consumption and risk of stomach cancer: a meta-analysis. Chem. Biol. Interact. 336 , 109365 (2021).
Wang, P. L. et al. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget 8 , 99013–99023 (2017).
Rota, M. et al. Alcohol consumption and gastric cancer risk — a pooled analysis within the StoP project consortium. Int. J. Cancer 141 , 1950–1962 (2017).
Lim, H. Y. et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin. Cancer Res. 6 , 519–525 (2000).
Oba, M. et al. Chemoprevention of glandular stomach carcinogenesis through duodenogastric reflux in rats by a COX-2 inhibitor. Int. J. Cancer 123 , 1491–1498 (2008).
Huang, X. Z. et al. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: a dose-response meta-analysis. Oncotarget 8 , 4781–4795 (2017).
Epplein, M. et al. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am. J. Epidemiol. 170 , 507–514 (2009).
Follet, J. et al. The association of statins and taxanes: an efficient combination trigger of cancer cell apoptosis. Br. J. Cancer 106 , 685–692 (2012).
Singh, P. P. & Singh, S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann. Oncol. 24 , 1721–1730 (2013).
Wu, X. D. et al. Statins are associated with reduced risk of gastric cancer: a meta-analysis. Eur. J. Clin. Pharmacol. 69 , 1855–1860 (2013).
MacArthur, T. A. et al. Association of common medications and the risk of early-onset gastric cancer: a population-based matched study. J. Cancer Epidemiol. 2021 , 2670502 (2021).
Segna, D. et al. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Ther. Adv. Gastroenterol. 14 , 17562848211051463 (2021).
Gonzalez, C. A. & Agudo, A. Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int. J. Cancer 130 , 745–753 (2012).
Gullo, I., van der Post, R. S. & Carneiro, F. Recent advances in the pathology of heritable gastric cancer syndromes. Histopathology 78 , 125–147 (2021).
Blair, V. R. et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 21 , e386–e397 (2020).
Li, J. et al. Point mutations in exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am. J. Hum. Genet. 98 , 830–842 (2016).
Vogelaar, I. P. et al. Gastric cancer in three relatives of a patient with a biallelic IL12RB1 mutation. Fam. Cancer 14 , 89–94 (2015).
Capelle, L. G. et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology 138 , 487–492 (2010).
Oliveira, C., Seruca, R. & Carneiro, F. Genetics, pathology, and clinics of familial gastric cancer. Int. J. Surg. Pathol. 14 , 21–33 (2006).
Camargo, M. C. et al. Determinants of Epstein–Barr virus-positive gastric cancer: an international pooled analysis. Br. J. Cancer 105 , 38–43 (2011).
Bizzaro, N. & Antico, A. Diagnosis and classification of pernicious anemia. Autoimmun. Rev. 13 , 565–568 (2014).
Weis, V. G. & Goldenring, J. R. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 12 , 189–197 (2009).
Zamcheck, N. et al. Occurrence of gastric cancer among patients with pernicious anemia at the Boston City Hospital. N. Engl. J. Med. 252 , 1103–1110 (1955).
Li, D. et al. Risks and predictors of gastric adenocarcinoma in patients with gastric intestinal metaplasia and dysplasia: a population-based study. Am. J. Gastroenterol. 111 , 1104–1113 (2016).
Lahner, E. et al. Gender–sex differences in autoimmune atrophic gastritis. Transl. Res. 248 , 1–10 (2022).
Landgren, A. M. et al. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer 117 , 1163–1171 (2011).
Song, M. et al. Autoimmune diseases and gastric cancer risk: a systematic review and meta-analysis. Cancer Res. Treat. 51 , 841–850 (2019).
Wolfsen, H. C., Carpenter, H. A. & Talley, N. J. Menetrier’s disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology 104 , 1310–1319 (1993).
Megged, O. & Schlesinger, Y. Cytomegalovirus-associated protein-losing gastropathy in childhood. Eur. J. Pediatr. 167 , 1217–1220 (2008).
Badov, D. et al. Helicobacter pylori as a pathogenic factor in Ménétrier’s disease. Am. J. Gastroenterol. 93 , 1976–1979 (1998).
Madsen, L. G. et al. Ménétrier’s disease and Helicobacter pylori : normalization of gastrointestinal protein loss after eradication therapy. Dig. Dis. Sci. 44 , 2307–2312 (1999).
