- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
Nih research matters.
February 3, 2014
Diet Beverages and Body Weight

Overweight and obese adults who drink diet beverages consume significantly more solid-food calories—particularly from snacks—than those who drink sugary beverages. The findings highlight the challenges in using diet beverages to help control weight.
Excess weight can raise your risk for type 2 and gestational diabetes, heart disease, cancer, and other health problems. But maintaining a healthy weight is difficult for many people. Body weight reflects a complicated balance between the amount of calories consumed and the amount of energy used by the body.
Diet beverage use has skyrocketed in recent decades. It’s now a common weight control strategy. It might make sense to think that diet beverages would help you lose weight due to their lack of calories. But the body’s mechanisms for maintaining weight are subtle and complex. Studies into how diet beverages affect weight control have found conflicting results.
To gain a better understanding of the relationship between diet beverage consumption and caloric intake, a research team led by Dr. Sara N. Bleich at the Johns Hopkins Bloomberg School of Public Health examined patterns of food and beverage consumption. They used data collected between 1999 and 2010 in the National Health and Nutrition Examination Survey (NHANES), a periodic survey of the health and habits of the U.S. population by the Centers for Disease Control and Prevention. The analysis was funded by NIH’s National Heart, Lung, and Blood Institute (NHLBI).
The researchers studied almost 24,000 adults, age 20 and older, who reported all the food and beverages they had consumed in a previous 24-hour period. Results appeared online in the American Journal of Public Health on January 16, 2014.
The team found that 11% of healthy-weight, 19% of overweight, and 22% of obese adults drank diet beverages. Diet drinks appeared to help healthy-weight adults maintain their weight. These adults consumed less food and significantly fewer total calories on a typical day than did healthy-weight adults who drank sugared drinks.
The total calories consumed by overweight and obese adults who drank diet beverages, however, were similar to that of those who drank sugary beverages. Heavier adults who drank diet beverages tended to eat more calories in the form of solid food. Overweight and obese adults who drank diet beverages consumed 88 and 194 more calories from solid foods per day, respectively, than those who drank sugared beverages.
To understand these differences in solid-food intake, the scientists took a closer look at patterns of solid-food consumption. Notably, obese adults who consumed diet drinks ate significantly more snacks than those who had sugared drinks. Those who drank diet beverages consumed 131 calories per day in salty snacks and 243 in sweet snacks, compared to 107 and 213, respectively, for obese adults who drank sugared drinks.
“The results of our study suggest that overweight and obese adults looking to lose or maintain their weight—who have already made the switch from sugary to diet beverages—may need to look carefully at other components of their solid-food diet, particularly sweet snacks, to potentially identify areas for modification,” Bleich says.
Controlled studies would be needed to confirm these results. Nevertheless, the research highlights the need for heavier adults who drink diet beverages to closely monitor their food intake.
—by Harrison Wein, Ph.D.
Related Links
- Gut Microbes and Diet Interact to Affect Obesity
- Genes, Junk Food and Weight
- Certain Foods Linked to Long-term Weight Gain
- Weight-control Information Network
- Overweight and Obesity
- Aim for a Healthy Weight
- Energy Balance: Weight and Obesity, Physical Activity, Diet
References: Diet-Beverage Consumption and Caloric Intake Among US Adults, Overall and by Body Weight. Bleich SN, Wolfson JA, Vine S, Wang YC. Am J Public Health . 2014 Jan 16. [Epub ahead of print]. PMID: 24432876.
Funding: NIH’s National Heart, Lung, and Blood Institute (NHLBI).
Connect with Us
- More Social Media from NIH
Advertisement
Impact of soft drinks to health and economy: a critical review
- Original Scientific Article
- Open access
- Published: 08 June 2019
- Volume 21 , pages 109–117, ( 2020 )
Cite this article
You have full access to this open access article

- J. F. Tahmassebi 1 &
- A. BaniHani 1
96k Accesses
60 Citations
89 Altmetric
Explore all metrics
To provide information regarding the different types of soft drinks and critically reviewing their risk on the dental and general health of children and adolescents, as well as the cost associated with such drinks.
The literature was reviewed using electronic databases, Medline, Embase, Cochrane library, and was complemented by cross-referencing using published references list from reviewed articles. Search words; soft drinks, juices, carbonated drinks, sports and energy drinks, soft drink and dental diseases, soft drink and health, cost of soft drinks, soft drink advertising, sugar tax on soft drinks were used for this review. In total, 104 papers were reviewed by both authors; of these, 62 papers were found to have relevant information.
The consumption of soft drinks was found to have increased dramatically over the past several decades. The greatest increase in soft drink consumption has been among children and adolescents. Some commercial soft drinks are high in sugar content and acidity. In addition, they supply energy only and are of little nutritional benefit and lack micro-nutrients, vitamins and minerals. Soft drink consumption can contribute to detrimental oral and general health. Efforts have been made by manufacturers and government agencies to reduce the potential harmful effects of sugar-containing soft drinks on teeth and general health. These include banning the sale of soft drinks in schools, restricting soft drinks advertising, modifying the composition of soft drinks and introducing tax on sugar-containing soft drinks.
Conclusions
The consumption of soft drinks with high sugar content and acidity can contribute to detrimental oral health and may also affect general health. Therefore, it is necessary to educate patients about the harmful effects of different types of soft drinks as it is not always easy for individuals to identify from drink labelling the ingredients which they contain.
Similar content being viewed by others
The top five selling UK energy drinks: implications for dental and general health
The impact of sugar-sweetened beverages tax on oral health-related outcomes: a systematic review of the current evidence

Association between Sugar Intake, Oral Health, and the Impact on Overall Health: Raising Public Awareness
Avoid common mistakes on your manuscript.
Introduction
Soft drinks include carbonated drinks, still and juice drinks, dilutables, fruit juices, bottled waters, sports and energy drinks (British Soft Drinks Association Annual Report 2016 ). According to the British Soft Drinks Association Annual Report ( 2016 ), the overall consumption of soft drinks in the UK has increased slightly from 2010 to 2015 by 0.2%. In 2015; 13.3 billion litres of soft drinks were consumed compared with 13.2 in 2010 with more than half (58%) of the consumption was of no or low calorie types (0–20 kcal per 100 ml).
Commercial soft drinks first appeared in 1884 when a product called “Moxie” was made by a drugstore owner in Lisbon Falls in the USA (Tahmassebi et al. 2006 ). Soon afterwards, similar products appeared including Coca-Cola ® and Pepsi-Cola ® . Over the past century, soft drinks have changed dramatically from being a local pharmacy product to worldwide industry that earns $60 billion and produce 1 billion litres per year. These changes have been due to advances in manufacturing technology and marketing innovations (Shenkin et al. 2003 ).
Some soft drinks have been suggested to have a harmful effect on the dental and general health of people including children and adolescents (Al-Majed et al. 2002 ; Sayegh et al. 2002 ; Harding et al. 2003 ; Luo et al. 2005 ; Tahmassebi et al. 2006 ; Cheng et al. 2009 ; Vartanian et al. 2011 ; Malik et al. 2010 ; Chi and Scott 2019 ). The high content of sugar and acids, which have cariogenic and acidogenic potential, can contribute to dental caries, tooth erosion, as well as contributing to health effects such as overweight and obesity and may be associated with an increased risk of type 2 diabetes. Efforts have been made by manufacturers and government agencies to reduce the potential harmful effects of sugar-containing soft drinks on teeth and general health. These include banning the sale of soft drinks in schools, restricting soft drinks advertising, modifying the composition of soft drinks and introducing tax on sugar-containing soft drinks.
This paper aims to provide information regarding the different types of soft drinks and their risk on the dental and general health of children and adolescents and the use of artificial sweeteners in soft drinks and a discussion of the cost associated with such drinks.
Materials and methods
Research question.
What are the different types of soft drinks and their risk on the dental and general health of children and adolescents including the use of artificial sweeteners as well as the cost associated with such drinks?
Search strategy
The literature was reviewed by both authors (AB and JT) using electronic databases, Ovid Medline, Embase, Cochrane library and was complemented by cross-referencing using published references list from reviewed articles. Search words included soft drinks, juices, carbonated drinks, sports and energy drinks, soft drink and dental diseases, soft drink and health, cost of soft drinks, soft drink advertising, and sugar tax on soft drinks were used for this review. For Ovid Medline, Embase, and Cochrane library, studies related to the MeSH heading of ‘soft drinks’ or the terms ‘juices’, ‘carbonated drinks’, or ‘sports and energy drinks’ together with the MeSH headings of ‘soft drink and dental diseases’, ‘soft drink and health’, ‘cost of soft drink’, ‘soft drink advertising’, or ‘sugar tax on soft drinks’ were combined. Papers were initially reviewed by assessing the title and abstract followed by the full paper. In total, 104 papers were reviewed; of these, 62 papers were found to have relevant information.
The search strategy is summarised in Fig. 1 . The inclusion and exclusion criteria are summarised in Table 1 .
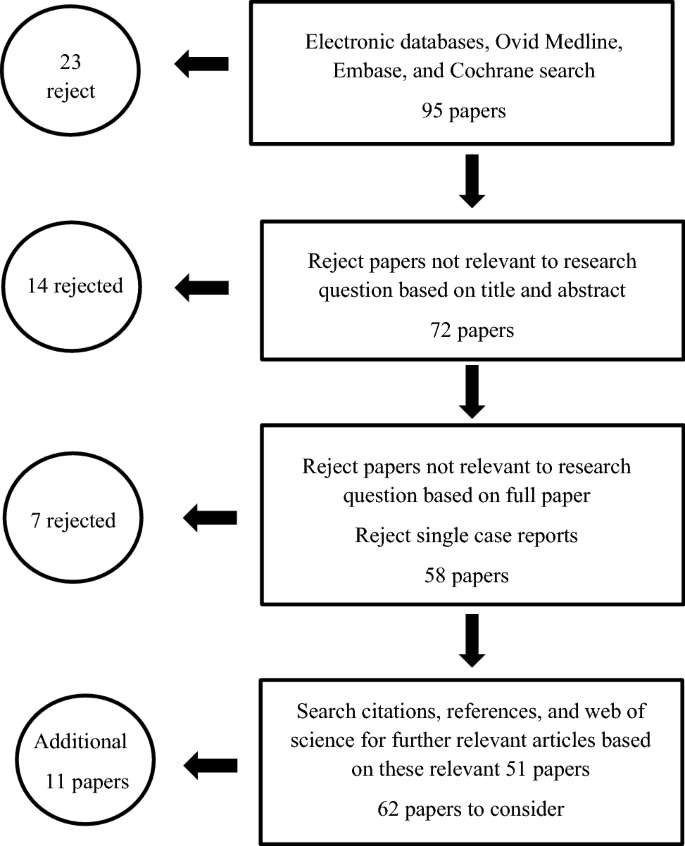
Summary of search strategy with inclusions and exclusions
Different types of soft drinks
Modern drinks now contain carbon dioxide for carbonation. Carbonated soft drinks accounted for the largest category of these drinks in 2016, with a market share of 38% in the UK (British Soft Drinks Association Annual Report 2016 ). Carbon dioxide, a common factor to all carbonates, is added to make drinks fizzy. Other ingredients include water, sugar (sucrose, glucose, and fructose), intense sweeteners (discussed later), acid (citric acid, malic acid, and phosphoric acid), fruit juice, preservatives, flavourings, and colours. Currently, low- and no-calorie drinks make up 45% of the category, with a further 5% being mid-calorie (British Soft Drinks Association Annual Report 2016 ).
Some drinks are made with concentrates that require dilution to taste by consumers, such as squashes, and cordials, accounted for the second largest share (22%) of overall soft drink consumption in 2016. There is a dominance of low- and no-calorie variants within this category (87%), providing lower calorie refreshment for adults and children alike (British Soft Drinks Association Annual Report 2016 ). Fruit juice is 100% pure juice which is made from the flesh of fresh fruit or from whole fruit, depending on the type used. No sugar, sweeteners, preservatives, flavourings or colourings are added to fruit juice. They contain cells or bits of fruit pulps and vitamin C (ascorbic acid). Fruit juice accounted for 7% of total soft drink consumption in UK (British Soft Drinks Association Annual Report 2016 ).
Sport drinks are another popular drinks especially amongst adolescents and young adults and they contain water, carbohydrate mainly glucose, maltodextrin as well as fructose, and electrolytes such as sodium, potassium and chloride (Coombes and Hamilton 2002 ; Coombes 2005 ; British Soft Drinks Association Annual Report 2016 ). The electrolytes are added to improve palatability and to help maintain fluid/electrolyte balance. Sport drinks aim to prevent dehydration, and enhance the athletic physical performance before, during or after sporting activity. They replace fluids and electrolytes/minerals lost by sweating and supply a boost of carbohydrates. The additional benefits of sport drinks over water alone in reducing the effect of dehydration resulting from exercise on cardiovascular dynamics, temperature regulation and exercise performance have been questioned (Coombes and Hamilton 2002 ; Coombes 2005 ; Seifert et al. 2011 ; Jean 2017 ). For most individuals engaged in physical activity, no clear evidence was found to support the additional performance benefits of soft drinks over water alone (Coombes and Hamilton 2002 ; Coombes 2005 ; Jean 2017 ). Although these drinks are designed to help athletes, they have become popular over recent years with the general population especially the younger generation and are being consumed socially (Coombes and Hamilton 2002 ; Coombes 2005 ).
Conversely, energy drinks are glucose based that supply a boost of energy from caffeine, guarana, taurine, and ginseng (British Soft Drinks Association Annual Report 2016 ). Energy drinks contain high amount of sugar and caffeine; therefore, they can enhance the mental and physical performance, improve alertness, concentration, endurance and mood (Bunting et al. 2013 ). The caffeine content and concentration vary widely between various brands and labelling of the amount of caffeine presents in these drinks is not mandated by the Food and Drug Administration of the USA (Rath 2012 ).
Currently, the sport and energy drinks sector in the UK market has a share of 6% and worth over £2 billion (Bunting et al. 2013 ; British Soft Drinks Association Annual Report 2016 ). Low- and no-calorie made up only 5% of the category with 62% of the energy drinks sold in the market are of the regular type.
It is encouraging to see that the consumption of bottled waters in UK has increased significantly from 2 billion litres (14.8%) in 2010 to nearly 3 billion litres (19.3%) in 2015. Likewise, in USA, total consumption of bottled water increased from 54 billion litres in 2015 to 58 billion litres in 2016, an increase of nearly 9%. The sales of bottled water surpassed carbonated soft drinks to become the largest beverage category by volume in the USA in 2016 (International Bottled Water Association 2017 ).
Effect of soft drinks on dental health
Dental caries is a multifactorial disease that is affected by several factors including salivary flow and composition, exposure to fluoride, consumption of dietary sugars, and by oral hygiene practices (González-Aragón Pineda et al. 2019 ).
Regular (non-diet)-soft drinks excluding bottled waters contain large amounts of sucrose or high-fructose corn syrup that have cariogenic potential; a typical 350-ml can of regular carbonated soft drink contains approximately 10 teaspoons (40 g) of these sugars (Table 2 ). Long-term and frequent consumption of regular-soft drinks with high sugar content may induce dental caries. Many studies have shown a positive relationship between caries and intake of soft drinks (Al-Majed et al. 2002 ; Sayegh et al. 2002 ; Harding et al. 2003 ; Luo et al. 2005 ; Cheng et al. 2009 ; Chi and Scott 2019 ). The greatest risk for caries development in children is associated with the consumption of soft drink between meals rather than with meals.
Unfortunately, dental caries is the most common reason for children aged 5–9 years to be admitted to hospital in UK when poor oral health is largely preventable (The Royal College of Surgeons of England 2015 ; BaniHani et al. 2019 ). In 2013–2014, nearly 46,500 children and young people under 19 years old in England were admitted to hospital for a primary diagnosis of dental caries from which over 55% of the cases were between 5 and 9 years old. This figure has increased by 14% from 2010 to 2011 and it continues to increase year on year. Dental rehabilitation under general anaesthesia (GA) is considered as a distressing experience for many children and their parents, and it carries risk of morbidity including postoperative pain, sleepiness, dizziness, nausea and vomiting, and mortality (Atan et al. 2004 ). This approach to dental care comes at a cost to health services as well. For example, in 2012–2013, the NHS spent £30 million on hospital-based tooth extractions for children aged 18 years and under with average cost of £837 for treatment under GA (The Royal College of Surgeons of England 2015 ; BaniHani et al. 2019 ).
The solubility of dental tissues is affected by a pH and titratable acidity of both the oral cavity and the soft drink. When oral pH drops below the pH of 5.5, enamel dissolution occurs (Chowdhury et al. 2018 ). Most soft drinks excluding bottled waters have a pH that ranges from 2.5 to 3.5 with an average pH of 3.44 for the carbonated drinks and fruit juices (Table 2 ) (Chowdhury et al. 2018 ). In addition, they contain acids that have erosive potential mainly carbonic acid, phosphoric acid, malic acid, and citric acid (Shenkin et al. 2003 ; González-Aragón Pineda et al. 2019 ). Therefore, the consumption of soft drinks with high acidic content, both regular- and diet/zero-calories types, is significantly associated with dental erosion (Al-Majed et al. 2002 ; Sayegh et al. 2002 ; Harding et al. 2003 ; Luo et al. 2005 ; Cheng et al. 2009 ; Tahmassebi et al. 2014 ; Pachori et al. 2018 ). Dental erosion can contribute to significant tooth surface loss (TSL) not only in adults but also in children and adolescents resulting in teeth sensitivity, eating and drinking difficulties as well as dissatisfaction with appearance (Milosevic 2017 ).
The total acid level rather than the pH of the beverage, known as titratable acid, determines the actual hydrogen ion availability for interaction with the tooth surface, and is considered as an important factor in development of dental erosion (Tahmassebi et al. 2014 ). Other important factors include the type of acid and its calcium chelating properties, exposure time to the acidic drink, temperature, and the concentration of the modifying substances in the acidic beverage including the calcium, phosphate and fluoride (Zero 1996 ) (Table 3 ).
It has been shown that dental erosion is associated with the drinking methods. Frequent consumption of fruit drink, carbonated beverage and fruit juice as well as bedtime consumption increased the severity of dental erosion (Milosevic 2017 ). Holding the drink longer and swishing it around the mouth lead to a more pronounced pH drop (Eisenburger and Addy 2003 ). The latter can be enhanced by higher temperature of the acid; whereas, the use of a straw while drinking has been shown to reduce the risk of acid erosion (Tahmassebi and Duggal 1997 ).
In an attempt to reduce overweight, obesity and dental caries among populations, diet soft drinks were introduced. Diet (alternatively marketed as sugar-free, zero-calorie or low-calorie) drinks are sugar-free, artificially sweetened versions of carbonated soft drinks with virtually no calories. They are generally marketed toward health conscious people, diabetics, athletes, and other people who want to lose weight, improve physical fitness, or reduce their sugar intake (Weihrauch and Diehl 2004 ; Whitehouse et al. 2008 ; Tandel 2011 ; Gardner et al. 2012 ; Pearlman et al. 2017 ). Although diet soft drinks are non-cariogenic as they contain artificial sweeteners, they contain phosphoric and citric acid at a similar level as the regular beverages which contribute to the total acidic challenge potential on enamel (Roos and Donly 2002 ; Shenkin et al. 2003 ). Diet soft drinks often have a high erosive potential that can enhance enamel demineralisation and contribute to dental erosion as sugar-containing soft drinks (Tahmassebi et al. 2006 ). Ali and Tahmassebi ( 2014 ) reported in an in vitro study that diet-Coca cola ® was acidic with an inherent pH value (pH 2.61) and low titratable acidity.
The management of dental erosion is an area of clinical practice that is undoubtedly expanding. Depending on the degree of tooth wear and symptoms, management can range from monitoring and fluoride treatment to tooth restoration including the placement of composite resin, glass ionomer fillings, and veneers (Milosevic 2017 ). The cost of placing and replacing a restorative material can be high.
Effect of soft drinks on general health
Soft drinks are often high in sugar content and acidity (Table 2 ). Each gram of sugar contains 4 calories. In addition, they supply energy only and are of little nutritional benefit (Bucher and Siegrist 2015 ; Chi and Scott 2019 ). Several studies have shown that soft drink with high sugar and acid content consumption can contribute to detrimental general and oral health effects on children and adolescents including an increasing risk of overweight, obesity, type 2 diabetes, dental caries and dental erosion (Scientific Advisory Committee on Nutrition 2015 ; Chi and Scott 2019 ).
Obesity has recently emerged as a major global health problem. The World Health Organisation (WHO) and Scientific Advisory Committee on Nutrition (SACN) recommend a diet where a maximum 5% of the energy comes from free sugars. The SACN ( 2015 ) reported that nearly a third of children aged 2–15 years living in the UK are overweight or obese, and that younger generations are becoming obese at earlier ages and staying so for longer. In the USA, two out of three adults and one out of three children are overweight or obese with over 18% of 6–19 year olds are above the 95th percentiles of body mass index (BMI), for age and gender (Ogden et al. 2014 ).
A rising consumption of sugar-containing soft drinks has been suggested as a major contributor to the obesity epidemic. The increase in intake of sugar-containing soft drink has coincided with rising body weights and energy intakes in several populations. In the USA, the per capita annual consumption of regular soft drink increased by 86% between 1970 and 1997 alone. During that period of time, the prevalence of obesity rose by 112% (Flegal et al. 2000 ).
Overweight and obesity can have major costs for individuals and their families as well as for the health care systems. It increases the risk of developing type 2 diabetes and heart disease as well as doubles the risk of dying prematurely (Pischon et al. 2008 ).
Type 2 diabetes has also emerged as a global public health concern, parallel to the global trends in the prevalence of obesity. Along with the increased consumption of soft drinks, there has been a rapid and large increase in the reported incidence of type 2 diabetes (Hu and Malik 2010 ; Greenwood et al. 2014 ).
In a systematic review by Vartanian et al. ( 2011 ), high consumption of soft drinks was related to low consumption of milk, calcium, fruit and dietary fibres contributing to an overall poorer diet. In addition, in two studies by Whiting et al. ( 2001 ) and McGartland et al. ( 2003 ), the high intake of carbonated soft drinks during adolescence was significantly associated with reduced bone mineral density among girls aged 12 and 15 years. Calcium is found mainly in dairy products and is an essential nutrient for the structural integrity of bone and for maintaining bone density throughout life (Shenkin et al. 2003 ); whereas, carbonated soft drinks contain mostly empty calories (Whiting et al. 2001 ).
Energy drinks are often high in caffeine to enhance the mental and physical performance, improve alertness, and concentration (Bunting et al. 2013 ). The amount of caffeine in most of the energy drinks is usually three times the concentration in cola drinks. They are available in the market of more than 140 countries and are the fastest growing soft drink sector not only in the USA and UK but also worldwide (Seifert et al. 2011 ).
Although moderate consumption of caffeine can be tolerated by most healthy people, studies showed that its high consumption (> 400 mg per day) has been associated with adverse effects on health including anxiety, restlessness, aggression, headaches, and depression. A prolonged exposure to high intakes of caffeine, levels greater than 500–600 mg a day, can result in chronic toxicity leading to nervousness, nausea, vomiting, seizures and cardiovascular symptoms in severe cases (Seifert et al. 2011 ; Bunting et al. 2013 ; Jean 2017 ).
Artificial sweeteners in soft drinks and general health
Several artificial sweeteners are used to give diet soft drinks a sweet taste without sugar. They are called sugar substitutes because they provide the sweetness of sugars without the added calories, thus reducing the risk for obesity, and dental caries. However, their safety has been controversial (Whitehouse et al. 2008 ). The breakdown product of these sweeteners has controversial health and metabolic effects (Whitehouse et al. 2008 ). Some research has linked the consumption of artificial sweeteners with adverse health conditions including obesity, lymphomas, leukemias, cancers of the bladder, and brain, chronic fatigue syndrome, Parkinson`s disease, Alzheimer`s disease, multiple sclerosis, autism, and systemic lupus (Whitehouse et al. 2008 ). The carcinogenic potential of artificial sweeteners, mainly aspartame and saccharine, has been investigated. Exposure to these chemicals was associated with an increased risk of brain tumours and cancer of the bladder, in both male and female mice, respectively (Olney et al. 1996 ; Weihrauch and Diehl 2004 ). Another sweetener Saccharine ® was prohibited in Canada and banned in the USA following the results of two-generation study published by Arnold et al. ( 1983 ). However, the ban on Saccharine ® use in the USA was withdrawn in 1991; nevertheless, all food and soft drinks containing Saccharine ® have to carry a warning label to indicate that “Saccharine ® is a potential cancer causing agent”. Conversely, future research has failed to conclude that there is a clear causal relationship between aspartame, saccharine and other approved artificial sweeteners consumption, with health risks in humans at normal doses (Chattopadhyay et al. 2014 ). Therefore, the FDA has concluded that these sweeteners are safe at current levels of consumption and, as a result, the decision of placing warning labels on all products that contain saccharine was overturned in 2000 (Tandel 2011 ).
Some commercial soft drinks are high in sugar content and acidity and, therefore, their consumption can contribute to detrimental oral and general health. There is a clear association of soft drink intake with increased energy intake and body weight is evident in the literature (Malik et al. 2010 ; Vartanian et al. 2011 ; Basu et al. 2013 ; Powell et al. 2017 ). Soft drinks apart from the low- and zero-calories categories contain high sugar content. A daily addition of one 350-ml can of sugar-sweetened carbonated soft drink which contains 150 kcl and 40–50 g of sugar to a typical diet with no reduction in other caloric sources can lead to a weight gain of 6.75 kg within 1 year in adults (Apovian 2004 ). Moreover, soft drinks increase hunger, decrease satiety, and condition people to a high level of sweetness that produces a preference in other foods leading to excess energy intake. If normal dietary intake does not decrease by an equivalent amount of calories obtained from consuming soft drinks, then weight gain is very much to be expected (Malik et al. 2010 ; Vartanian et al. 2011 ).
Soft drinks can also contribute to type 2 diabetes through several mechanisms mainly by their ability to induce a weight gain, which is a risk factor for the development of the condition. In addition in the USA, some of these drinks contain high amounts of rapidly absorbable carbohydrates such as sucrose and high-fructose corn syrup (HFCS), a key ingredient in some of sugar-sweetened beverages. Though HFCS is not currently a key ingredient in sugar-sweetened beverages in the UK or EU, changes to the EU quota system on sugar policy since 2017 may influence addition of HFCS in the soft drinks in the future. These types of carbohydrates can lead to hepatic lipogenesis and high dietary glycaemic load resulting in inflammation, insulin resistance and impaired B cell function, thereby fuelling the development of type 2 diabetes (Hu and Malik 2010 ; Caprio 2012 ; Greenwood et al. 2014 ).
The economic costs of obesity and its related ill-health are great too. In 2014/2015, it was estimated that the National Health Service (NHS) in the UK spent nearly £5.1 billion on the treatment of obesity and its related ill-health. A higher figure was reported in the USA where healthcare expenditures on overweight and obesity were estimated to be between $150 billion and $190 billion, attributing to 20% of total healthcare costs per year (Scharf and DeBoer 2016 ).
Several artificial sweeteners are used to give diet soft drinks a sweet taste without sugar. The consumption of artificial sweeteners has been found to promote weight gain rather than weight loss in several studies (Hampton 2008 ; Swithers and Davidson 2008 ; Pearlman et al. 2017 ). These studies showed that these sweeteners induce insulin production into the blood and in the absence of blood sugar, hypoglycaemia and increased food intake occur resulting in overweight and obesity.
Actions have been taken by few countries across the globe to tackle the obesity and dental caries. These include banning the sale of soft drinks in schools, restricting soft drinks advertising, modifying the composition of soft drinks and introducing tax on sugar-containing soft drinks. Sugar-containing soft drinks are banned for sale in schools in many countries.
In the UK, strict rules on sales of high-sugar and -acid content soft drinks in school were instigated in 2007. Beverages with added sugar including energy drinks are not permitted. Also, some schools have banned their students from bringing energy drinks into school from outside (British Soft Drinks Association Annual Report 2016 ).
Furthermore, governments in some countries such as the UK applied restrictions in marketing soft drinks to children online and on television (Al-Mazyad et al. 2017 ). Advertising is essential to the marketing of soft drinks with millions of dollars spent to promote their consumption. Food and beverage advertising increases the total demand and motivates brand switching (Powell et al. 2017 ). Children and youths are exposed to advertising from not only television, but also billboards, magazines, signs in stores and public places such as airports and subway stations, and now increasingly on technology such as iPad apps, and video games as well as social media (Scharf and DeBoer 2016 ). Social media are a relatively new medium through which soft drink manufacturers can uniquely target young people. The increased usage and importance of social media for young people make them vulnerable to highly personalised and targeted digital marketing campaigns by the food and beverage industry. Brownbill et al. ( 2018 ) explored how soft drinks are marketed to Australian young people, aged 13–25 years, through soft drink brand Facebook pages. The authors found that soft drink brands share highly engaging content on Facebook which seamlessly integrates their content into the lives of young people. Brands were found to align their products with common sociocultural values and practices such as masculinity, femininity, friendship, and leisure, which are regarded as important by young people today, thus portraying their products as having a normal place within their everyday lives. The results of the study suggested the need to monitor advertising via social media and the importance of understanding the exposure to, and impact on young people.
Australia, Sweden, and Belgium as well as UK are among the countries that have banned television advertisement of food high in sugar and fat during children’s programmes (Story and French 2004 ).
A number of countries across the globe have introduced a tax on sugar-containing soft drinks in an effort to reduce childhood obesity and dental caries including France, Finland, Hungary and Mexico (World Cancer Research Fund International 2008 ). Colchero et al. ( 2016 ) reported a 10% decrease of sugar-based soft drinks consumption and a 4% increase in the purchase of healthier alternatives such as bottled plain water among the Mexican population following the introduction of a tax on soft drinks in 2014. In addition, 39 states in USA have applied a tax on sugar-containing soft drinks sold either in food premises and/or vending machines (Centre for Science in the Public Interest 2011 ).
A new sugar tax on soft drinks, known as the soft drinks industry levy, was introduced in April 2018 on soft drinks with added sugar in UK to help tackle childhood obesity by reducing the consumption of soft drinks with added sugars. The levy applies to soft drinks that contain 5 grams or more of added sugar per 100 ml. Revenue from the levy is planned to be used to develop programmes that aim to reduce obesity and encourage physical activity for school age children (HM Revenue & Customs 2016 ). This action is expected to reduce the consumption of sugar-containing soft drinks by 1.6%, and it is hoped that it will encourage soft drinks manufacturer to reduce the sugar content of their products.
The consumption of soft drinks was found to have increased dramatically over the past several decades with the greatest increase among children and adolescents. Excessive intake of soft drinks with high sugar and acid content both regular and diet could cause detrimental impacts on dental and general health including dental caries, dental erosion, overweight, obesity and increased risk of type 2 diabetes. The sugar tax has raised the level of awareness; however, it is necessary to educate patients about the harmful effects of different types of soft drink as it is not always easy for individuals to know from drink labelling what they actually contain.
Ali H, Tahmassebi JF. The effects of smoothies on enamel erosion: an in situ study. Int J Paediatr Dent. 2014;24:184–91.
PubMed Google Scholar
Al-Majed I, Maguire A, Murray JJ. Risk factors for dental erosion in 5~6 year old and 12~14 year old boys in Saudi Arabia. Community Dent Oral Epidemiol. 2002;30:38–46.
Al-Mazyad M, Flannigan N, Burnside G, Higham S, Boyland E. Food advertisements on UK television popular with children: a content analysis in relation to dental health. Br Dent J. 2017;222:171–6.
Apovian C. Sugar-sweetened soft drinks, obesity, and type 2 diabetes. JAMA. 2004;292:978–9.
Arnold DL, Krewski D, Munro IC. Saccharin: a toxicological and historical perspective. Toxicology. 1983;27:179–256.
Atan S, Ashley P, Gilthorpe MS, et al. Morbidity following dental treatment of children under intubation general anaesthesia in a day-stay unit. Int J Paediatr Dent. 2004;14:9–16.
Ayers KM, Drummond BK, Thomson WM, Kieser JA. Risk indicators for tooth wear in New Zealand school children. Int Dent J. 2002;52:41–6.
BaniHani A, Deery C, Toumba J, Duggal M. Effectiveness, costs and patient acceptance of a conventional and a biological treatment approach for carious primary teeth in children. Caries Res. 2019;53:65–75.
Basu S, McKee M, Galea G, Stuckler D. Relationship of soft drink consumption to global overweight, obesity, and diabetes: a cross-national analysis of 75 countries. Am J Public Health. 2013;103:2071–7.
PubMed PubMed Central Google Scholar
Bottled water—the nation’s healthiest beverage—sees accelerated growth and consumption. International Bottled Water Association. 2017. http://www.bottledwater.org/bottled-water-%E2%80%93-nation%E2%80%99s-healthiest-beverage-%E2%80%93-sees-accelerated-growth-and-consumption . Accessed Dec 2017.
British Soft Drink Association. Leading the Way. UK soft drinks annual report. 2016. http://www.britishsoftdrinks.com/write/MediaUploads/Publications/BSDA_Annual_report_2016pdf . Accessed Sept 2017.
Brownbill AL, Miller CL, Braunack-Mayer AJ. The marketing of sugar-sweetened beverages to young people on Facebook. Aust N Z J Public Health. 2018;42(4):354–60.
Bucher T, Siegrist M. Children’s and parents’ health perception of different soft drinks. Br J Nutr. 2015;113:526–35.
Bunting H, Baggett A, Grigor J. Adolescent and young adult perceptions of caffeinated energy drinks. A qualitative approach. Appetite. 2013;65:132–8.
Caprio S. Calories from soft drinks—do they matter. N Engl J Med. 2012;367:1462–3.
Carbohydrates and Health. Scientific Advisory Committee on Nutrition. 2015. https://www.gov.uk/government/publications/sacn-carbohydrates-and-health-report . Accessed Sept 2017.
Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners—a review. J Food Sci Technol. 2014;51:611–21.
Cheng R, Yang H, Shao MY, Hu T, Zhou XD. Dental erosion and severe tooth decay related to soft drinks: a case report and literature review. J Zhejiang Univ Sci B. 2009;10:395–9.
Child Dental Health Survey 2013: Gov.Uk. 2015. http://www.gov.uk . Accessed Sept 2017.
Chi DL, Scott JM. Added sugar and dental caries in children: a scientific update and future steps. Dent Clin N Am. 2019;63:17–33.
Chowdhury CR, Shahnawaz K, Kumari PD, et al. Highly acidic pH values of carbonated sweet drinks, fruit juices, mineral waters and unregulated fluoride levels in oral care products and drinks in India: a public health concern. Perspect Public Health. 2018;1:1–9.
Google Scholar
Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704.
Coombes JS, Hamilton KL. The effectiveness of commercially available sports drinks. Sports Med. 2002;29:181–209.
Coombes JS. Sports drinks and dental. Am J Dent. 2005;18:101–4.
Deery C, Wagner ML, Longbottom C, Simon R, Nugent ZL. The prevalence of dental erosion in United States and a United Kingdom sample of adolescents. Paediatr Dent. 2000;22:505–10.
Eisenburger M, Addy M. Influence of liquid temperature and flow rate on enamel erosion and surface softening. J Oral Rehabil. 2003;30:1076–80.
Existing soft drink taxes. Center for Science in the Public Interest. 2011. http://cspinet.org/liquidcandy/existingtaxes.html . Accessed Oct 2017.
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2000;288:1723–7.
Ganss C, Kilmek J, Giese K. Dental erosion in children and adolescents -a cross-sectional and logitudinal investigation using study models. Community Dent Oral Epideniol. 2001;29:264–71.
Gardner C, Wylie-Rosett J, Gidding CC, et al. Nonnutritive sweeteners: current use and health perspectives. Circulation. 2012;126:509–19.
Greenwood DC, Threapleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Br J Nutr. 2014;112:725–34.
González-Aragón Pineda ÁE, Borges-Yáñez SA, Irigoyen-Camacho ME, Lussi A. Relationship between erosive tooth wear and beverage consumption among a group of schoolchildren in Mexico City. Clin Oral Investig. 2019;23:715–23.
Hampton T. Sugar substitutes linked to weigh gain. JAMA. 2008;299:2137–8.
Harding MA, Whelton H, O’Mullane DM, Cronin M. Dental erosion in 5-year-old Irish school children and associated factors: a pilot study. Community Dent Health. 2003;20:165–70.
Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100:47–54.
Jean G. How can we restrict the sale of sports and energy drinks to children? A proposal for a World Health Organization-sponsored framework convention to restrict the sale of sports and energy drinks. Aust Dent J. 2017;62(4):420–5.
Kazoullis S, Seow WK, Holcombe T, Newman B, Ford D. Common dental conditions associated with dental erosion in school children in Australia. Pediatr Dent. 2007;29:33–9.
Luo Y, Zeng XJ, Du MQ, Bedi R. The prevalence of dental erosion in preschool children in China. J Dent. 2005;33:115–21.
Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;12:1356–64.
McGartland C, Robson PJ, Murray G, et al. Carbonated soft drink consumption and bone mineral density in adolescence: the Northern Ireland Young Hearts project. J Bone Miner Res. 2003;18:1563–9.
Milosevic A. Acid erosion: an increasingly relevant dental problem. Risk factors, management and restoration. Prim Dent J. 2017;6(1):37–45.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311N:806–14.
Olney JW, Farber NB, Spitznagel E, Robins LN. Increasing brain tumor rates: is there a link to aspartame? J Neuropathol Exp Neurol. 1996;55:1115–23.
Pachori A, Kambalimath H, Maran S, et al. Evaluation of changes in salivary pH after intake of different eatables and beverages in children at different time intervals. Int J Clin Pediatr Dent. 2018;11(3):177–82.
Pearlman M, Obert J, Casey L. The association between artificial sweeteners and obesity. Curr Gastroenterol Rep. 2017;19(12):64.
Pischon T, Boeing H, Hoffmann KM, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20.
Powell LM, Wada R, Khan T, Emery SL. Food and beverage television advertising exposure and youth consumption, body mass index and adiposity outcomes. Can J Econ. 2017;50(2):345–64.
Rath M. Energy drinks: what is all the hype? The dangers of energy drink consumption. J Am Acad Nurse Pract. 2012;24:70–6.
Roos EH, Donly KJ. In vivo dental plaque pH variation with regular and diet soft drinks. Pediatr Dent. 2002;24:350–3.
Sayegh A, Dini EL, Holt RD, Bedi R. Food and drink consumption, sociodemographic factors and dental caries in 4–5-year-old children in Amman, Jordan. Br Dent J. 2002;193:37–42.
Scharf RJ, DeBoer MD. Sugar-sweetened beverages and children’s health. Annu Rev Public Health. 2016;37:273–93.
Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127:511–28.
Shenkin JD, Heller KE, Warren JJ, Marshall TA. Soft drink consumption and caries risk in children and adolescents. Gen Dent. 2003;51:30–6.
Soft drinks industry levy. HM Revenue & Customs. 2016. https://www.gov.uk/government/publications/soft-drinks-industry-levy/soft-drinks-industry-levy . Accessed Sept 2017.