Remes-Troche, J. M. et al. Early gastric cancer in Menetrier’s disease. BMJ Case Rep. 2009 , bcr07.2008.0453 (2009).
Kim, J. et al. Menetrier’s disease in Korea: report of two cases and review of cases in a gastric cancer prevalent region. Yonsei Med. J. 45 , 555–560 (2004).
Kim, H. J. et al. Comparison between resectable Helicobacter pylori -negative and -positive gastric cancers. Gut Liver 10 , 212–219 (2016).
Nguyen, T. H. et al. Prevalence of Helicobacter pylori positive non-cardia gastric adenocarcinoma is low and decreasing in a US population. Dig. Dis. Sci. 65 , 2403–2411 (2020).
Matsuo, T. et al. Low prevalence of Helicobacter pylori -negative gastric cancer among Japanese. Helicobacter 16 , 415–419 (2011).
Kageyama, S. et al. Characteristics of the salivary microbiota in patients with various digestive tract cancers. Front. Microbiol. 10 , 1780 (2019).
Sjöstedt, S. et al. Microbial colonization of tumors in relation to the upper gastrointestinal tract in patients with gastric carcinoma. Ann. Surg. 207 , 341–346 (1988).
Engstrand, L. & Graham, D. Y. Microbiome and gastric cancer. Dig. Dis. Sci. 65 , 865–873 (2020).
Yamamoto, Y. et al. Helicobacter pylori -negative gastric cancer: characteristics and endoscopic findings. Dig. Endosc. 27 , 551–561 (2015).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513 , 202–209 (2014).
Soerjomataram, I. et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380 , 1840–1850 (2012).
Cai, Q. et al. Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut 68 , 1576–1587 (2019).
Thrift, A. P., Kanwal, F. & El-Serag, H. B. Prediction models for gastrointestinal and liver diseases: too many developed, too few validated. Clin. Gastroenterol. Hepatol. 14 , 1678–1680 (2016).
Rokkas, T. & Graham, D. Y. How widespread and convenient H. pylori susceptibility testing will result in pharmacological opportunities. Expert Rev. Gastroenterol. Hepatol. 17 , 1–7 (2023).
Ford, A. C., Yuan, Y. & Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 69 , 2113–2121 (2020).
Chiang, T. H. et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 70 , 243–250 (2021).
Piazuelo, M. B. et al. The Colombian Chemoprevention Trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology 160 , 1106–1117.e3 (2021).
Kumar, S. et al. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology 158 , 527–536.e7 (2020).
Doorakkers, E. et al. Eradication of Helicobacter pylori and gastric cancer: a systematic review and meta-analysis of cohort studies. J. Natl. Cancer Inst. 108 , djw132 (2016).
Pan, K. F. et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 65 , 9–18 (2016).
Khan, M. Y. et al. Effectiveness of Helicobacter pylori eradication in preventing metachronous gastric cancer and preneoplastic lesions. A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 32 , 686–694 (2020).
PubMed Google Scholar
Zeng, M. et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386 , 1457–1464 (2015).
IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer (IARC Working Group Reports, No. 8) (IARC, 2014).
Areia, M. et al. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter 18 , 325–337 (2013).
El-Serag, H. B. et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin. Gastroenterol. Hepatol. 16 , 992–1002 (2018).
Sugano, K. Screening of gastric cancer in Asia. Best. Pract. Res. Clin. Gastroenterol. 29 , 895–905 (2015).
Choi, K. S. et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS ONE 7 , e50041 (2012).
Yeh, J. M. et al. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut 65 , 563–574 (2016).
Gupta, N. et al. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest. Endosc. 74 , 610–624.e2 (2011).
Saumoy, M. et al. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology 155 , 648–660 (2018).
Tashiro, A. et al. Comparing mass screening techniques for gastric cancer in Japan. World J. Gastroenterol. 12 , 4873–4874 (2006).
PubMed PubMed Central Google Scholar
Choi, K. S. & Suh, M. Screening for gastric cancer: the usefulness of endoscopy. Clin. Endosc. 47 , 490–496 (2014).
Asaka, M. A new approach for elimination of gastric cancer deaths in Japan. Int. J. Cancer 132 , 1272–1276 (2013).
Asaka, M., Kato, M. & Sakamoto, N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J. Gastroenterol. 49 , 1–8 (2014).
World Health Organization. International Classification of Diseases for Oncology (ICD-O), 3rd edn, 1st revision (WHO, 2013).
Download references
Author information
Authors and affiliations.