Story M, French S. Food advertising and marketing directed at children and adolescents in the US. Int J Behav Nutr Phys Act. 2004;1:1–3.
Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–73.
Tahmassebi JF, Duggal MS. The effect of different methods of drinking on the pH of dental plaque in vivo. Int J Paediatr Dent. 1997;7:249–53.
Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: a review of the current literature. J Dent. 2006;34:2–11.
Tahmassebi JF, Kandiah P, Sukeri S. The effects of fruit smoothies on enamel erosion. Eur Arch Paediatr Dent. 2014;15:175–81.
Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55:358–67.
Tandel KR. Sugar substitutes: health controversy over perceived benefits. J Pharmacol Pharmacother. 2011;2:236–43.
The state of children’s oral health in England. Royal College of Surgeons; England, 2015. https://www.rcseng.ac.uk/library-and-publications/college-publications/docs/report-childrens-oral-health/ . Accessed Sept 2017.
Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2011;97:667–75.
Weihrauch MR, Diehl V. Artificial sweeteners—do they bear a carcinogenic risk? Ann Oncol. 2004;15:1460–5.
Whitehouse CR, Boullata J, McCauley LA. The potential toxicity of artificial sweeteners. AAOHN J. 2008;56:251–61.
Whiting SJ, Healey A, Psiuk S, et al. Relationship between carbonated and other low nutrient dense beverages and bone mineral content of adolescents. Nutr Res. 2001;21:1107–15.
World Cancer Research Fund International, “Economic Tools”. 2008. http://www.wcrf.org/int/policy/nourishing-framework/use-economic-tools . Accessed Sept 2017.
Zero DT. Etiology of dental erosion—extrinsic factors. Eur J Oral Sci. 1996;104:162–77.
Download references
Author information
Authors and affiliations.
Leeds School of Dentistry/Faculty of Medicine and Health, University of Leeds, Level 6, Worsley Building, Clarendon Way, Leeds, LS2 9LU, UK
J. F. Tahmassebi & A. BaniHani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to J. F. Tahmassebi .
Ethics declarations
Conflict of interest.
The authors have no conflicts of interest to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Tahmassebi, J.F., BaniHani, A. Impact of soft drinks to health and economy: a critical review. Eur Arch Paediatr Dent 21 , 109–117 (2020). https://doi.org/10.1007/s40368-019-00458-0
Download citation
Received : 19 February 2019
Accepted : 03 June 2019
Published : 08 June 2019
Issue Date : February 2020
DOI : https://doi.org/10.1007/s40368-019-00458-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Carbonated drink
- Dental caries
- Dental erosion
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 July 2017
Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study
- Anika Knüppel ORCID: orcid.org/0000-0003-1049-4836 1 ,
- Martin J. Shipley 1 ,
- Clare H. Llewellyn 1 &
- Eric J. Brunner 1
Scientific Reports volume 7 , Article number: 6287 ( 2017 ) Cite this article
97k Accesses
134 Citations
1980 Altmetric
Metrics details
- Epidemiology
- Risk factors
Intake of sweet food, beverages and added sugars has been linked with depressive symptoms in several populations. Aim of this study was to investigate systematically cross-sectional and prospective associations between sweet food/beverage intake, common mental disorder (CMD) and depression and to examine the role of reverse causation (influence of mood on intake) as potential explanation for the observed linkage. We analysed repeated measures (23,245 person-observations) from the Whitehall II study using random effects regression. Diet was assessed using food frequency questionnaires, mood using validated questionnaires. Cross-sectional analyses showed positive associations. In prospective analyses, men in the highest tertile of sugar intake from sweet food/beverages had a 23% increased odds of incident CMD after 5 years (95% CI: 1.02, 1.48) independent of health behaviours, socio-demographic and diet-related factors, adiposity and other diseases. The odds of recurrent depression were increased in the highest tertile for both sexes, but not statistically significant when diet-related factors were included in the model (OR 1.47; 95% CI: 0.98, 2.22). Neither CMD nor depression predicted intake changes. Our research confirms an adverse effect of sugar intake from sweet food/beverage on long-term psychological health and suggests that lower intake of sugar may be associated with better psychological health.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls

Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial
Introduction.
Sugar consumption is increasingly discussed as an intervention target to reduce prevalence of obesity, diabetes and other non-communicable diseases 1 , 2 . In Britain, adults consume approximately double, and in the U.S. triple, the recommended level of added sugar for additional health benefits (5% of energy intake), with sweet foods and drinks contributing three-quarters of the intake 1 , 3 , 4 . Meanwhile, major depression is predicted to become the leading cause of disability in high income countries by 2030 5 .
Higher sugar consumption was linked to higher depression prevalence in several ecological and cross-sectional studies 6 , 7 , 8 . To date, few studies have investigated the prospective association of sweet food and beverage intake with depression 9 , 10 , 11 , 12 . Although all studies found an increased risk of depression with higher baseline consumption of added sugars, soft drinks, juices and pastries; none examined the role of ‘reverse causation’ in producing the observed association. Reverse causation refers, in this context, to the possibility that a mood disorder may lead to higher sugar intake, so that the diet-mental health association is wholly or partly the result of poor mental health rather than of high sugar intake 13 , 14 , 15 . A prospective study with repeat measures of food intake and mental health provides the opportunity to examine the bidirectional nature of the association, and to contribute novel evidence on the effect of sugar dense diet on depression in the general population.
There are several plausible biological explanations for an association of habitual sugar intake and subsequent risk of depression, in the long-term. Firstly, low levels of the growth factor brain derived neurotrophic factor (BDNF) have been discussed as facilitating neurogenesis and hippocampal atrophy in depression 16 . Rodents fed high-fat high-sugar diets, but not high-fat diets only, show a decrease in BDNF level 17 , 18 , 19 , which could be a mechanistic link between diets high in sugar and depression. Secondly, carbohydrate consumption has been associated with increased circulating inflammatory markers, which may depress mood 20 , 21 . Thirdly, high sugar diets could induce hypoglycaemia through an exaggerated insulin response and thereby influence hormone levels and potentially mood states 22 . Fourthly, addiction-like effects of sugar suggest dopaminergic neurotransmission mechanisms might connect frequent sugar intake with depression 23 , 24 , 25 . Lastly, obesity could be a mediating factor between a sugar-dense diet and depression 26 , 27 not only via inflammatory but also psychosocial factors like weight discrimination 28 .
The aim of this study is to investigate whether sugar intake from sweet food/beverages is positively associated with the risk of both incident and recurrent mood disorders, and to establish the role of the reverse effect in the Whitehall II cohort, using prospective, repeat measures data collected over a 22 year period.
Study cohort
The Whitehall Study II consists of non-industrial civil servants, who were recruited in London at age 35 to 55 years during 1985–1988 (phase 1). The initial sample size was 10,308 individuals (33.1% female and 66.9% male). The participants were followed up via questionnaire in 1989–1990 (phase 2), 1991–1993 (phase 3), 1995–1996 (phase 4), 1997–1999 (phase 5), 2001 (phase 6), 2003–2004 (phase 7), 2006 (phase 8), 2008–2009 (phase 9) and 2012–2013 (phase 11). In phases 1, 3, 5, 7, 9 and 11 they were additionally invited for screening in a research clinic 29 . Phase 10 (2011) consisted of a smaller sample of participants used for a pilot study. The study was approved by the Joint UCL/UCLH Committee on the Ethics of Human Research and carried out in accordance with the ethical principles set out in the Declaration of Helsinki. Further all participants have been asked for informed consent at every follow-up.
Ascertainment of sugar intake from sweet food/beverages
Diet was assessed at phases 3, 5, 7 and 9 using a 127-item machine-readable semi-quantitative food frequency questionnaire (FFQ) which originates from the tool used in the US Nurses’ Health Study, a self-administered questionnaire on habitual diet over the past 12 months 30 , 31 . In order to reflect most diets in the UK it has been modified and anglicized 32 . This FFQ has been validated against a 7 day diet diary in a stratified random sample of 865 participants in the Whitehall Study II at collection phase 3 30 . Sweet food and beverage intake was measured with 15 items such as cakes, biscuits, added sugar to coffee or tea, and fizzy soft drinks (see Supplementary Table S1 ). Sugar intake was calculated by multiplying sweet food/beverage consumption frequencies per day by their sugar content and portion size based on McCance and Widdowson’s The Composition of Foods, 5th edition 33 .
Depressive symptom assessment
The 30-item General Health Questionnaire (GHQ) measures depressive and somatic symptoms over the past two weeks 34 . Caseness was defined as reporting ≥5 symptoms and is referred to as common mental disorder (CMD). This measure was included in follow-up questionnaires at all phases apart from phase 4. In addition, the 20-item Center of Epidemiologic Studies Depression Scale (CES-D), a self-report measure of depressive symptoms in the general population over the past week 35 , was administered at phases 7, 9 and 11. Individuals scoring ≥16 were considered cases of depression 36 . Lastly, a clinical interview using the Revised Clinical Interview Schedule (CIS-R) was administered at phase 11 with participants assessed according to International Classification of Diseases (ICD-10) F32 criteria. The computerized self-completion version of the CIS-R included questions on depressive symptoms that were present for at least 2 weeks 37 , 38 , 39 . The GHQ and CES-D have been validated against the CIS-R in this cohort and showed high sensitivity and specificity in measuring depressive episodes 39 .
Potential confounders were chosen based on review of the literature and restricted to variables available at all phases used in the analyses. All estimates were initially adjusted for age, ethnicity (White/ South Asian/ Black) and sex, with an interaction of sex and age where both sexes included. Socio-demographic variables consisted of marital status (married/cohabiting, single or divorced/widowed) and last employment grade level within the civil service, (high, intermediate, low). Health behaviours included smoking (never, former, current), alcohol intake (none: ≤1 unit/weeks, moderate, heavy: ≥14 units/week) self-reported physical activity (vigorous, moderate and non/mild) 40 and duration of sleep (5 categories from ≤5 hours to ≥9 hours/day). Diet-related factors comprised energy intake, diet quality, fish, coffee and tea intake based on FFQ data. Energy intake was used to ascertain dietary misreporting. Misreporting was considered where the log ratio of energy intake to estimated energy expenditure was outside of 3 SD of the log mean. This definition was adopted by Mosdol et al . 2007 and based on basal metabolic rate equations of the Department of Health 41 , 42 , 43 . Since sugar intake from sweet food/beverages was strongly correlated with energy intake (r = 0.61, P < 0.001), energy intake was adjusted for with the partition method by using energy intake from other foods 44 . Diet quality was assessed using the Dietary Approaches to Stop Hypertension (DASH) diet score modified by excluding a measure for sweet drinks 45 . DASH diet score, coffee and tea intake were analysed as continuous variables, fish intake per day as quintiles and all dichotomized for descriptive analyses. Body mass index (BMI) (kg/m 2 ) and central obesity (in women waist circumference ≥88 cm and in men ≥102 cm) were both measured by trained staff 46 . Physical health was defined as diabetes and cardiovascular disease (coronary heart disease and stroke, CVD) based on self-reports which were validated using the study clinical examination, Hospital Episode Statistics data, and by contacting general practitioners for confirmation when no other external source existed. Cancer was based on cancer registration data 29 . Finally, doctor diagnosis of depression was based on self-report at phases 1 to 4 and on self-reported antidepressant intake at all phases after phase 4.
Statistical analysis
At each phase, participants were included if they had answered at least 8 of the FFQ sweet food and beverage items 47 (less than 5% of eligible sample had one missing item and about 1% two or more), their ethnicity was known to be either White, Black or South Asian, and participants were not energy misreporters (see above). In addition, participants were also excluded from analyses if they had incomplete data on GHQ-, CES-D- or CIS-R caseness for outcome-specific analyses, respectively. Supplementary Fig. S1 shows how the included sample was reached (see Online).
Three binary outcomes were analysed, GHQ caseness, CES-D caseness and CIS-R caseness. Daily sweet food and beverage intake was modelled as sex-specific tertiles of sugar intake from sweet food/beverages based on the distribution at phase 3 (in men <39.5, ≥39.5 to <67.0 and ≥67.0 g/day; in women <30.0, ≥30.0 to <51.0 and ≥51.0 g/day). To describe the sample at phase 3, GHQ cases, non-cases and tertiles of sugar intake by covariate were compared. To examine the prospective association of sugar intake from sweet food and beverages, a random effects logistic regression model (REM) was performed using the STATA command xtlogit 48 , with exposures at phases 3, 5, 7 and 9 for GHQ caseness, and at phases 7 and 9 for CES-D caseness. The applicability of the REM was tested by introducing study phase-interactions and likelihood ratio tests (LRT). The prospective effect of sugar intake from sweet food/beverages on incident and recurrent CMD and depression was examined using REMs in 2, 5 and 10-year cycles 49 . Figure 1 shows the included phases for analyses using GHQ caseness as the outcome. For example, the association between sugar intake and GHQ status 2 years later was conducted by combining the associations between sugar intake at Phase 5 and incident GHQ caseness at Phase 6, and between sugar intake at Phase 7 and incident GHQ caseness at Phase 8. For all depression outcomes, incidence was assumed if no CMD was apparent at each baseline, and recurrence if CMD was apparent at each baseline. For the analyses of depression, two 5-year cycles (to Phase 9 and 11) and three 10 year cycles (to Phases 7, 9 and 11) were used. For clinical depression, one 5-year cycle and one 10-year cycle were used.
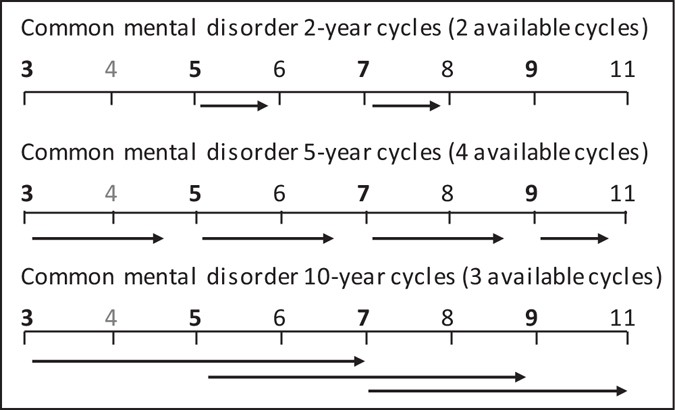
Modes of analysis using cycle approach for common mental disorder a . Numbers indicate study phases. Phases with food frequency data in bold; no data on common mental disorder available at Phase 4. a Common mental disorder measured using the 30-item General Health Questionnaire.
To check for reverse causation, that depressive symptoms may affect subsequent sugar intake from sweet food/beverages, linear regression models of 5-year change and multinomial logistic regression for change groups were fitted for each cycle, from phases 3 to 5, 5 to 7 and 7 to 9, with CMD at phases 3, 5, 7 respectively, and for change from phase 7 to 9 with depression at phase 7. Normal distribution of change in sugar intake from sweet food/beverages was verified using a histogram. Change groups were created by subtracting tertiles of sugar intake at baseline (t) from sugar intake from sweet food/beverages at follow-up (t + 5 y) and coding −2/−1 as decrease, 0 as no change and +1/+2 as increase in sugar intake from sweet food/beverages.
All analyses were performed using Stata 14 50 . Interactions of CMD and depression with sex in the initial model (Model 0 per sex-specific tertile trend: adjusted for age and ethnicity) were tested using LRT since sex-differences have been reported in a prior study on the association of diet and depression in the Whitehall II cohort 51 . Further adjustments were grouped into four hierarchical models: baseline socio-demographic factors and health behaviours (Model 1), diet-related factors (Model 2), BMI and central obesity (Model 3), and physical health (Model 4). In sensitivity analyses, main analyses were repeated by: (a) excluding participants with unknown or reported doctor diagnosis of depression at each baseline (at phases 3/5/7/9: 166/156/193/209 individuals) and: (b) excluding participants with extreme values of sugar intake (>7 SD) at phases 3/5/7/9: 5/3/4/4 individuals.
Table 1 shows the prevalence of CMD and tertiles of reported sugar consumption from sweet food/beverages according to covariates at phase 3. CMD was more prevalent in women: under 50-years old, divorced/widowed, physically inactive, current smokers and those with fewer hours of sleep. Women with CMD were more likely to be in a lower grade level in civil service ( P < 0.001; not depicted). Sugar consumption was associated with socio-demographic factors, health behaviours, physical health and diet-related factors (Table 1 ). Unexpectedly, participants in the highest tertile of sweet food/beverage intake had the highest prevalence of normal weight and lowest prevalence of overweight and obesity as well as the lowest prevalence of abdominal obesity in men (both P = 0.002; not depicted).
Incidence of CMD was around 9 to 15%, highest in the first cycle but did not differ greatly by cycle length. Depression and clinical depression incidence were approximately 8% and 2%, respectively. About 44% of participants who were CMD cases at baseline of each cycle remained recurrent CMD cases, 47% became recurrent depression cases and 58% recurrent clinical depression cases.
Cross-sectional results
Cross-sectional analyses showed strong positive associations between sugar intake from sweet food/beverages and common mental disorder from the GHQ, as well as CES-D caseness, when adjusted for age, sex and ethnicity (Table 2 ). There was no evidence for any interaction with sex ( P = 0.8 for GHQ and P = 0.7 for CES-D). The association with CMD was robust whereas for depression it was removed on adjustment for socio-demographic factors, health behaviours and diet-related factors (Table 2 ). Further adjustments for central obesity and physical health (not shown), exclusion of 709 person-observations (377 in CES-D analysis) with reported doctor diagnosis of depression and person-observations with extreme values of sugar intake at baseline did not change the results.
Prospective results
Prospective analyses regarding incident CMD were stratified by sex, since interactions with sex were observed in the 5 years later model (LR test for sex interaction: GHQ 2 years later, P = 0.26: GHQ 5 years later, P = 0.05). In women, no associations were found for incident CMD with tertiles of sugar intake from sweet food/beverages (after 2 years, highest vs. lowest tertile OR: 0.98; 95% CI 0.72, 1.34; P for tertile trend = 0.90; after 5 years, highest vs. lowest tertile OR: 0.94; 95% CI 0.74, 1.19; P for tertile trend = 0.59). In men, after adjustment for age and ethnicity, sugar intake was associated with incident CMD 2 and 5 years later (Table 3 ). In further models, the association with 2-year incidence attenuated but the association with 5-year incidence remained (Table 3 ) and further adjustments for BMI, central obesity and physical health (not shown) resulted in an OR for highest vs. lowest tertile of: 1.23, 95% CI: 1.02, 1.48, P for trend = 0.03. Excluding participants who reported a doctor diagnosis of depression at each baseline strengthened the association (Model 4 for CMD after 5 years, Person observations = 10944; highest vs. lowest tertile OR; 1.25; 95% CI 1.03, 1.50; P for trend = 0.02, Supplementary Table S2 ) and exclusion of person observations with extremely high sugar intakes did not affect the results. In men and women, no association between sugar intake from sweet food/beverages and incident depression or clinical depression 5 years later was observed (Model 0, highest vs. lowest tertile OR, depression 0.92; 95% CI: 0.71, 1.18; P for tertile trend = 0.44; clinical depression: 0.95; 95% CI: 0.51, 1.75; P for trend = 0.84). The same exposure contrast was not associated with incident CMD or depression caseness after 10 years (Model 0, highest vs. lowest tertile OR: 1.10; 95% CI: 0.92, 1.31; P for tertile trend = 0.31; and 1.17; 95% CI: 0.91, 1.50, P for tertile trend = 0.25, respectively). However, sugar intake was positively associated with incident clinical depression after 10 years in men (Model 2 Person observations = 2572, cases = 35; P for tertile trend = 0.67), but negatively in women (Person observations = 848, cases = 28; P for tertile trend = 0.02).
Prospective analyses regarding the associations of sugar intake from sweet food/beverages and recurrent mood disorders showed no evidence for sex interaction for CMD, CES-D depression or clinical depression 5 years later. Sugar intake from sweet food/beverages was positively associated with recurrent depression after 5 years (Model 0, highest vs. lowest tertile OR: 1.81; 95% CI: 1.23, 2.66; P for tertile trend = 0.003, Table 4 ). The association was attenuated when adjusted for other diet-related factors. Moreover, there was some evidence that sugar intake from sweet food/beverages was associated with recurrent clinical depression in both sexes combined (highest vs. lowest tertile OR: 1.66; 95% CI: 0.96, 2.87 and P for tertile trend = 0.07) when adjusted for age, sex and ethnicity (Supplementary Table S3 ). This association attenuated when further factors were introduced to the model. No statistically significant association was found for sugar intake from sweet food/beverages and recurrent GHQ caseness after 2 and 5 years (Model 0 for CMD after 2 years, highest vs. lowest tertile OR: 1.05; 95% CI 0.76, 1.45; P for tertile trend = 0.83; for CMD after 5 years: 1.16; 95% CI 0.93, 1.46; P for tertile trend = 0.20).
Analyses of recurrent CMD, depression and recurrent clinical depression after 10 years showed no associations with sugar intake from sweet food/beverages.
Sensitivity analyses excluding extreme values of sugar intake and excluding person-observations with self-reported doctor diagnosis at baseline attenuated the association of sugar intake from sweet food/beverages and recurrent depression slightly (before P for tertile trend 0.003 after 0.022 and 0.010, respectively). Similarly, associations with clinical depression weakened when participants with depression diagnosis at baseline were excluded (Model 0, Person observations = 573; cases = 78; P for tertile trend = 0.17).
Analysis of reverse causation
Sugar intake from sweet food/beverages decreased by 2.00 (SD 28.8; 95% CI 1.20, 2.79) grams per day from phase 3 to 5, by 3.44 (SD 28.0; 95% CI 2.59, 4.30) grams from phase 5 to 7 and by 1.57 (SD 26.0; 95% CI 0.91, 2.33) grams from phase 7 to 9, and was normally distributed. Mean 5-year change was approximately 31 g sugar from sweet food/beverages per day in the decrease group, −0.7 g in the stable intake group and 29 g in the increase group. Neither CMD, nor depression predicted 5-year changes in sugar intake (Table 5 ).
The present long-term prospective study is the first to investigate the association of sugar consumption from sweet food/beverages with prevalent, incident and recurrent mood disorders, while also examining the effect these disorders might have on subsequent habitual sugar intake. We found an adverse effect of higher sugar intake on mental health cross-sectionally and 5 years later in a study based on 23,245 repeated measures in men and women aged between 39 and 83. Further, we found an increased likelihood for incident CMD in men and some evidence of recurrent depression in both sexes with higher intakes of sugar from sweet food/beverages. These associations with incident CMD could not be explained by socio-demo graphic factors, other diet-related factors, adiposity and other diseases although the association with recurrent depression was explained by other diet-related factors.
In our study we were able to exclude potential ‘reverse causation’ as the reason for the observed link between high sugar intake and low mood. Over years and decades, it could be that those susceptible to depression tend to increase their sugar intake. This group may tend to report higher consumption at study baseline even in the absence of depression at the time of the questionnaire, while having an increased risk of future depression compared to other participants 14 , 15 . However, there was no support for this alternative hypothesis, since the observed associations in our analysis were not the result of secondary changes in consumption of sugary food and drinks. Our study findings are consistent with the hypothesis that high sugar intake plays a causal role in the risks of both incident and recurrent depression and CMD.
Higher sugar intake from sweet food/beverages was associated with increased likelihood of incident CMD after 5 years in men. The association in men was in line with results from previous prospective studies in American and Spanish cohorts 9 , 10 , 11 . There are several potential explanations for the observed sex differences. First, the associations in men for incident CMD show a stronger effect with a bigger sample (comparing analysis of 2-year CMD with 5-year CMD), suggesting the lower number of female participants in our sample could have impaired the power of the analysis. Second, the results might reflect differences in pathways of depression by sex and type of depressive symptomatology 52 , 53 , 54 . Third, differences could be due to limitations of the study or to chance.
As described in the Introduction there are four potential mechanisms for an association of habitual sugar intake and subsequent depression risk. Sugar intake could increase depression risk over its potential influence on BDNF levels 16 and inflammation 20 which are both discussed as potential biological explanations for depression 17 , 21 . Furthermore postprandial hypoglycaemia 22 and addiction-like effects of sugar influencing neurotransmitters 23 , 24 , 25 could link sugar intake with low mood. The pathway of postprandial hypoglycaemia is also relevant in the context of Glycaemic index, which has been shown to be associated with depression prevalence and incidence 55 , 56 . However, it is a complex issue to tease apart the effects of a single nutrient in epidemiological studies since foods represent a mix of macro- and micronutrients. In this study associations were attenuated when adjusted for diet-related factors providing evidence of confounding and suggesting that the effect of sugar intake from sweet food/beverages could be partly explained by other components of the diet. Also, given that we analysed sugar intake from aggregated sweet foods and beverages we cannot rule out that certain types of foods and their particular components such as saturated fat content may have affected our findings. In our analysis, the association of sugar intake and recurrent depression was attenuated by measures of body fatness in participants without doctor diagnosis of depression at baseline supporting the hypothesis of an indirect effect mediated by adiposity 26 , 27 , 28 driving the association of sugar intake and recurrent depression.
Meanwhile, there are several sources of possible error. Our study was based on an occupational cohort but sugar intakes from sweet food/beverages were close to those reported previously in a representative cohort in the UK (approximately 40 grams), and Batty et al . showed that effects found in Whitehall II were comparable to those observed in population-representative cohorts 3 , 57 . A major limitation was the use of FFQ to derive diet data. FFQ data is subject to misreporting and underreporting, which have been found to differ by food group, depressive mood and BMI 58 , 59 , 60 . As reported previously in Whitehall, we showed a clear trend of lower sugar intake with higher BMI in men 60 . Nutrient content was based on food composition tables from 1991 and has to be considered as a source of error, since food composition especially of highly processed food is likely to change over the course of 18 years. In this long-term follow-up study, sugar intake from sweet food/beverages, which are consistently high in sugar content, has been used as the exposure measure. Compared to a measure of intake that includes processed foods 61 , this method may involve less information bias. Furthermore, this FFQ is meant to reflect habitual diet over the course of a year and therefore might not pick up short-term diet changes or occasional binge eating 30 . Although we adjusted for a number of potential confounders, we cannot rule out residual confounding through unknown or unmeasured factors. Finally, not all depression measures were obtained in all phases and selective dropout due to depressive symptoms might have influenced case numbers 62 .
In conclusion, our study provides evidence that sugar intake from sweet food/beverages increases the chance of incident mood disorders in men and limited evidence regarding recurrent mood disorders in both sexes. With a high prevalence of mood disorders, and sugar intake commonly two to three times the level recommended, our findings indicate that policies promoting the reduction of sugar intake could additionally support primary and secondary prevention of depression. To elucidate the association further, especially regarding observed sex differences our study should be replicated in representative prospective cohorts.
WHO. Guideline: Sugar intake for adults and children. WHO Document Production Services (2015).
Public Health England. Sugar Reduction The evidence for action , https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/470179/Sugar_reduction_The_evidence_for_action.pdf (2015).
Public Health England. National Diet and Nutrition Survey: Results from Years 1–4 (combined) of the Rolling Programme (2008/2009 -2011/12) (2014).
Welsh, J. A., Sharma, A. J., Grellinger, L. & Vos, M. B. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 94 , 726–734 (2011).
Article CAS PubMed PubMed Central Google Scholar
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3 , e442 (2006).
Article PubMed PubMed Central Google Scholar
El Ansari, W., Adetunji, H. & Oskrochi, R. Food and mental health: Relationship between food and perceived stress and depressive symptoms among university students in the United Kingdom. Cent. Eur. J. Public Health 22 , 90–97 (2014).
Article PubMed Google Scholar
Yu, B. et al . Soft drink consumption is associated with depressive symptoms among adults in China. Journal of Affective Disorders 172 , 422–427 (2015).
Westover, A. N. & Marangell, L. B. A cross-national relationship between sugar consumption and major depression? Depression and Anxiety 16 , 118–120 (2002).
Gangwisch, J. E. et al . High glycemic index diet as a risk factor for depression: analyses from the Women’s Health Initiative. Am J Clin Nutr 102 , 454–463 (2015).
Guo, X. et al . Sweetened Beverages, Coffee, and Tea and Depression Risk among Older US Adults. PLoS ONE 9 , e94715, doi: 10.1371/journal.pone.0094715 (2014).
Article ADS PubMed PubMed Central Google Scholar
Sánchez-Villegas, A. et al . Fast-food and commercial baked goods consumption and the risk of depression. Public health nutrition 15 , 424–432 (2012).
Sanchez-Villegas, A. et al . Validity of a self-reported diagnosis of depression among participants in a cohort study using the Structured Clinical Interview for DSM-IV (SCID-I). BMC psychiatry 8 , 1–8 (2008).
Article Google Scholar
Jeffery, R. W. et al . Reported food choices in older women in relation to body mass index and depressive symptoms. Appetite 52 , 238–240 (2009).
Singh, M. Mood, food, and obesity. Frontiers in Psychology 5 (2014).
Macht, M. How emotions affect eating: A five-way model. Appetite 50 , 1–11 (2008).
Sen, S., Duman, R. & Sanacora, G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biological Psychiatry 64 , 527–532 (2008).
Molteni, R., Barnard, R. J., Ying, Z., Roberts, C. K. & Gomez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112 , 803–814 (2002).
Article CAS PubMed Google Scholar
Gainey, S. J. et al . Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide. Front Behav Neurosci 10 , 156 (2016).
Heyward, F. D. et al . Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem 98 , 25–32 (2012).
Calder, P. C. et al . Dietary factors and low-grade inflammation in relation to overweight and obesity. British Journal of Nutrition 106 , S1–S78 (2011).
Kivimaki, M. et al . Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psychiatry 19 , 149–150 (2014).
Schwartz, N. S., Clutter, W. E., Shah, S. D. & Cryer, P. E. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. Journal of Clinical Investigation 79 , 777–781 (1987).
Avena, N. M., Rada, P. & Hoebel, B. G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews 32 , 20–39 (2008).
Article CAS Google Scholar
Dunlop, B. W. & Nemeroff, C. B. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64 , 327–337 (2007).
Grant, B. F., Stinson, F. S. & Dawson, D. A. et al . Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry 61 , 807–816 (2004).
Te Morenga, L., Mallard, S. & Mann, J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Bmj 346 , e7492 (2013).
Luppino, F. S., de Wit, L. M. & Bouvy, P. F. et al . Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry 67 , 220–229 (2010).
Jackson, S. E., Beeken, R. J. & Wardle, J. Obesity, perceived weight discrimination, and psychological well-being in older adults in England. Obesity (Silver.Spring) 23 , 1105–1111 (2015).
Marmot, M. & Brunner, E. Cohort Profile: The Whitehall II study. International Journal of Epidemiology 34 , 251–256 (2005).
Brunner, E., Juneja, M. & Marmot, M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. British Journal of Nutrition 86 , 405–414 (2001).
Willett, W. C. et al . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122 , 51–65 (1985).
Bingham, S. A. et al . Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers International Journal of Epidemiology 26 , S137 (1997).
Holland, B., Unwin, I., Buss, D., Pauk, A. & Southgate, D. McCance and Widdowson’s The Composition of Foods, 5th edition . (Royal Society of Chemistry 1991).
Goldberg, D. P. The detection of psychiatric illness by questionnaire: A technique for the identification and assessment of non-psychotic psychiatric illness. (Oxford U. Press, 1972).
Radloff, L. S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement 1 , 385–401 (1977).
Stansfeld, S., Head, J., Bartley, M. & Fonagy, P. Social position, early deprivation and the development of attachment. Soc Psychiat Epidemiol 43 , 516–526 (2008).
Lewis, G., Pelosi, A. J., Araya, R. & Dunn, G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 22 , 465–486 (1992).
Lewis, G. et al . The development of a computerized assessment for minor psychiatric disorder. Psychological Medicine 18 , 737–745 (1988).
Head, J. et al . Use of self-administered instruments to assess psychiatric disorders in older people: validity of the General Health Questionnaire, the Center for Epidemiologic Studies Depression Scale and the self-completion version of the revised Clinical Interview Schedule. Psychol Med 43 , 2649–2656 (2013).
Kumari, M., Head, J. & Marmot, M. Prospective Study of Social and Other Risk Factors for Incidence of Type 2 Diabetes in the Whitehall II Study. Archives of Internal Medicine 164 , 1873–1880 (2004).
Schofield, W. N. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39 (Suppl 1), 5–41 (1985).
PubMed Google Scholar
Mosdøl, A., Witte, D. R., Frost, G., Marmot, M. G. & Brunner, E. J. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. The American Journal of Clinical Nutrition 86 , 988–994 (2007).
Department of Health Great Britain. Dietary reference values for food energy and nutrients for the United Kingdom: Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. (H.M.S.O., 1991).
Willett, W. C., Howe, G. R. & Kushi, L. H. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65, 1220S–1228S, discussion 1229S–1231S (1997).
Fung, T. T. et al . Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168 , 713–720 (2008).
WHO. Obesity: preventing and managing the global epidemic. WHO Document Production Services (2000).
Willett, W. Nutritional epidemiology (2013).
Twisk, J. W. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol 19 , 769–776 (2004).
Brunner, E. J. et al . Depressive disorder, coronary heart disease, and stroke: dose-response and reverse causation effects in the Whitehall II cohort study. Eur J Prev Cardiol 21 , 340–346 (2014).
Stata Statistical Software: Release 14 (StataCorp LP, College Station, TX, 2015).
Akbaraly, T. N., Sabia, S., Shipley, M. J., Batty, G. D. & Kivimaki, M. Adherence to healthy dietary guidelines and future depressive symptoms: evidence for sex differentials in the Whitehall II study. Am J Clin Nutr 97 , 419–427 (2013).
Rahe, C. et al . Associations between depression subtypes, depression severity and diet quality: cross-sectional findings from the BiDirect Study. BMC Psychiatry 15 , 38 (2015).
Agurs-Collins, T. & Fuemmeler, B. F. Dopamine polymorphisms and depressive symptoms predict foods intake. Results from a nationally representative sample. Appetite 57 , 339–348 (2011).
CAS PubMed Google Scholar
Verhagen, M. et al . Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol. Psychiatry 15 , 260–271 (2010).
Gopinath, B., Flood, V. M., Burlutksy, G., Louie, J. C. Y. & Mitchell, P. Association between carbohydrate nutrition and prevalence of depressive symptoms in older adults. British Journal of Nutrition 116 , 2109–2114 (2016).
Gangwisch, J. E. et al . High glycemic index diet as a risk factor for depression: analyses from the Women’s Health Initiative. Am. J. Clin. Nutr (2015).
Batty, G. D. et al . Generalizability of Occupational Cohort Study Findings. Epidemiology 25 , 932–933 (2014).
Lutomski, J. E., van den Broeck, J., Harrington, J., Shiely, F. & Perry, I. J. Sociodemographic, lifestyle, mental health and dietary factors associated with direction of misreporting of energy intake. Public health nutrition 14 , 532–541 (2011).
Millen, A. E. et al . Differences between Food Group Reports of Low-Energy Reporters and Non–Low-Energy Reporters on a Food Frequency Questionnaire. Journal of the American Dietetic Association 109 , 1194–1203 (2009).
Stallone, D. D., Brunner, E. J., Bingham, S. A. & Marmot, M. G. Dietary assessment in Whitehall II: the influence of reporting bias on apparent socioeconomic variation in nutrient intakes. Eur J Clin Nutr 51 , 815–825 (1997).
Louie, J. C. et al . A systematic methodology to estimate added sugar content of foods. Eur J Clin Nutr 69 , 154–161 (2015).
Jokela, M. et al . Natural course of recurrent psychological distress in adulthood. J Affect Disord 130 , 454–461 (2011).
Download references
Acknowledgements
We thank all participating women and men in the Whitehall II Study, as well as all Whitehall II research scientists, study and data managers and clinical and administrative staff who make the study possible. The UK Medical Research Council, British Heart Foundation, and the US National Institutes of Health (R01HL36310, R01AG013196) have supported collection of data in the Whitehall II Study. This research is part of the Multi-country collaborative project on the role of Diet, Food-related behaviour, and Obesity in the prevention of Depression (MooDFOOD) and was supported by the Seventh Framework Programme of the European Commission (FP7-KKBE-2013-2-1-01). MJS is partly supported by the British Heart Foundation.
Author information
Authors and affiliations.
Department of Epidemiology and Public Health, University College London, London, WC1E 6BT, UK
Anika Knüppel, Martin J. Shipley, Clare H. Llewellyn & Eric J. Brunner
You can also search for this author in PubMed Google Scholar
Contributions
E.J.B., A.K. and M.J.S. designed research; A.K. analysed the data; A.K., E.J.B., M.J.S., C.H.L. wrote the paper; E.B. had primary responsibility for final content. All authors read and approved the final manuscript.

Corresponding author
Correspondence to Anika Knüppel .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Knüppel, A., Shipley, M.J., Llewellyn, C.H. et al. Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Sci Rep 7 , 6287 (2017). https://doi.org/10.1038/s41598-017-05649-7
Download citation
Received : 21 November 2016
Accepted : 01 June 2017
Published : 27 July 2017
DOI : https://doi.org/10.1038/s41598-017-05649-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Association between dietary sugar intake and depression in us adults: a cross-sectional study using data from the national health and nutrition examination survey 2011–2018.
- Haiyang Sun
- Yuanxiang Liu
BMC Psychiatry (2024)
The impact of non-caloric artificial sweetener aspartame on female reproductive system in mice model
- Ab Qayoom Naik
- Tabassum Zafar
- Vinoy K Shrivastava
Reproductive Biology and Endocrinology (2023)
The association between healthy beverage index and quality of life among overweight and obese women: a cross-sectional study
- Niloufar Rasaei
- Rasool Ghaffarian-Ensaf
- Khadijeh Mirzaei
BMC Public Health (2023)
Secondary analysis of a randomized trial testing community health educator interventions for diabetes prevention among refugees with depression: effects on nutrition, physical activity and sleep
- Julie A. Wagner
- Angela Bermúdez-Millán
- Mary F. Scully
International Journal of Behavioral Nutrition and Physical Activity (2023)
The associations of dietary patterns with depressive and anxiety symptoms: a prospective study
BMC Medicine (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Article Collection Archive
- Author Guidelines
- Submission Site
- Open Access
- Call for Papers
- Why Publish?
- About Nutrition Reviews
- About International Life Sciences Institute
- Editorial Board
- Early Career Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, physiological effects of dehydration, hydration and chronic diseases, water consumption and requirements and relationships to total energy intake, water requirements: evaluation of the adequacy of water intake, acknowledgments, water, hydration, and health.
- Article contents
- Figures & tables
- Supplementary Data
Barry M Popkin, Kristen E D'Anci, Irwin H Rosenberg, Water, hydration, and health, Nutrition Reviews , Volume 68, Issue 8, 1 August 2010, Pages 439–458, https://doi.org/10.1111/j.1753-4887.2010.00304.x
- Permissions Icon Permissions
This review examines the current knowledge of water intake as it pertains to human health, including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, and the effects of variation in water intake on health and energy intake, weight, and human performance and functioning. Water represents a critical nutrient, the absence of which will be lethal within days. Water's importance for the prevention of nutrition-related noncommunicable diseases has received more attention recently because of the shift toward consumption of large proportions of fluids as caloric beverages. Despite this focus, there are major gaps in knowledge related to the measurement of total fluid intake and hydration status at the population level; there are also few longer-term systematic interventions and no published randomized, controlled longer-term trials. This review provides suggestions for ways to examine water requirements and encourages more dialogue on this important topic.