Section of Epidemiology and Population Sciences, Department of Medicine, Baylor College of Medicine, Houston, TX, USA
Aaron P. Thrift
Dan L Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, TX, USA
Section of Gastroenterology and Hepatology, Department of Medicine, Baylor College of Medicine, Houston, TX, USA
Theresa Nguyen Wenker & Hashem B. El-Serag
Center for Innovations in Quality, Effectiveness and Safety (IQuESt), Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of article preparation.
Corresponding author
Correspondence to Hashem B. El-Serag .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Clinical Oncology thanks J. Bornschein, M. Leja, P. Malfertheine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
SEER: www.seer.cancer.gov
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Thrift, A.P., Wenker, T.N. & El-Serag, H.B. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 20 , 338–349 (2023). https://doi.org/10.1038/s41571-023-00747-0
Download citation
Accepted : 21 February 2023
Published : 23 March 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41571-023-00747-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Plasma metabolites and risk of seven cancers: a two-sample mendelian randomization study among european descendants.
- Yaohua Yang
BMC Medicine (2024)
LncRNA WFDC21P interacts with SEC63 to promote gastric cancer malignant behaviors by regulating calcium homeostasis signaling pathway
- Jinyao Dong
- Yongqiang Lv
- Yanyan Zhang
Cancer Cell International (2024)
Spatial and single-cell analyses uncover links between ALKBH1 and tumor-associated macrophages in gastric cancer
- Renin Chang
- Kuan-Hao Tsui
- Chia-Jung Li
Noxa inhibits oncogenesis through ZNF519 in gastric cancer and is suppressed by hsa-miR-200b-3p
Scientific Reports (2024)
HOOK3 suppresses proliferation and metastasis in gastric cancer via the SP1/VEGFA axis
- Weichang Chen
Cell Death Discovery (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

COMMENTS
Background. Gastric cancer is a deadly disease with poor overall survival statistics throughout the world. The majority of new diagnoses per year of gastric cancer occur mainly in Asian and South American countries ().Within the United States, there are a projected 27,000 new cases to be diagnosed in 2020 ().It is only recently that researchers started to understand how heterogenous gastric ...
Find research articles on stomach cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies. ... For some people with advanced stomach cancer, the drug nivolumab (Opdivo) plus chemotherapy may improve how long they live, results from a large clinical trial show. ...
Stomach cancer, also known as gastric cancer, is the fifth most frequent type of cancer and the third-leading cause of cancer-related death worldwide, responsible for over 1,000,000 new cases and an estimated 783,000 deaths in 2018 . Many factors may play a role in the development of stomach cancer.
Gastric stump cancer. Gastric stump cancer (GSC) is a separate subtype of GC, defined as a carcinoma that occurs in the gastric remnant at least 5 years after the surgery for peptic ulcer. 64 GSC represents from 1.1% to 7% of all GCs (Figure 1), and males are more prone to them than woman. 55, 65, 66 Gastrectomy is a well-established risk factor for GSC, even long time after the initial ...
Stomach cancer is a common malignant tumor that poses a serious disease burden worldwide 1.As the fourth leading cause of cancer-related death and the fifth most commonly diagnosed cancer in the ...
Gastric cancer articles from across Nature Portfolio. Gastric cancer, or stomach cancer, is a type of cancer that begins in the mucus-producing cells on the inside lining of the stomach. The most ...
Gastric cancer represents a global health-care challenge. With an estimated 1,089,103 new cases and 768,793 deaths from gastric cancer in 2020, it is the fifth most common cancer and the fourth ...
Introduction. Although gastric cancer is not in the top 10 malignancies ranked by either incidence or mortality in the United States, it does represent the second most common cause of cancer death worldwide. 1, 2 Therefore, the advances we make in gastric cancer treatment, even in low-incidence countries, can have global implications. Caution must be exercised, however, in applying findings ...
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become ...
Groundbreaking research offers early clues to stomach cancer development Date: December 11, 2023 Source: Duke-NUS Medical School Summary: Scientists have decoded critical genetic factors in ...
Gastric cancer is the fifth most common cancer and the third most common cause of cancer death globally. Risk factors for the condition include Helicobacter pylori infection, age, high salt intake, and diets low in fruit and vegetables. Gastric cancer is diagnosed histologically after endoscopic biopsy and staged using CT, endoscopic ultrasound, PET, and laparoscopy. It is a molecularly and ...
Newer chemotherapy treatments. Chemotherapy with multiple combinations of drugs is being increasingly used for people with stomach cancer. Drug combinations work slightly better than single drugs. As outlined in the Types of Treatment section, drugs such as 5-FU, paclitaxel, docetaxel, irinotecan, oxaliplatin, as well as oral medications such ...
Randomized controlled trial comparing the costs of gastric cancer screening systems between serological risk-based upper gastrointestinal endoscopy and the existing barium photofluorography: gastric cancer screening labeled by serum examination in place of aged gastric cancer organized screening systems (GALAPAGOS study) Takuji Gotoda.
2. Epidemiology and Risk Factors. Gastric Cancer is the 5th most common and the 3rd deadliest cancer worldwide, according to the latest report of the International Agency for research on Cancer [].Its geographic incidence remains heterogenous with most cases occurring in Eastern Asia (619,226 in 2018), and men being twice as much affected as women.
Key Points. Question Is anti-Helicobacter pylori treatment associated with enhanced postgastrectomy survival prospects for patients diagnosed with gastric cancer who have a preoperative confirmation of H pylori infection?. Findings In this cohort study of 1293 patients, the anti-H pylori treatment group had a significant survival advantage compared with the non-anti-H pylori treatment ...
Gastric cancer remains a major unmet clinical problem with over 1 million new cases worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. A major fraction of gastric cancer has been linked to variety of pathogenic infections including but not limited to Helicobacter pylori (H. pylori) or Epstein Barr virus (EBV). Strategies ...
1 INTRODUCTION. Similar to various other solid tumors, human gastric tumorigenesis is a complex and multifactorial process, regulated by the tumor microenvironment (TME). 1, 2 The TME is an intricate system involving various cell types, such as tumor cells, immune cells, fibroblasts, and vascular endothelial cells. Interactions among these components play a crucial role in tumor initiation ...
Gastric and gastro-oesophageal cancer is a leading cause of cancer-related death worldwide with a poor prognosis. This Review provides a comprehensive overview of current treatment strategies set ...
NCAPD3 loss can directly inhibit CCND1 and ESR1 expression to downregulate the expression of downstream targets CDK6 and IRS1 and inhibit the proliferation of gastric cancer cells. Moreover, NCAPD3 loss activates IRF7 and DDIT3 to regulate apoptosis in gastric cancer cells.Our study revealed that NCAPD3 silencing attenuates malignant phenotypes ...
Examples include having an infection in the stomach, having long-standing acid reflux and eating a lot of salty foods. Not everyone with these risk factors gets stomach cancer, though. So more research is needed to find out exactly what causes it. Stomach cancer begins when something hurts cells in the inner lining of the stomach.
Dr. Yoon, a robotic surgery specialist noted for his treatment of stomach cancers, has helped bring in nearly $5 million in federal research money over his career.. The latest retractions from his ...
The incidence of gastric cancer is declining in the U.S. but it remains the fifth most common type of cancer globally (per World Cancer Research Fund International). Being over 60 years of age ...
Abdominal cancers are on the rise in the under-45s, doctors say Credit: Sebastian Kaulitzki/Science Photo Library. Leading doctors have warned of a mysterious new "epidemic" of abdominal ...
Objective To examine and interpret trends in UK cancer incidence and mortality for all cancers combined and for the most common cancer sites in adults aged 35-69 years. Design Retrospective secondary data analysis. Data sources Cancer registration data, cancer mortality and national population data from the Office for National Statistics, Public Health Wales, Public Health Scotland, Northern ...
Catherine, Princess of Wales visiting Northern Ireland in October. Her royal schedule has been suspended while she receives cancer treatment. Reuters. Gina Kolata previously reported on King ...
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why. Marisa Peters had been experiencing symptoms for years: blood on her toilet paper after ...
The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) summarized that fruit and vegetables are protectors against GC development, whereas broiled and charbroiled animal meats, salt-preserved foods and smoked foods probably enhance GC progression . Food carcinogens might interact with gastric epithelial cells and ...
Technology with broad potential. Strand's first clinical trial is targeting solid tumors like melanoma and triple-negative breast cancer. The company is also actively developing mRNA therapies that could be used to treat blood cancers. "We'll be expanding into new areas as we continue to de-risk the translation of the science and create ...
Gastric cancer remains a major cause of cancer-related mortality worldwide. The temporal trends for this malignancy, however, are dynamic, and reports from the past decade indicate important ...