Water is essential for life. From the time that primeval species ventured from the oceans to live on land, a major key to survival has been the prevention of dehydration. The critical adaptations cross an array of species, including man. Without water, humans can survive only for days. Water comprises from 75% body weight in infants to 55% in the elderly and is essential for cellular homeostasis and life. 1 Nevertheless, there are many unanswered questions about this most essential component of our body and our diet. This review attempts to provide some sense of our current knowledge of water, including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, the effects of variation in water intake on health and energy intake, weight, and human performance and functioning.
Recent statements on water requirements have been based on retrospective recall of water intake from food and beverages among healthy, noninstitutionalized individuals. Provided here are examples of water intake assessment in populations to clarify the need for experimental studies. Beyond these circumstances of dehydration, it is not fully understood how hydration affects health and well-being, even the impact of water intakes on chronic diseases. Recently, Jéquier and Constant 2 addressed this question based on human physiology, but more knowledge is required about the extent to which water intake might be important for disease prevention and health promotion.
As noted later in the text, few countries have developed water requirements and those that exist are based on weak population-level measures of water intake and urine osmolality. 3 , 4 The European Food Safety Authority (EFSA) was recently asked to revise existing recommended intakes of essential substances with a physiological effect, including water since this nutrient is essential for life and health. 5
The US Dietary Recommendations for water are based on median water intakes with no use of measurements of the dehydration status of the population to assist. One-time collection of blood samples for the analysis of serum osmolality has been used by the National Health and Nutrition Examination Survey program. At the population level, there is no accepted method of assessing hydration status, and one measure some scholars use, hypertonicity, is not even linked with hydration in the same direction for all age groups. 6 Urine indices are used often but these reflect the recent volume of fluid consumed rather than a state of hydration. 7 Many scholars use urine osmolality to measure recent hydration status. 8 , – 12 Deuterium dilution techniques (isotopic dilution with D 2 O, or deuterium oxide) allow measurement of total body water but not water balance status. 13 Currently, there are no completely adequate biomarkers to measure hydration status at the population level.
In discussing water, the focus is first and foremost on all types of water, whether it be soft or hard, spring or well, carbonated or distilled. Furthermore, water is not only consumed directly as a beverage; it is also obtained from food and to a very small extent from oxidation of macronutrients (metabolic water). The proportion of water that comes from beverages and food varies according to the proportion of fruits and vegetables in the diet. The ranges of water content in various foods are presented in Table 1 . In the United States it is estimated that about 22% of water intake comes from food while the percentages are much higher in European countries, particularly a country like Greece with its higher intake of fruits and vegetables, or in South Korea. 3 , – 15 The only in-depth study performed in the United States of water use and water intrinsic to food found a 20.7% contribution from food water; 16 , 17 however, as shown below, this research was dependent on poor overall assessment of water intake.
Ranges of water content for selected foods.
Data from the USDA national nutrient database for standard reference, release 21, as provided in Altman. 126
This review considers water requirements in the context of recent efforts to assess water intake in US populations. The relationship between water and calorie intake is explored both for insights into the possible displacement of calories from sweetened beverages by water and to examine the possibility that water requirements would be better expressed in relation to calorie/energy requirements with the dependence of the latter on age, size, gender, and physical activity level. Current understanding of the exquisitely complex and sensitive system that protects land animals against dehydration is covered and commentary is provided on the complications of acute and chronic dehydration in man, against which a better expression of water requirements might complement the physiological control of thirst. Indeed, the fine intrinsic regulation of hydration and water intake in individuals mitigates prevalent underhydration in populations and its effects on function and disease.
Regulation of fluid intake
To prevent dehydration, reptiles, birds, vertebrates, and all land animals have evolved an exquisitely sensitive network of physiological controls to maintain body water and fluid intake by thirst. Humans may drink for various reasons, particularly for hedonic ones, but drinking is most often due to water deficiency that triggers the so-called regulatory or physiological thirst. The mechanism of thirst is quite well understood today and the reason nonregulatory drinking is often encountered is related to the large capacity of the kidneys to rapidly eliminate excesses of water or to reduce urine secretion to temporarily economize on water. 1 But this excretory process can only postpone the necessity of drinking or of ceasing to drink an excess of water. Nonregulatory drinking is often confusing, particularly in wealthy societies that have highly palatable drinks or fluids that contain other substances the drinker seeks. The most common of these are sweeteners or alcohol for which water is used as a vehicle. Drinking these beverages is not due to excessive thirst or hyperdipsia, as can be shown by offering pure water to individuals instead and finding out that the same drinker is in fact hypodipsic (characterized by abnormally diminished thirst). 1
Fluid balance of the two compartments
Maintaining a constant water and mineral balance requires the coordination of sensitive detectors at different sites in the body linked by neural pathways with integrative centers in the brain that process this information. These centers are also sensitive to humoral factors (neurohormones) produced for the adjustment of diuresis, natriuresis, and blood pressure (angiotensin mineralocorticoids, vasopressin, atrial natriuretic factor). Instructions from the integrative centers to the “executive organs” (kidney, sweat glands, and salivary glands) and to the part of the brain responsible for corrective actions such as drinking are conveyed by certain nerves in addition to the above-mentioned substances. 1
Most of the components of fluid balance are controlled by homeostatic mechanisms responding to the state of body water. These mechanisms are sensitive and precise, and are activated with deficits or excesses of water amounting to only a few hundred milliliters. A water deficit produces an increase in the ionic concentration of the extracellular compartment, which takes water from the intracellular compartment causing cells to shrink. This shrinkage is detected by two types of brain sensors, one controlling drinking and the other controlling the excretion of urine by sending a message to the kidneys, mainly via the antidiuretic hormone vasopressin to produce a smaller volume of more concentrated urine. 18 When the body contains an excess of water, the reverse processes occur: the lower ionic concentration of body fluids allows more water to reach the intracellular compartment. The cells imbibe, drinking is inhibited, and the kidneys excrete more water.
The kidneys thus play a key role in regulating fluid balance. As discussed later, the kidneys function more efficiently in the presence of an abundant water supply. If the kidneys economize on water and produce more concentrated urine, they expend a greater amount of energy and incur more wear on their tissues. This is especially likely to occur when the kidneys are under stress, e.g., when the diet contains excessive amounts of salt or toxic substances that need to be eliminated. Consequently, drinking a sufficient amount of water helps protect this vital organ.
Regulatory drinking
Most drinking occurs in response to signals of water deficit. Apart from urinary excretion, the other main fluid regulatory process is drinking, which is mediated through the sensation of thirst. There are two distinct mechanisms of physiological thirst: the intracellular and the extracellular mechanisms. When water alone is lost, ionic concentration increases. As a result, the intracellular space yields some of its water to the extracellular compartment. Once again, the resulting shrinkage of cells is detected by brain receptors that send hormonal messages to induce drinking. This association with receptors that govern extracellular volume is accompanied by an enhancement of appetite for salt. Thus, people who have been sweating copiously prefer drinks that are relatively rich in Na+ salts rather than pure water. When excessive sweating is experienced, it is also important to supplement drinks with additional salt.
The brain's decision to start or stop drinking and to choose the appropriate drink is made before the ingested fluid can reach the intra- and extracellular compartments. The taste buds in the mouth send messages to the brain about the nature, and especially the salt content, of the ingested fluid, and neuronal responses are triggered as if the incoming water had already reached the bloodstream. These are the so-called anticipatory reflexes: they cannot be entirely “cephalic reflexes” because they arise from the gut as well as the mouth. 1
The anterior hypothalamus and pre-optic area are equipped with osmoreceptors related to drinking. Neurons in these regions show enhanced firing when the inner milieu gets hyperosmotic. Their firing decreases when water is loaded in the carotid artery that irrigates the neurons. It is remarkable that the same decrease in firing in the same neurons takes place when the water load is applied on the tongue instead of being injected into the carotid artery. This anticipatory drop in firing is due to communication from neural pathways that depart from the mouth and converge onto neurons that simultaneously sense the blood's inner milieu.
Nonregulatory drinking
Although everyone experiences thirst from time to time, it plays little role in the day-to-day control of water intake in healthy people living in temperate climates. In these regions, people generally consume fluids not to quench thirst, but as components of everyday foods (e.g., soup, milk), as beverages used as mild stimulants (tea, coffee), and for pure pleasure. A common example is alcohol consumption, which can increase individual pleasure and stimulate social interaction. Drinks are also consumed for their energy content, as in soft drinks and milk, and are used in warm weather for cooling and in cold weather for warming. Such drinking seems to also be mediated through the taste buds, which communicate with the brain in a kind of “reward system”, the mechanisms of which are just beginning to be understood. This bias in the way human beings rehydrate themselves may be advantageous because it allows water losses to be replaced before thirst-producing dehydration takes place. Unfortunately, this bias also carries some disadvantages. Drinking fluids other than water can contribute to an intake of caloric nutrients in excess of requirements, or in alcohol consumption that, in some people, may insidiously bring about dependence. For example, total fluid intake increased from 79 fluid ounces in 1989 to 100 fluid ounces in 2002 among US adults, with the difference representing intake of caloric beverages. 19
Effects of aging on fluid intake regulation
The thirst and fluid ingestion responses of older persons to a number of stimuli have been compared to those of younger persons. 20 Following water deprivation, older individuals are less thirsty and drink less fluid compared to younger persons. 21 , 22 The decrease in fluid consumption is predominantly due to a decrease in thirst, as the relationship between thirst and fluid intake is the same in young and old persons. Older persons drink insufficient amounts of water following fluid deprivation to replenish their body water deficit. 23 When dehydrated older persons are offered a highly palatable selection of drinks, this also fails to result in increased fluid intake. 23 The effects of increased thirst in response to an osmotic load have yielded variable responses, with one group reporting reduced osmotic thirst in older individuals 24 and one failing to find a difference. In a third study, young individuals ingested almost twice as much fluid as old persons, even though the older subjects had a much higher serum osmolality. 25
Overall, these studies support small changes in the regulation of thirst and fluid intake with aging. Defects in both osmoreceptors and baroreceptors appear to exist as do changes in the central regulatory mechanisms mediated by opioid receptors. 26 Because the elderly have low water reserves, it may be prudent for them to learn to drink regularly when not thirsty and to moderately increase their salt intake when they sweat. Better education on these principles may help prevent sudden hypotension and stroke or abnormal fatigue, which can lead to a vicious circle and eventually hospitalization.
Thermoregulation
Hydration status is critical to the body's process of temperature control. Body water loss through sweat is an important cooling mechanism in hot climates and in periods of physical activity. Sweat production is dependent upon environmental temperature and humidity, activity levels, and type of clothing worn. Water losses via skin (both insensible perspiration and sweating) can range from 0.3 L/h in sedentary conditions to 2.0 L/h in high activity in the heat, and intake requirements range from 2.5 to just over 3 L/day in adults under normal conditions, and can reach 6 L/day with high extremes of heat and activity. 27 , 28 Evaporation of sweat from the body results in cooling of the skin. However, if sweat loss is not compensated for with fluid intake, especially during vigorous physical activity, a hypohydrated state can occur with concomitant increases in core body temperature. Hypohydration from sweating results in a loss of electrolytes, as well as a reduction in plasma volume, and this can lead to increased plasma osmolality. During this state of reduced plasma volume and increased plasma osmolality, sweat output becomes insufficient to offset increases in core temperature. When fluids are given to maintain euhydration, sweating remains an effective compensation for increased core temperatures. With repeated exposure to hot environments, the body adapts to heat stress and cardiac output and stroke volume return to normal, sodium loss is conserved, and the risk for heat-stress-related illness is reduced. 29 Increasing water intake during this process of heat acclimatization will not shorten the time needed to adapt to the heat, but mild dehydration during this time may be of concern and is associated with elevations in cortisol, increased sweating, and electrolyte imbalances. 29
Children and the elderly have differing responses to ambient temperature and different thermoregulatory concerns than healthy adults. Children in warm climates may be more susceptible to heat illness than adults due to their greater surface area to body mass ratio, lower rate of sweating, and slower rate of acclimatization to heat. 30 , 31 Children may respond to hypohydration during activity with a higher relative increase in core temperature than adults, 32 and with a lower propensity to sweat, thus losing some of the benefits of evaporative cooling. However, it has been argued that children can dissipate a greater proportion of body heat via dry heat loss, and the concomitant lack of sweating provides a beneficial means of conserving water under heat stress. 30 Elders, in response to cold stress, show impairments in thermoregulatory vasoconstriction, and body water is shunted from plasma into the interstitial and intracellular compartments. 33 , 34 With respect to heat stress, water lost through sweating decreases the water content of plasma, and the elderly are less able to compensate for increased blood viscosity. 33 Not only do they have a physiological hypodipsia, but this can be exaggerated by central nervous system disease 35 and by dementia. 36 In addition, illness and limitations in daily living activities can further limit fluid intake. When reduced fluid intake is coupled with advancing age, there is a decrease in total body water. Older individuals have impaired renal fluid conservation mechanisms and, as noted above, have impaired responses to heat and cold stress. 33 , 34 All of these factors contribute to an increased risk of hypohydration and dehydration in the elderly.
With regard to physiology, the role of water in health is generally characterized in terms of deviations from an ideal hydrated state, generally in comparison to dehydration. The concept of dehydration encompasses both the process of losing body water and the state of dehydration. Much of the research on water and physical or mental functioning compares a euhydrated state, usually achieved by provision of water sufficient to overcome water loss, to a dehydrated state, which is achieved via withholding of fluids over time and during periods of heat stress or high activity. In general, provision of water is beneficial in individuals with a water deficit, but little research supports the notion that additional water in adequately hydrated individuals confers any benefit.
Physical performance
The role of water and hydration in physical activity, particularly in athletes and in the military, has been of considerable interest and is well-described in the scientific literature. 37 , – 39 During challenging athletic events, it is not uncommon for athletes to lose 6–10% of body weight through sweat, thus leading to dehydration if fluids have not been replenished. However, decrements in the physical performance of athletes have been observed under much lower levels of dehydration, i.e., as little as 2%. 38 Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. 40 , 41 Rehydration can reverse these deficits and reduce the oxidative stress induced by exercise and dehydration. 42 Hypohydration appears to have a more significant impact on high-intensity and endurance activity, such as tennis 43 and long-distance running, 44 than on anaerobic activities, 45 such as weight lifting, or on shorter-duration activities, such as rowing. 46
During exercise, individuals may not hydrate adequately when allowed to drink according to thirst. 32 After periods of physical exertion, voluntary fluid intake may be inadequate to offset fluid deficits. 1 Thus, mild-to-moderate dehydration can persist for some hours after the conclusion of physical activity. Research performed on athletes suggests that, principally at the beginning of the training season, they are at particular risk for dehydration due to lack of acclimatization to weather conditions or suddenly increased activity levels. 47 , 48 A number of studies show that performance in temperate and hot climates is affected to a greater degree than performance in cold temperatures. 41 , – 50 Exercise in hot conditions with inadequate fluid replacement is associated with hyperthermia, reduced stroke volume and cardiac output, decreases in blood pressure, and reduced blood flow to muscle. 51
During exercise, children may be at greater risk for voluntary dehydration. Children may not recognize the need to replace lost fluids, and both children as well as coaches need specific guidelines for fluid intake. 52 Additionally, children may require more time to acclimate to increases in environmental temperature than adults. 30 , 31 Recommendations are for child athletes or children in hot climates to begin athletic activities in a well-hydrated state and to drink fluids over and above the thirst threshold.
Cognitive performance
Water, or its lack (dehydration), can influence cognition. Mild levels of dehydration can produce disruptions in mood and cognitive functioning. This may be of special concern in the very young, very old, those in hot climates, and those engaging in vigorous exercise. Mild dehydration produces alterations in a number of important aspects of cognitive function such as concentration, alertness, and short-term memory in children (10–12 y), 32 young adults (18–25 y), 53 , – 56 and the oldest adults (50–82 y). 57 As with physical functioning, mild-to-moderate levels of dehydration can impair performance on tasks such as short-term memory, perceptual discrimination, arithmetic ability, visuomotor tracking, and psychomotor skills. 53 , – 56 However, mild dehydration does not appear to alter cognitive functioning in a consistent manner. 53 , – 58 In some cases, cognitive performance was not significantly affected in ranges from 2% to 2.6% dehydration. 56 , 58 Comparing across studies, performance on similar cognitive tests was divergent under dehydration conditions. 54 , 56 In studies conducted by Cian et al., 53 , 54 participants were dehydrated to approximately 2.8% either through heat exposure or treadmill exercise. In both studies, performance was impaired on tasks examining visual perception, short-term memory, and psychomotor ability. In a series of studies using exercise in conjunction with water restriction as a means of producing dehydration, D'Anci et al. 56 observed only mild decrements in cognitive performance in healthy young men and women athletes. In these experiments, the only consistent effect of mild dehydration was significant elevations of subjective mood score, including fatigue, confusion, anger, and vigor. Finally, in a study using water deprivation alone over a 24-h period, no significant decreases in cognitive performance were seen with 2.6% dehydration. 58 It is therefore possible that heat stress may play a critical role in the effects of dehydration on cognitive performance.
Reintroduction of fluids under conditions of mild dehydration can reasonably be expected to reverse dehydration-induced cognitive deficits. Few studies have examined how fluid reintroduction may alleviate the negative effects of dehydration on cognitive performance and mood. One study 59 examined how water ingestion affected arousal and cognitive performance in young people following a period of 12-h water restriction. While cognitive performance was not affected by either water restriction or water consumption, water ingestion affected self-reported arousal. Participants reported increased alertness as a function of water intake. Rogers et al. 60 observed a similar increase in alertness following water ingestion in both high- and low-thirst participants. Water ingestion, however, had opposite effects on cognitive performance as a function of thirst. High-thirst participants' performance on a cognitively demanding task improved following water ingestion, but low-thirst participants' performance declined. In summary, hydration status consistently affected self-reported alertness, but effects on cognition were less consistent.
Several recent studies have examined the utility of providing water to school children on attentiveness and cognitive functioning in children. 61 , – 63 In these experiments, children were not fluid restricted prior to cognitive testing, but were allowed to drink as usual. Children were then provided with a drink or no drink 20–45 min before the cognitive test sessions. In the absence of fluid restriction and without physiological measures of hydration status, the children in these studies should not be classified as dehydrated. Subjective measures of thirst were reduced in children given water, 62 and voluntary water intake in children varied from 57 mL to 250 mL. In these studies, as in the studies in adults, the findings were divergent and relatively modest. In the research led by Edmonds et al., 61 , 62 children in the groups given water showed improvements in visual attention. However, effects on visual memory were less consistent, with one study showing no effects of drinking water on a spot-the-difference task in 6–7-year-old children 61 and the other showing a significant improvement in a similar task in 7–9-year-old children. 62 In the research described by Benton and Burgess, 63 memory performance was improved by provision of water but sustained attention was not altered with provision of water in the same children.
Taken together, these studies indicate that low-to-moderate dehydration may alter cognitive performance. Rather than indicating that the effects of hydration or water ingestion on cognition are contradictory, many of the studies differ significantly in methodology and in measurement of cognitive behaviors. These variances in methodology underscore the importance of consistency when examining relatively subtle chances in overall cognitive performance. However, in those studies in which dehydration was induced, most combined heat and exercise; this makes it difficult to disentangle the effects of dehydration on cognitive performance in temperate conditions from the effects of heat and exercise. Additionally, relatively little is known about the mechanism of mild dehydration's effects on mental performance. It has been proposed that mild dehydration acts as a physiological stressor that competes with and draws attention from cognitive processes. 64 However, research on this hypothesis is limited and merits further exploration.
Dehydration and delirium
Dehydration is a risk factor for delirium and for delirium presenting as dementia in the elderly and in the very ill. 65 , – 67 Recent work shows that dehydration is one of several predisposing factors for confusion observed in long-term-care residents 67 ; however, in this study, daily water intake was used as a proxy measure for dehydration rather than other, more direct clinical assessments such as urine or plasma osmolality. Older people have been reported as having reduced thirst and hypodipsia relative to younger people. In addition, fluid intake and maintenance of water balance can be complicated by factors such as disease, dementia, incontinence, renal insufficiency, restricted mobility, and drug side effects. In response to primary dehydration, older people have less thirst sensation and reduced fluid intakes in comparison to younger people. However, in response to heat stress, while older people still display a reduced thirst threshold, they do ingest comparable amounts of fluid to younger people. 20
Gastrointestinal function
Fluids in the diet are generally absorbed in the proximal small intestine, and the absorption rate is determined by the rate of gastric emptying to the small intestine. Therefore, the total volume of fluid consumed will eventually be reflected in water balance, but the rate at which rehydration occurs is dependent upon factors affecting the rate of delivery of fluids to the intestinal mucosa. The gastric emptying rate is generally accelerated by the total volume consumed and slowed by higher energy density and osmolality. 68 In addition to water consumed in food (1 L/day) and beverages (circa 2–3 L/day), digestive secretions account for a far greater portion of water that passes through and is absorbed by the gastrointestinal tract (circa 8 L/day). 69 The majority of this water is absorbed by the small intestine, with a capacity of up to 15 L/day with the colon absorbing some 5 L/day. 69
Constipation, characterized by slow gastrointestinal transit, small, hard stools, and difficulty in passing stool, has a number of causes, including medication use, inadequate fiber intake, poor diet, and illness. 70 Inadequate fluid consumption is touted as a common culprit in constipation, and increasing fluid intake is a frequently recommended treatment. Evidence suggests, however, that increasing fluids is only useful to individuals in a hypohydrated state, and is of little utility in euhydrated individuals. 70 In young children with chronic constipation, increasing daily water intake by 50% did not affect constipation scores. 71 For Japanese women with low fiber intake, concomitant low water intake in the diet is associated with increased prevalence of constipation. 72 In older individuals, low fluid intake is a predictor for increased levels of acute constipation, 73 , 74 with those consuming the least amount of fluid having over twice the frequency of constipation episodes than those consuming the most fluid. In one trial, researchers compared the utility of carbonated mineral water in reducing functional dyspepsia and constipation scores to tap water in individuals with functional dyspepsia. 75 When comparing carbonated mineral water to tap water, participants reported improvements in subjective gastric symptoms, but there were no significant improvements in gastric or intestinal function. The authors indicate it is not possible to determine to what degree the mineral content of the two waters contributed to perceived symptom relief, as the mineral water contained greater levels of magnesium and calcium than the tap water. The available evidence suggests that increased fluid intake should only be indicated in individuals in a hypohydrated state. 69 , 71
Significant water loss can occur through the gastrointestinal tract, and this can be of great concern in the very young. In developing countries, diarrheal diseases are a leading cause of death in children, resulting in approximately 1.5–2.5 million deaths per year. 76 Diarrheal illness results not only in a reduction in body water, but also in potentially lethal electrolyte imbalances. Mortality in such cases can many times be prevented with appropriate oral rehydration therapy, by which simple dilute solutions of salt and sugar in water can replace fluid lost by diarrhea. Many consider application of oral rehydration therapy to be one of the significant public health developments of the last century. 77
Kidney function
As noted above, the kidney is crucial in regulating water balance and blood pressure as well as removing waste from the body. Water metabolism by the kidney can be classified into regulated and obligate. Water regulation is hormonally mediated, with the goal of maintaining a tight range of plasma osmolality (between 275 and 290 mOsm/kg). Increases in plasma osmolality and activation of osmoreceptors (intracellular) and baroreceptors (extracellular) stimulate hypothalamic release of arginine vasopressin (AVP). AVP acts at the kidney to decrease urine volume and promote retention of water, and the urine becomes hypertonic. With decreased plasma osmolality, vasopressin release is inhibited, and the kidney increases hypotonic urinary output.
In addition to regulating fluid balance, the kidneys require water for the filtration of waste from the bloodstream and excretion via urine. Water excretion via the kidney removes solutes from the blood, and a minimum obligate urine volume is required to remove the solute load with a maximum output volume of 1 L/h. 78 This obligate volume is not fixed, but is dependent upon the amount of metabolic solutes to be excreted and levels of AVP. Depending on the need for water conservation, basal urine osmolality ranges from 40 mOsm/kg to a maximum of 1,400 mOsm/kg. 78 The ability to both concentrate and dilute urine decreases with age, with a lower value of 92 mOsm/kg and an upper range falling between 500 and 700 mOsm/kg for individuals over the age of 70 years. 79 , – 81 Under typical conditions, in an average adult, urine volume of 1.5 to 2.0 L/day would be sufficient to clear a solute load of 900 to 1,200 mOsm/day. During water conservation and the presence of AVP, this obligate volume can decrease to 0.75–1.0 L/day and during maximal diuresis up to 20 L/day can be required to remove the same solute load. 78 , – 81 In cases of water loading, if the volume of water ingested cannot be compensated for with urine output, having overloaded the kidney's maximal output rate, an individual can enter a hyponatremic state.
Heart function and hemodynamic response
Blood volume, blood pressure, and heart rate are closely linked. Blood volume is normally tightly regulated by matching water intake and water output, as described in the section on kidney function. In healthy individuals, slight changes in heart rate and vasoconstriction act to balance the effect of normal fluctuations in blood volume on blood pressure. 82 Decreases in blood volume can occur, through blood loss (or blood donation), or loss of body water through sweat, as seen with exercise. Blood volume is distributed differently relative to the position of the heart, whether supine or upright, and moving from one position to the other can lead to increased heart rate, a fall in blood pressure, and, in some cases, syncope. This postural hypotension (or orthostatic hypotension) can be mediated by drinking 300–500 mL of water. 83 , 84 Water intake acutely reduces heart rate and increases blood pressure in both normotensive and hypertensive individuals. 85 These effects of water intake on the pressor effect and heart rate occur within 15–20 min of drinking water and can last for up to 60 min. Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope. 86 The effect of water intake in these situations is thought to be due to effects on the sympathetic nervous system rather than to changes in blood volume. 83 , 84 Interestingly, in rare cases, individuals may experience bradycardia and syncope after swallowing cold liquids. 87 , – 89 While swallow syncope can be seen with substances other than water, swallow syncope further supports the notion that the result of water ingestion in the pressor effect has both a neural component as well as a cardiac component.
Water deprivation and dehydration can lead to the development of headache. 90 Although this observation is largely unexplored in the medical literature, some observational studies indicate that water deprivation, in addition to impairing concentration and increasing irritability, can serve as a trigger for migraine and can also prolong migraine. 91 , 92 In those with water deprivation-induced headache, ingestion of water provided relief from headache in most individuals within 30 min to 3 h. 92 It is proposed that water deprivation-induced headache is the result of intracranial dehydration and total plasma volume. Although provision of water may be useful in relieving dehydration-related headache, the utility of increasing water intake for the prevention of headache is less well documented.
The folk wisdom that drinking water can stave off headaches has been relatively unchallenged, and has more traction in the popular press than in the medical literature. Recently, one study examined increased water intake and headache symptoms in headache patients. 93 In this randomized trial, patients with a history of different types of headache, including migraine and tension headache, were either assigned to a placebo condition (a nondrug tablet) or the increased water condition. In the water condition, participants were instructed to consume an additional volume of 1.5 L water/day on top of what they already consumed in foods and fluids. Water intake did not affect the number of headache episodes, but it was modestly associated with reduction in headache intensity and reduced duration of headache. The data from this study suggest that the utility of water as prophylaxis is limited in headache sufferers, and the ability of water to reduce or prevent headache in the broader population remains unknown.
One of the more pervasive myths regarding water intake is its relation to improvements of the skin or complexion. By improvement, it is generally understood that individuals are seeking to have a more “moisturized” look to the surface skin, or to minimize acne or other skin conditions. Numerous lay sources such as beauty and health magazines as well as postings on the Internet suggest that drinking 8–10 glasses of water a day will “flush toxins from the skin” and “give a glowing complexion” despite a general lack of evidence 94 , 95 to support these proposals. The skin, however, is important for maintaining body water levels and preventing water loss into the environment.
The skin contains approximately 30% water, which contributes to plumpness, elasticity, and resiliency. The overlapping cellular structure of the stratum corneum and lipid content of the skin serves as “waterproofing” for the body. 96 Loss of water through sweat is not indiscriminate across the total surface of the skin, but is carried out by eccrine sweat glands, which are evenly distributed over most of the body surface. 97 Skin dryness is usually associated with exposure to dry air, prolonged contact with hot water and scrubbing with soap (both strip oils from the skin), medical conditions, and medications. While more serious levels of dehydration can be reflected in reduced skin turgor, 98 , 99 with tenting of the skin acting as a flag for dehydration, overt skin turgor in individuals with adequate hydration is not altered. Water intake, particularly in individuals with low initial water intake, can improve skin thickness and density as measured by sonogram, 100 offsets transepidermal water loss, and can improve skin hydration. 101 Adequate skin hydration, however, is not sufficient to prevent wrinkles or other signs of aging, which are related to genetics and to sun and environmental damage. Of more utility to individuals already consuming adequate fluids is the use of topical emollients; these will improve skin barrier function and improve the look and feel of dry skin. 102 , 103
Many chronic diseases have multifactorial origins. In particular, differences in lifestyle and the impact of environment are known to be involved and constitute risk factors that are still being evaluated. Water is quantitatively the most important nutrient. In the past, scientific interest with regard to water metabolism was mainly directed toward the extremes of severe dehydration and water intoxication. There is evidence, however, that mild dehydration may also account for some morbidities. 4 , 104 There is currently no consensus on a “gold standard” for hydration markers, particularly for mild dehydration. As a consequence, the effects of mild dehydration on the development of several disorders and diseases have not been well documented.
There is strong evidence showing that good hydration reduces the risk of urolithiasis (see Table 2 for evidence categories). Less strong evidence links good hydration with reduced incidence of constipation, exercise asthma, hypertonic dehydration in the infant, and hyperglycemia in diabetic ketoacidosis. Good hydration is associated with a reduction in urinary tract infections, hypertension, fatal coronary heart disease, venous thromboembolism, and cerebral infarct, but all these effects need to be confirmed by clinical trials. For other conditions such as bladder or colon cancer, evidence of a preventive effect of maintaining good hydration is not consistent (see Table 3 ).
Categories of evidence used in evaluating the quality of reports.
Data adapted from Manz. 104
Summary of evidence for association of hydration status with chronic diseases.
Categories of evidence: described in Table 2 .
Water consumption, water requirements, and energy intake are linked in fairly complex ways. This is partially because physical activity and energy expenditures affect the need for water but also because a large shift in beverage consumption over the past century or more has led to consumption of a significant proportion of our energy intake from caloric beverages. Nonregulatory beverage intake, as noted earlier, has assumed a much greater role for individuals. 19 This section reviews current patterns of water intake and then refers to a full meta-analysis of the effects of added water on energy intake. This includes adding water to the diet and water replacement for a range of caloric and diet beverages, including sugar-sweetened beverages, juice, milk, and diet beverages. The third component is a discussion of water requirements and suggestions for considering the use of mL water/kcal energy intake as a metric.
Patterns and trends of water consumption
Measurement of total fluid water consumption in free-living individuals is fairly new in focus. As a result, the state of the science is poorly developed, data are most likely fairly incomplete, and adequate validation of the measurement techniques used is not available. Presented here are varying patterns and trends of water intake for the United States over the past three decades followed by a brief review of the work on water intake in Europe.
There is really no existing information to support an assumption that consumption of water alone or beverages containing water affects hydration differentially. 3 , 105 Some epidemiological data suggest water might have different metabolic effects when consumed alone rather than as a component of caffeinated or flavored or sweetened beverages; however, these data are at best suggestive of an issue deserving further exploration. 106 , 107 As shown below, the research of Ershow et al. indicates that beverages not consisting solely of water do contain less than 100% water.
One study in the United States has attempted to examine all the dietary sources of water. 16 , 17 These data are cited in Table 4 as the Ershow study and were based on National Food Consumption Survey food and fluid intake data from 1977–1978. These data are presented in Table 4 for children aged 2–18 years (Panel A) and for adults aged 19 years and older (Panel B). Ershow et al. 16 , 17 spent a great deal of time working out ways to convert USDA dietary data into water intake, including water absorbed during the cooking process, water in food, and all sources of drinking water.
Beverage pattern trends in the United States for children aged 2–18 years and adults aged 19 years and older, (nationally representative).
Note: The data are age and sex adjusted to 1965.
Values stem from the Ershow calculations. 16
These researchers created a number of categories and used a range of factors measured in other studies to estimate the water categories. The water that is found in food, based on food composition table data, was 393 mL for children. The water that was added as a result of cooking (e.g., rice) was 95 mL. Water consumed as a beverage directly as water was 624 mL. The water found in other fluids, as noted, comprised the remainder of the milliliters, with the highest levels in whole-fat milk and juices (506 mL). There is a small discrepancy between the Ershow data regarding total fluid intake measures for these children and the normal USDA figures. That is because the USDA does not remove milk fats and solids, fiber, and other food constituents found in beverages, particularly juice and milk.
A key point illustrated by these nationally representative US data is the enormous variability between survey waves in the amount of water consumed (see Figure 1 , which highlights the large variation in water intake as measured in these surveys). Although water intake by adults and children increased and decreased at the same time, for reasons that cannot be explained, the variation was greater among children than adults. This is partly because the questions the surveys posed varied over time and there was no detailed probing for water intake, because the focus was on obtaining measures of macro- and micronutrients. Dietary survey methods used in the past have focused on obtaining data on foods and beverages containing nutrient and non-nutritive sweeteners but not on water. Related to this are the huge differences between the the USDA surveys and the National Health and Nutrition Examination Survey (NHANES) performed in 1988–1994 and in 1999 and later. In addition, even the NHANES 1999–2002 and 2003–2006 surveys differ greatly. These differences reflect a shift in the mode of questioning with questions on water intake being included as part of a standard 24-h recall rather than as stand-alone questions. Water intake was not even measured in 1965, and a review of the questionnaires and the data reveals clear differences in the way the questions have been asked and the limitations on probes regarding water intake. Essentially, in the past people were asked how much water they consumed in a day and now they are asked for this information as part of a 24-h recall survey. However, unlike for other caloric and diet beverages, there are limited probes for water alone. The results must thus be viewed as crude approximations of total water intake without any strong research to show if they are over- or underestimated. From several studies of water and two ongoing randomized controlled trials performed by us, it is clear that probes that include consideration of all beverages and include water as a separate item result in the provision of more complete data.
Water consumption trends from USDA and NHANES surveys (mL/day/capita), nationally representative. Note: this includes water from fluids only, excluding water in foods. Sources for 1965, 1977–1978, 1989–1991, and 1994–1998, are USDA. Others are NHANES and 2005–2006 is joint USDA and NHANES.
Water consumption data for Europe are collected far more selectively than even the crude water intake questions from NHANES. A recent report from the European Food Safety Agency provides measures of water consumption from a range of studies in Europe. 4 , – 109 Essentially, what these studies show is that total water intake is lower across Europe than in the United States. As with the US data, none are based on long-term, carefully measured or even repeated 24-h recall measures of water intake from food and beverages. In an unpublished examination of water intake in UK adults in 1986–1987 and in 2001–2002, Popkin and Jebb have found that although intake increased by 226 mL/day over this time period, it was still only 1,787 mL/day in the latter period (unpublished data available from BP); this level is far below the 2,793 mL/day recorded in the United States for 2005–2006 or the earlier US figures for comparably aged adults.
A few studies have been performed in the United States and Europe utilizing 24-h urine and serum osmolality measures to determine total water turnover and hydration status. Results of these studies suggest that US adults consume over 2,100 mL of water per day while adults in Europe consume less than half a liter. 4 , 110 Data on total urine collection would appear to be another useful measure for examining total water intake. Of course, few studies aside from the Donald Study of an adolescent cohort in Germany have collected such data on population levels for large samples. 109
Effects of water consumption on overall energy intake
There is an extensive body of literature that focuses on the impact of sugar-sweetened beverages on weight and the risk of obesity, diabetes, and heart disease; however, the perspective of providing more water and its impact on health has not been examined. The literature on water does not address portion sizes; instead, it focuses mainly on water ad libitum or in selected portions compared with other caloric beverages. A detailed meta-analysis of the effects of water intake alone (i.e., adding additional water) and as a replacement for sugar-sweetened beverages, juice, milk, and diet beverages appears elsewhere. 111
In general, the results of this review suggest that water, when consumed in place of sugar-sweetened beverages, juice, and milk, is linked with reduced energy intake. This finding is mainly derived from clinical feeding studies but also from one very good randomized, controlled school intervention and several other epidemiological and intervention studies. Aside from the issue of portion size, factors such as the timing of beverage and meal intake (i.e., the delay between consumption of the beverage and consumption of the meal) and types of caloric sweeteners remain to be considered. However, when beverages are consumed in normal free-living conditions in which five to eight daily eating occasions are the norm, the delay between beverage and meal consumption may matter less. 112 , – 114
The literature on the water intake of children is extremely limited. However, the excellent German school intervention with water suggests the effects of water on the overall energy intake of children might be comparable to that of adults. 115 In this German study, children were educated on the value of water and provided with special filtered drinking fountains and water bottles in school. The intervention schoolchildren increased their water intake by 1.1 glasses/day ( P < 0.001) and reduced their risk of overweight by 31% (OR = 0.69, P = 0.40).
Classically, water data are examined in terms of milliliters (or some other measure of water volume consumed per capita per day by age group). This measure does not link fluid intake and caloric intake. Disassociation of fluid and calorie intake is difficult for clinicians dealing with older persons with reduced caloric intake. This milliliter water measure assumes some mean body size (or surface area) and a mean level of physical activity – both of which are determinants of not only energy expenditure but also water balance. Children are dependent on adults for access to water, and studies suggest that their larger surface area to volume ratio makes them susceptible to changes in skin temperatures linked with ambient temperature shifts. 116 One option utilized by some scholars is to explore food and beverage intake in milliliters per kilocalorie (mL/kcal), as was done in the 1989 US recommended dietary allowances. 4 , 117 This is an option that is interpretable for clinicians and which incorporates, in some sense, body size or surface area and activity. Its disadvantage is that water consumed with caloric beverages affects both the numerator and the denominator; however, an alternative measure that could be independent of this direct effect on body weight and/or total caloric intake is not presently known.
Despite its critical importance in health and nutrition, the array of available research that serves as a basis for determining requirements for water or fluid intake, or even rational recommendations for populations, is limited in comparison with most other nutrients. While this deficit may be partly explained by the highly sensitive set of neurophysiological adaptations and adjustments that occur over a large range of fluid intakes to protect body hydration and osmolarity, this deficit remains a challenge for the nutrition and public health community. The latest official effort at recommending water intake for different subpopulations occurred as part of the efforts to establish Dietary Reference Intakes in 2005, as reported by the Institute of Medicine of the National Academies of Science. 3 As a graphic acknowledgment of the limited database upon which to express estimated average requirements for water for different population groups, the Committee and the Institute of Medicine stated: “While it might appear useful to estimate an average requirement (an EAR) for water, an EAR based on data is not possible.” Given the extreme variability in water needs that are not solely based on differences in metabolism, but also on environmental conditions and activities, there is not a single level of water intake that would assure adequate hydration and optimum health for half of all apparently healthy persons in all environmental conditions. Thus, an adequate intake (AI) level was established in place of an EAR for water.
The AIs for different population groups were set as the median water intakes for populations, as reported in the National Health and Nutrition Examination Surveys; however, the intake levels reported in these surveys varied greatly based on the survey years (e.g., NHANES 1988–1994 versus NHANES 1999–2002) and were also much higher than those found in the USDA surveys (e.g., 1989–1991, 1994–1998, or 2005–2006). If the AI for adults, as expressed in Table 5 , is taken as a recommended intake, the wisdom of converting an AI into a recommended water or fluid intake seems questionable. The first problem is the almost certain inaccuracy of the fluid intake information from the national surveys, even though that problem may also exist for other nutrients. More importantly, from the standpoint of translating an AI into a recommended fluid intake for individuals or populations, is the decision that was made when setting the AI to add an additional roughly 20% of water intake, which is derived from some foods in addition to water and beverages. While this may have been a legitimate effort to use total water intake as a basis for setting the AI, the recommendations that derive from the IOM report would be better directed at recommendations for water and other fluid intake on the assumption that the water content of foods would be a “passive” addition to total water intake. In this case, the observations of the dietary reference intake committee that it is necessary for water intake to meet needs imposed by metabolism and environmental conditions must be extended to consider three added factors, namely body size, gender, and physical activity. Those are the well-studied factors that allow a rather precise measurement and determination of energy intake requirements. It is, therefore, logical that those same factors might underlie recommendations to meet water intake needs in the same populations and individuals. Consideration should also be given to the possibility that water intake needs would best be expressed relative to the calorie requirements, as is done regularly in the clinical setting, and data should be gathered to this end through experimental and population research.
Water requirements expressed in relation to energy recommendations.
AI for total fluids derived from dietary reference intakes for water, potassium, sodium, chloride, and sulphate.
Ratios for water intake based on the AI for water in liters/day calculated using EER for each range of physical activity. EER adapted from the Institute of Medicine Dietary Reference Intakes Macronutrients Report, 2002.
It is important to note that only a few countries include water on their list of nutrients. 118 The European Food Safety Authority is developing a standard for all of Europe. 105 At present, only the United States and Germany provide AI values for water. 3 , 119
Another approach to the estimation of water requirements, beyond the limited usefulness of the AI or estimated mean intake, is to express water intake requirements in relation to energy requirements in mL/kcal. An argument for this approach includes the observation that energy requirements for each age and gender group are strongly evidence-based and supported by extensive research taking into account both body size and activity level, which are crucial determinants of energy expenditure that must be met by dietary energy intake. Such measures of expenditure have used highly accurate methods, such as doubly labeled water; thus, estimated energy requirements have been set based on solid data rather than the compromise inherent in the AIs for water. Those same determinants of energy expenditure and recommended intake are also applicable to water utilization and balance, and this provides an argument for pegging water/fluid intake recommendations to the better-studied energy recommendations. The extent to which water intake and requirements are determined by energy intake and expenditure is understudied, but in the clinical setting it has long been practice to supply 1 mL/kcal administered by tube to patients who are unable to take in food or fluids. Factors such as fever or other drivers of increased metabolism affect both energy expenditure and fluid loss and are thus linked in clinical practice. This concept may well deserve consideration in the setting of population intake goals.
Finally, for decades there has been discussion about expressing nutrient requirements per 1,000 kcal so that a single number would apply reasonably across the spectrum of age groups. This idea, which has never been adopted by the Institute of Medicine and the National Academies of Science, may lend itself to an improved expression of water/fluid intake requirements, which must eventually replace the AIs. Table 5 presents the IOM water requirements and then develops a ratio of mL/kcal based on them. The European Food Safety Agency refers positively to the possibility of expressing water intake recommendations in mL/kcal as a function of energy requirements. 105 Outliers in the adult male categories, which reach ratios as high as 1.5, may well be based on the AI data from the United States, which are above those in the more moderate and likely more accurate European recommendations.
The topic of utilizing mL/kcal to examine water intake and water gaps is explored in Table 6 , which takes the full set of water intake AIs for each age-gender grouping and examines total intake. The data suggest a high level of fluid deficiency. Since a large proportion of fluids in the United States is based on caloric beverages and this proportion has changed markedly over the past 30 years, fluid intake increases both the numerator and the denominator of this mL/kcal relationship. Nevertheless, even using 1 mL/kcal as the AI would leave a gap for all children and adolescents. The NHANES physical activity data were also translated into METS/day to categorize all individuals by physical activity level and thus varying caloric requirements. Use of these measures reveals a fairly large fluid gap, particularly for adult males as well as children ( Table 6 ).
Water intake and water intake gaps based on US Water Adequate Intake Recommendations (based on utilization of water and physical activity data from NHANES 2005–2006).
Note: Recommended water intake for actual activity level is the upper end of the range for moderate and active.
A weighted average for the proportion of individuals in each METS-based activity level.
This review has pointed out a number of issues related to water, hydration, and health. Since water is undoubtedly the most important nutrient and the only one for which an absence will prove lethal within days, understanding of water measurement and water requirements is very important. The effects of water on daily performance and short- and long-term health are quite clear. The existing literature indicates there are few negative effects of water intake while the evidence for positive effects is quite clear.
Little work has been done to measure total fluid intake systematically, and there is no understanding of measurement error and best methods of understanding fluid intake. The most definitive US and European documents on total water requirements are based on these extant intake data. 3 , 105 The absence of validation methods for water consumption intake levels and patterns represents a major gap in knowledge. Even varying the methods of probing in order to collect better water recall data has been little explored.
On the other side of the issue is the need to understand total hydration status. There are presently no acceptable biomarkers of hydration status at the population level, and controversy exists about the current knowledge of hydration status among older Americans. 6 , 120 Thus, while scholars are certainly focused on attempting to create biomarkers for measuring hydration status at the population level, the topic is currently understudied.
As noted, the importance of understanding the role of fluid intake on health has emerged as a topic of increasing interest, partially because of the trend toward rising proportions of fluids being consumed in the form of caloric beverages. The clinical, epidemiological, and intervention literature on the effects of added water on health are covered in a related systematic review. 111 The use of water as a replacement for sugar-sweetened beverages, juice, or whole milk has clear effects in that energy intake is reduced by about 10–13% of total energy intake. However, only a few longer-term systematic interventions have investigated this topic and no randomized, controlled, longer-term trials have been published to date. There is thus very minimal evidence on the effects of just adding water to the diet and of replacing water with diet beverages.
There are many limitations to this review. One certainly is the lack of discussion of potential differences in the metabolic functioning of different types of beverages. 121 Since the literature in this area is sparse, however, there is little basis for delving into it at this point. A discussion of the potential effects of fructose (from all caloric sweeteners when consumed in caloric beverages) on abdominal fat and all of the metabolic conditions directly linked with it (e.g., diabetes) is likewise lacking. 122 , – 125 A further limitation is the lack of detailed review of the array of biomarkers being considered to measure hydration status. Since there is no measurement in the field today that covers more than a very short time period, except for 24-hour total urine collection, such a discussion seems premature.
Some ways to examine water requirements have been suggested in this review as a means to encourage more dialogue on this important topic. Given the significance of water to our health and of caloric beverages to our total energy intake, as well as the potential risks of nutrition-related noncommunicable diseases, understanding both the requirements for water in relation to energy requirements, and the differential effects of water versus other caloric beverages, remain important outstanding issues.
This review has attempted to provide some sense of the importance of water to our health, its role in relationship to the rapidly increasing rates of obesity and other related diseases, and the gaps in present understanding of hydration measurement and requirements. Water is essential to our survival. By highlighting its critical role, it is hoped that the focus on water in human health will sharpen.
The authors wish to thank Ms. Frances L. Dancy for administrative assistance, Mr. Tom Swasey for graphics support, Dr. Melissa Daniels for assistance, and Florence Constant (Nestle's Water Research) for advice and references.
This work was supported by the Nestlé Waters, Issy-les-Moulineaux, France, 5ROI AGI0436 from the National Institute on Aging Physical Frailty Program, and NIH R01-CA109831 and R01-CA121152.
Declaration of interest
The authors have no relevant interests to declare.
Nicolaidis S . Physiology of thirst . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 247 .
Google Scholar
Jequier E Constant F . Water as an essential nutrient: the physiological basis of hydration . Eur J Clin Nutr. 2010 ; 64 : 115 – 123 .
Institute of Medicine . Panel on Dietary Reference Intakes for Electrolytes and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate . Washington, DC: The National Academies Press ; 2005 .
Manz F Wentz A . Hydration status in the United States and Germany . Nutr Rev. 2005 ; 63 :(Suppl): S55 – S62 .
European Food Safety Authority . Scientific opinion of the Panel on Dietetic Products Nutrition and Allergies. Draft dietary reference values for water . EFSA J. 2008 : 2 – 49 .
Stookey JD . High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III . J Am Diet Assoc. 2005 ; 105 : 1231 – 1239 .
Armstrong LE . Hydration assessment techniques . Nutr Rev. 2005 ; 63 (Suppl): S40 – S54 .
Bar-David Y Urkin J Kozminsky E . The effect of voluntary dehydration on cognitive functions of elementary school children . Acta Paediatr. 2005 ; 94 : 1667 – 1673 .
Shirreffs S . Markers of hydration status . J Sports Med Phys Fitness. 2000 ; 40 : 80 – 84 .
Popowski L Oppliger R Lambert G Johnson R Johnson A Gisolfi C . Blood and urinary measures of hydration status during progressive acute dehydration . Med Sci Sports Exerc. 2001 ; 33 : 747 – 753 .
Bar-David Y Landau D Bar-David Z Pilpel D Philip M . Urine osmolality among elementary schoolchildren living in a hot climate: implications for dehydration . Ambulatory Child Health. 1998 ; 4 : 393 – 397 .
Fadda R Rapinett G Grathwohl D Parisi M Fanari R Schmitt J . The Benefits of Drinking Supplementary Water at School on Cognitive Performance in Children . Washington, DC: International Society for Developmental Psychobiology ; 2008 : Abstract from poster session at the 41st Annual Conference of International Society for Psychobiology. Abstracts published in: Developmental Psychobiology 2008: v. 50(7): 726 . http://www3.interscience.wiley.com/cgi-bin/fulltext/121421361/PDFSTART
Eckhardt CL Adair LS Caballero B , et al . Estimating body fat from anthropometry and isotopic dilution: a four-country comparison . Obes Res. 2003 ; 11 : 1553 – 1562 .
Moreno LA Sarria A Popkin BM . The nutrition transition in Spain: a European Mediterranean country . Eur J Clin Nutr. 2002 ; 56 : 992 – 1003 .
Lee MJ Popkin BM Kim S . The unique aspects of the nutrition transition in South Korea: the retention of healthful elements in their traditional diet . Public Health Nutr. 2002 ; 5 : 197 – 203 .
Ershow AG Brown LM Cantor KP . Intake of tapwater and total water by pregnant and lactating women . Am J Public Health. 1991 ; 81 : 328 – 334 .
Ershow AG Cantor KP . Total Water and Tapwater Intake in the United States: Population-Based Estimates of Quantities and Sources . Bethesda, MD: FASEB/LSRO ; 1989 .
Ramsay DJ . Homeostatic control of water balance . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 9 – 18 .
Duffey K Popkin BM . Shifts in patterns and consumption of beverages between 1965 and 2002 . Obesity. 2007 ; 15 : 2739 – 2747 .
Morley JE Miller DK Zdodowski C Guitierrez B Perry IIIHM . Fluid intake, hydration and aging . In: Arnaud MJ , ed. Hydration Throughout Life: International Conference Vittel (France) . Montrouge: John Libbey Eurotext ; 1998 : 247 .
Phillips PA Rolls BJ Ledingham JG , et al . Reduced thirst after water deprivation in healthy elderly men . N Engl J Med. 1984 ; 311 : 753 – 759 .
Mack GW Weseman CA Langhans GW Scherzer H Gillen CM Nadel ER . Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation . J Appl Physiol. 1994 ; 76 : 1615 – 1623 .
Phillips PA Johnston CI Gray L . Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people . Age Ageing. 1993 ; 22 : S26 – S33 .
Phillips PA Bretherton M Johnston CI Gray L . Reduced osmotic thirst in healthy elderly men . Am J Physiol. 1991 ; 261 : R166 – R171 .
Davies I O'Neill PA McLean KA Catania J Bennett D . Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load . Age Ageing. 1995 ; 24 : 151 – 159 .
Silver AJ Morley JE . Role of the opioid system in the hypodipsia associated with aging . J Am Geriatr Soc. 1992 ; 40 : 556 – 560 .
Sawka MN Latzka WA Matott RP Montain SJ . Hydration effects on temperature regulation . Int J Sports Med. 1998 ; 19 (Suppl 2 ): S108 – S110 .
Sawka MN Cheuvront SN Carter R 3rd . Human water needs . Nutr Rev. 2005 ; 63 (Suppl): S30 – S39 .
Armstrong LE. Heat acclimatization . In: TD Fahey, ed. Encyclopedia of Sports Medicine and Science . Internet Society for Sport Science: http://sportsci.org . 10 March 1998 .
Falk B Dotan R . Children's thermoregulation during exercise in the heat: a revisit . Appl Physiol Nutr Metab. 2008 ; 33 : 420 – 427 .
Bytomski JR Squire DL . Heat illness in children . Curr Sports Med Rep. 2003 ; 2 : 320 – 324 .
Bar-Or O Dotan R Inbar O Rotshtein A Zonder H . Voluntary hypohydration in 10- to 12-year-old boys . J Appl Physiol. 1980 ; 48 : 104 – 108 .
Vogelaere P Pereira C . Thermoregulation and aging . Rev Port Cardiol. 2005 ; 24 : 747 – 761 .
Thompson-Torgerson CS Holowatz LA Kenney WL . Altered mechanisms of thermoregulatory vasoconstriction in aged human skin . Exerc Sport Sci Rev. 2008 ; 36 : 122 – 127 .
Miller PD Krebs RA Neal BJ McIntyre DO . Hypodipsia in geriatric patients . Am J Med. 1982 ; 73 : 354 – 356 .
Albert SG Nakra BR Grossberg GT Caminal ER . Drinking behavior and vasopressin responses to hyperosmolality in Alzheimer's disease . Int Psychogeriatr. 1994 ; 6 : 79 – 86 .
Maughan RJ Shirreffs SM Watson P . Exercise, heat, hydration and the brain . J Am Coll Nutr. 2007 ; 26 (Suppl): S604 – S612 .
Murray B . Hydration and physical performance . J Am Coll Nutr. 2007 ; 26 (Suppl): S542 – S548 .
Sawka MN Noakes TD . Does dehydration impair exercise performance? Med Sci Sports Exerc. 2007 ; 39 : 1209 – 1217 .
Montain SJ Coyle EF . Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise . J Appl Physiol. 1992 ; 73 : 1340 – 1350 .
Cheuvront SN . Carter R, 3rd, Sawka MN. Fluid balance and endurance exercise performance . Curr Sports Med Rep. 2003 ; 2 : 202 – 208 .
Paik IY Jeong MH Jin HE , et al . Fluid replacement following dehydration reduces oxidative stress during recovery . Biochem Biophys Res Commun. 2009 ; 383 : 103 – 107 .
Kovacs MS . A review of fluid and hydration in competitive tennis . Int J Sports Physiol Perform. 2008 ; 3 : 413 – 423 .
Cheuvront SN Montain SJ Sawka MN . Fluid replacement and performance during the marathon . Sports Med. 2007 ; 37 : 353 – 357 .
Cheuvront SN . Carter R, 3rd, Haymes EM, Sawka MN. No effect of moderate hypohydration or hyperthermia on anaerobic exercise performance . Med Sci Sports Exerc. 2006 ; 38 : 1093 – 1097 .
Penkman MA Field CJ Sellar CM Harber VJ Bell GJ . Effect of hydration status on high-intensity rowing performance and immune function . Int J Sports Physiol Perform. 2008 ; 3 : 531 – 546 .
Bergeron MF McKeag DB Casa DJ , et al . Youth football: heat stress and injury risk . Med Sci Sports Exerc. 2005 ; 37 : 1421 – 1430 .
Godek SF Godek JJ Bartolozzi AR . Hydration status in college football players during consecutive days of twice-a-day preseason practices . Am J Sports Med. 2005 ; 33 : 843 – 851 .
Cheuvront SN Carter R 3rd Castellani JW Sawka MN . Hypohydration impairs endurance exercise performance in temperate but not cold air . J Appl Physiol. 2005 ; 99 : 1972 – 1976 .
Kenefick RW Mahood NV Hazzard MP Quinn TJ Castellani JW . Hypohydration effects on thermoregulation during moderate exercise in the cold . Eur J Appl Physiol. 2004 ; 92 : 565 – 570 .
Maughan RJ Watson P Shirreffs SM . Heat and cold: what does the environment do to the marathon runner? Sports Med. 2007 ; 37 : 396 – 399 .
American Academy of Pediatrics . Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness . Pediatrics. 2000 ; 106 : 158 – 159 .
Cian C Barraud PA Melin B Raphel C . Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration . Int J Psychophysiol. 2001 ; 42 : 243 – 251 .
Cian C Koulmann PA Barraud PA Raphel C Jimenez C Melin B . Influence of variations of body hydration on cognitive performance . J Psychophysiol. 2000 ; 14 : 29 – 36 .
Gopinathan PM Pichan G Sharma VM . Role of dehydration in heat stress-induced variations in mental performance . Arch Environ Health. 1988 ; 43 : 15 – 17 .
D'Anci KE Vibhakar A Kanter JH Mahoney CR Taylor HA . Voluntary dehydration and cognitive performance in trained college athletes . Percept Mot Skills. 2009 ; 109 : 251 – 269 .
Suhr JA Hall J Patterson SM Niinisto RT . The relation of hydration status to cognitive performance in healthy older adults . Int J Psychophysiol. 2004 ; 53 : 121 – 125 .
Szinnai G Schachinger H Arnaud MJ Linder L Keller U . Effect of water deprivation on cognitive-motor performance in healthy men and women . Am J Physiol Regul Integr Comp Physiol. 2005 ; 289 : R275 – R280 .
Neave N Scholey AB Emmett JR Moss M Kennedy DO Wesnes KA . Water ingestion improves subjective alertness, but has no effect on cognitive performance in dehydrated healthy young volunteers . Appetite. 2001 ; 37 : 255 – 256 .
Rogers PJ Kainth A Smit HJ . A drink of water can improve or impair mental performance depending on small differences in thirst . Appetite. 2001 ; 36 : 57 – 58 .
Edmonds CJ Jeffes B . Does having a drink help you think? 6–7-year-old children show improvements in cognitive performance from baseline to test after having a drink of water . Appetite. 2009 ; 53 : 469 – 472 .
Edmonds CJ Burford D . Should children drink more water?: the effects of drinking water on cognition in children . Appetite. 2009 ; 52 : 776 – 779 .
Benton D Burgess N . The effect of the consumption of water on the memory and attention of children . Appetite. 2009 ; 53 : 143 – 146 .
Cohen S . After effects of stress on human performance during a heat acclimatization regimen . Aviat Space Environ. Med. 1983 ; 54 : 709 – 713 .
Culp KR Wakefield B Dyck MJ Cacchione PZ DeCrane S Decker S . Bioelectrical impedance analysis and other hydration parameters as risk factors for delirium in rural nursing home residents . J Gerontol A Biol Sci Med Sci. 2004 ; 59 : 813 – 817 .
Lawlor PG . Delirium and dehydration: some fluid for thought? Support Care Cancer. 2002 ; 10 : 445 – 454 .
Voyer P Richard S Doucet L Carmichael PH . Predisposing factors associated with delirium among demented long-term care residents . Clin Nurs Res. 2009 ; 18 : 153 – 171 .
Leiper JB . Intestinal water absorption – implications for the formulation of rehydration solutions . Int J Sports Med. 1998 ; 19 (Suppl 2 ): S129 – S132 .
Ritz P Berrut G . The importance of good hydration for day-to-day health . Nutr Rev. 2005 ; 63 : S6 – S13 .
Arnaud MJ . Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003 ; 57 (Suppl): S88 – S95 .
Young RJ Beerman LE Vanderhoof JA . Increasing oral fluids in chronic constipation in children . Gastroenterol Nurs. 1998 ; 21 : 156 – 161 .
Murakami K Sasaki S Okubo H , et al . Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women . Eur J Clin Nutr. 2007 ; 61 : 616 – 622 .
Lindeman RD Romero LJ Liang HC Baumgartner RN Koehler KM Garry PJ . Do elderly persons need to be encouraged to drink more fluids? J Gerontol A Biol Sci Med Sci. 2000 ; 55 : M361 – M365 .
Robson KM Kiely DK Lembo T . Development of constipation in nursing home residents . Dis Colon Rectum. 2000 ; 43 : 940 – 943 .
Cuomo R Grasso R Sarnelli G , et al . Effects of carbonated water on functional dyspepsia and constipation . Eur J Gastroenterol Hepatol. 2002 ; 14 : 991 – 999 .
Kosek M Bern C Guerrant RL . The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000 . Bull World Health Organ. 2003 ; 81 : 197 – 204 .
Atia AN Buchman AL . Oral rehydration solutions in non-cholera diarrhea: a review . Am J Gastroenterol. 2009 ; 104 : 2596 – 2604 , quiz 2605.
Schoen EJ . Minimum urine total solute concentration in response to water loading in normal men . J Appl Physiol. 1957 ; 10 : 267 – 270 .
Sporn IN Lancestremere RG Papper S . Differential diagnosis of oliguria in aged patients . N Engl J Med. 1962 ; 267 : 130 – 132 .
Brenner BM , ed. Brenner and Rector's The Kidney , 8th edn. Philadelphia, PA: Saunders Elsevier ; 2007 .
Lindeman RD Van Buren HC Raisz LG . Osmolar renal concentrating ability in healthy young men and hospitalized patients without renal disease . N Engl J Med. 1960 ; 262 : 1306 – 1309 .
Shirreffs SM Maughan RJ . Control of blood volume: long term and short term regulation . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 31 – 39 .
Schroeder C Bush VE Norcliffe LJ , et al . Water drinking acutely improves orthostatic tolerance in healthy subjects . Circulation. 2002 ; 106 : 2806 – 2811 .
Lu CC Diedrich A Tung CS , et al . Water ingestion as prophylaxis against syncope . Circulation. 2003 ; 108 : 2660 – 2665 .
Callegaro CC Moraes RS Negrao CE , et al . Acute water ingestion increases arterial blood pressure in hypertensive and normotensive subjects . J Hum Hypertens. 2007 ; 21 : 564 – 570 .
Ando SI Kawamura N Matsumoto M , et al . Simple standing test predicts and water ingestion prevents vasovagal reaction in the high-risk blood donors . Transfusion. 2009 ; 49 : 1630 – 1636 .
Brick JE Lowther CM Deglin SM . Cold water syncope . South Med J. 1978 ; 71 : 1579 – 1580 .
Farb A Valenti SA . Swallow syncope . Md Med J. 1999 ; 48 : 151 – 154 .
Casella F Diana A Bulgheroni M , et al . When water hurts . Pacing Clin Electrophysiol. 2009 ; 32 : e25 – e27 .
Shirreffs SM Merson SJ Fraser SM Archer DT . The effects of fluid restriction on hydration status and subjective feelings in man . Br J Nutr. 2004 ; 91 : 951 – 958 .
Blau J . Water deprivation: a new migraine precipitant . Headache. 2005 ; 45 : 757 – 759 .
Blau JN Kell CA Sperling JM . Water-deprivation headache: a new headache with two variants . Headache. 2004 ; 44 : 79 – 83 .
Spigt MG Kuijper EC Schayck CP , et al . Increasing the daily water intake for the prophylactic treatment of headache: a pilot trial . Eur J Neurol. 2005 ; 12 : 715 – 718 .
Valtin H . “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 x 8”? Am J Physiol Regul Integr Comp Physiol. 2002 ; 283 : R993 – 1004 .
Negoianu D Goldfarb S . Just add water . J Am Soc Nephrol. 2008 ; 19 : 1041 – 1043 .
Madison KC . Barrier function of the skin: “la raison d'etre” of the epidermis . J Invest Dermatol. 2003 ; 121 : 231 – 241 .
Champion RH Burton JL Ebling FJG , eds. Textbook of Dermatology (Rook) . Oxford: Blackwell ; 1992 .
Vivanti A Harvey K Ash S Battistutta D . Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008 ; 47 : 340 – 355 .
Colletti JE Brown KM Sharieff GQ Barata IA Ishimine P Committee APEM . The management of children with gastroenteritis and dehydration in the emergency department . J Emerg Med. 2009 .
Williams S Krueger N Davids M Kraus D Kerscher M . Effect of fluid intake on skin physiology: distinct differences between drinking mineral water and tap water . Int J Cosmet Sci. 2007 ; 29 : 131 – 138 .
Mac-Mary S Creidi P Marsaut D , et al . Assessment of effects of an additional dietary natural mineral water uptake on skin hydration in healthy subjects by dynamic barrier function measurements and clinic scoring . Skin Res Technol. 2006 ; 12 : 199 – 205 .
Warner RR Stone KJ Boissy YL . Hydration disrupts human stratum corneum ultrastructure . J Invest Dermatol. 2003 ; 120 : 275 – 284 .
Loden M . Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders . Am J Clin Dermatol. 2003 ; 4 : 771 – 788 .
Manz F Wentz A . The importance of good hydration for the prevention of chronic diseases . Nutr Rev. 2005 ; 63 (Suppl): S2 – S5 .
Panel on Dietetic Products Nutrition and Allergies . Dietary reference values for water Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies (Question No EFSA-Q-2005-015a) . EFSA J. 2009 ; 8 ( 3 ): 1459 .
Stookey JD Constant F Gardner C Popkin B . Replacing sweetened caloric beverages with drinking water is associated with lower energy intake . Obesity. 2007 ; 15 : 3013 – 3022 .
Stookey JD Constant F Gardner C Popkin BM . Drinking water is associated with weight loss . Obesity. 2008 ; 16 : 2481 – 2488 .
Turrini A Saba A Perrone D Cialfa E D'Amicis D . Food consumption patterns in Italy: the INN-CA Study 1994–1996 . Eur J Clin Nutr. 2001 ; 55 : 571 – 588 .
Sichert-Hellert W Kersting M Manz F . Fifteen year trends in water intake in German children and adolescents: results of the DONALD Study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study . Acta Paediatr. 2001 ; 90 : 732 – 737 .
Raman A Schoeller DA Subar AF , et al . Water turnover in 458 American adults 40–79 yr of age . Am J Physiol Renal Physiol. 2004 ; 286 : F394 – F401 .
Daniels MC Popkin BM . The impact of water intake on energy intake and weight status: a review . Nutr Rev. 2010 ; 9 :in press.
Piernas C Popkin B . Snacking trends in U.S. adults between 1977 and 2006 . J Nutr . 2010 ; 140 : 325 – 332 .
Piernas C Popkin BM . Trends in snacking among U.S. children . Health Affairs. 2010 ; 29 : 398 – 404 .
Popkin BM Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States . Am J Clin Nutr . 2010 ; 91 : 1342 – 1347 .
Muckelbauer R Libuda L Clausen K Toschke AM Reinehr T Kersting M . Promotion and provision of drinking water in schools for overweight prevention: randomized, controlled cluster trial . Pediatrics. 2009 ; 123 : e661 – e667 .
Bar-or O . Temperature regulation during exercise in children and aolescents . In: Gisolfi C Lamb DR , eds. Youth, Exercise, and Sport: Symposium: Papers and Discussions . Indianapolis: Benchmark ; 1989 : 335 – 367 .
National Research Council . Recommended Dietary Allowances . Washington, DC: National Academy Press ; 1989 .
Prentice A Branca F Decsi T , et al . Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations . Br J Nutr. 2004 ; 92 (Suppl 2 ): S83 – 146 .
German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research Swiss Nutrition Association . Reference Values for Nutrient Intake (in English) , 1st edn. Frankfurt: Umschau Brau ; 2002 .
Stookey JD Pieper CF Cohen HJ . Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+years . Public Health Nutr. 2005 ; 8 : 1275 – 1285 .
Malik VS Popkin BM Bray G Després J-P Hu FB. Sugar sweetened beverages, obesity, type 2 diabetes and cardiovascular disease risk . Circulation 2010 ; 121 : 1356 – 1364 .
Teff KL Grudziak J Townsend RR , et al . Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses . J Clin Endocrinol Metab. 2009 ; 94 : 1562 – 1569 .
Stanhope KL . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans . J Clin Investigat. 2009 ; 119 : 1322 – 1334 .
Stanhope KL Havel PJ . Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup . Am J Clin Nutr. 2008 ; 88 (Suppl): S1733 – S1737 .
Stanhope KL Griffen SC Bair BR Swarbrick MM Keim NL Havel PJ . Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals . Am J Clin Nutr. 2008 ; 87 : 1194 – 1203 .
Altman P . Blood and Other Body Fluids . Washington, DC: Federation of American Societies for Experimental Biology ; 1961 .
Kalhoff H . Mild dehydration: a risk factor of broncho-pulmonary disorders? Eur J Clin Nutr. 2003 ; 57 (Suppl 2 ): S81 – S87 .
Taitz LS Byers HD . High calorie-osmolar feeding and hypertonic dehydration . Arch Dis Child. 1972 ; 47 : 257 – 260 .
Burge MR Garcia N Qualls CR Schade DS . Differential effects of fasting and dehydration in the pathogenesis of diabetic ketoacidosis . Metabolism. 2001 ; 50 : 171 – 177 .
Jayashree M Singhi S . Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country . Pediatr Crit Care Med. 2004 ; 5 : 427 – 433 .
Hebert LA Greene T Levey A Falkenhain ME Klahr S . High urine volume and low urine osmolality are risk factors for faster progression of renal disease . Am J Kidney Dis. 2003 ; 41 : 962 – 971 .
Bankir L Bardoux P Mayaudon H Dupuy O Bauduceau B . [Impaired urinary flow rate during the day: a new factor possibly involved in hypertension and in the lack of nocturnal dipping] . Arch Mal Coeur Vaiss. 2002 ; 95 : 751 – 754 .
Blanker MH Bernsen RM Ruud Bosch JL , et al . Normal values and determinants of circadian urine production in older men: a population based study . J Urol. 2002 ; 168 : 1453 – 1457 .
Chan J Knutsen SF Blix GG Lee JW Fraser GE . Water, other fluids, and fatal coronary heart disease: the Adventist Health Study . Am J Epidemiol. 2002 ; 155 : 827 – 833 .
Kelly J Hunt BJ Lewis RR , et al . Dehydration and venous thromboembolism after acute stroke . QJM. 2004 ; 97 : 293 – 296 .
Bhalla A Sankaralingam S Dundas R Swaminathan R Wolfe CD Rudd AG . Influence of raised plasma osmolality on clinical outcome after acute stroke . Stroke. 2000 ; 31 : 2043 – 2048 .
Longo-Mbenza B Phanzu-Mbete LB M'Buyamba-Kabangu JR , et al . Hematocrit and stroke in black Africans under tropical climate and meteorological influence . Ann Med Interne (Paris). 1999 ; 150 : 171 – 177 .
Diamond PT Gale SD Evans BA . Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study . Arch Phys Med Rehabil. 2003 ; 84 : 964 – 967 .
Mazzola BL von Vigier RO Marchand S Tonz M Bianchetti MG . Behavioral and functional abnormalities linked with recurrent urinary tract infections in girls . J Nephrol. 2003 ; 16 : 133 – 138 .
Wilde MH Carrigan MJ . A chart audit of factors related to urine flow and urinary tract infection . J Adv Nurs. 2003 ; 43 : 254 – 262 .
Altieri A La Vecchia C Negri E . Fluid intake and risk of bladder and other cancers . Eur J Clin Nutr. 2003 ; 57 (Suppl 2 ): S59 – S68 .
Donat SM Bayuga S Herr HW Berwick M . Fluid intake and the risk of tumor recurrence in patients with superficial bladder cancer . J Urol. 2003 ; 170 : 1777 – 1780 .
Radosavljevic V Jankovic S Marinkovic J Djokic M . Fluid intake and bladder cancer. A case control study . Neoplasma. 2003 ; 50 : 234 – 238 .
Math MV Rampal PM Faure XR Delmont JP . Gallbladder emptying after drinking water and its possible role in prevention of gallstone formation . Singapore Med J. 1986 ; 27 : 531 – 532 .
Aufderheide S Lax D Goldberg SJ . Gender differences in dehydration-induced mitral valve prolapse . Am Heart J. 1995 ; 129 : 83 – 86 .
Martin B Harris A Hammel T Malinovsky V . Mechanism of exercise-induced ocular hypotension . Invest Ophthalmol Vis Sci. 1999 ; 40 : 1011 – 1015 .
Brucculeri M Hammel T Harris A Malinovsky V Martin B . Regulation of intraocular pressure after water drinking . J Glaucoma. 1999 ; 8 : 111 – 116 .
- dehydration
- energy intake
- water drinking
- fluid intake
- water requirements
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1753-4887
- Print ISSN 0029-6643
- Copyright © 2024 International Life Sciences Institute
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Energy Drinks: A Contemporary Issues Paper
Affiliation.
- 1 McGovern Medical School at The University of Texas Health Science Center at Houston (UTHealth), Houston, TX.
- PMID: 29420350
- DOI: 10.1249/JSR.0000000000000454
Since their introduction in 1987, energy drinks have become increasingly popular and the energy drink market has grown at record pace into a multibillion-dollar global industry. Young people, students, office workers, athletes, weekend warriors, and service members frequently consume energy drinks. Both health care providers and consumers must recognize the difference between energy drinks, traditional beverages (e.g., coffee, tea, soft drinks/sodas, juices, or flavored water), and sports drinks. The research about energy drinks safety and efficacy is often contradictory, given the disparate protocols and types of products consumed: this makes it difficult to draw firm conclusions. Also, much of the available literature is industry-sponsored. After reports of adverse events associated with energy drink consumption, concerns including trouble sleeping, anxiety, cardiovascular events, seizures, and even death, have been raised about their safety. This article will focus on energy drinks, their ingredients, side effects associated with their consumption, and suggested recommendations, which call for education, regulatory actions, changes in marketing, and additional research.
- Athletic Performance
- Caffeine / adverse effects
- Energy Drinks / adverse effects*
- Energy Drinks / analysis*
- Energy Drinks / classification
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Health Sci (Qassim)
- v.9(4); 2015 Oct
Energy Drink Consumption: Beneficial and Adverse Health Effects
Consumption of energy drinks has been increasing dramatically in the last two decades, particularly amongst adolescents and young adults. Energy drinks are aggressively marketed with the claim that these products give an energy boost to improve physical and cognitive performance. However, studies supporting these claims are limited. In fact, several adverse health effects have been related to energy drink; this has raised the question of whether these beverages are safe. This review was carried out to identify and discuss the published articles that examined the beneficial and adverse health effects related to energy drink. It is concluded that although energy drink may have beneficial effects on physical performance, these products also have possible detrimental health consequences. Marketing of energy drinks should be limited or forbidden until independent research confirms their safety, particularly among adolescents.
Introduction
Energy drinks belong to a class of products, in liquid form, that typically contain caffeine, with or without other added dietary supplements. The first energy drink appeared in the U.S. in 1949 and was marketed as “Dr. Enuf”. ( 1 ) In Europe, they were launched for the first time in 1987; then the market expanded throughout the world, becoming very popular after the launch of Red Bull in 1997. ( 2 ) Since then, the energy drink market has grown dramatically, with various brands released worldwide. The annual consumption of energy drinks in 2013 exceeded 5.8 billion liters in around 160 countries. ( 3 ) The estimated total U.S. retail market value for energy drinks was around 12.5 billion USD in 2012 and the market increased 56% from 2006 to 2002. ( 2 )
Manufacturers recently have shifted their consumer focus from athletes to young people. Energy drinks are aggressively marketed in places popular with teens and young adults. Approximately, two thirds of energy drink consumers are 13–35 years old, and boys are two thirds of the market. ( 4 ) In the U.S., energy drinks are the second most common dietary supplement used by young people; about 30% consume energy drinks on a regular basis. ( 5 ) The popularity of energy drinks in the Kingdom of Saudi Arabia does not seem to differ from other parts of the world. Around half of the Saudi University students who participated in a survey admitted to regular consumption of energy. ( 6 )
Energy drinks are designed to give an “energy boost” to the drinker by a combination of stimulants and energy boosters. The major constituent in most energy drinks is caffeine. They usually contain 80–150 mg of caffeine per 8 ounces, which is equivalent to 5 ounces of coffee or two 12-ounce cans of caffeinated soda. ( 7 ) Most of the brands on the market contain large amounts of glucose while some brands offer artificially sweetened versions. Other commonly used constituents are taurine, methylxanthines, vitamin B, ginseng, guarana, yerba mate, acai, maltodextrin, inositol, carnitine, creatine, glucuronolactone, and ginkgo biloba.
Currently, significant concerns have been raised about the safety of these products. There have been several reports that showed adverse health effects associated with energy drink. Despite this, manufactures of energy drinks claim these products are suitable for consumers and that they are safe. In fact, the adverse health effects associated with energy drink remains controversial among scientists. There are limited comprehensive literature reviews that illustrate in detail the suitability and safety related to energy drink consumption, particularly among young adults. Here we review the available literature on the beneficial and adverse health effects related to energy drinks consumption.
Potential adverse effects of energy drinks in relation to their ingredients
Cardiovascular effect.
Several studies have shown an increase in heart rate and arterial blood pressure after energy drink consumption. These findings were attributed to the ergogenic effects of the caffeine content of the energy drink. In addition, significant cardiac manifestations such as ventricular arrhythmias, ST segment elevation and QT prolongation have been documented following energy drink overconsumption. ( 8 ) Additionally, atrial fibrillation has been reported after high energy drinks ingestion in two healthy boys, 14 and 16 years of age. ( 9 ) Recently, energy drink consumption has been related to myocardial infarction in healthy 17-and 19-year-old boys. ( 10 , 11 ) This observation has been supported by the findings that consuming energy drinks reduces endothelial function and stimulates platelet activity through arachidonic acid-induced platelet aggregation in healthy young adults. ( 12 ) Recent reports have demonstrated a relationship between energy drink overconsumption and arterial dilatation, aneurysm formation, dissection and rupture of large arteries. ( 13 )
Neurological and psychological effect
Individuals usually develop symptoms of caffeine intoxication in doses equal to or above 200 mg. Symptoms include anxiety, insomnia, gastrointestinal upset, muscle twitching, restlessness, and periods of inexhaustibility. ( 14 ) In addition, High caffeine intake is associated with acute and chronic daily headaches by stimulating a pro-nociceptive state of cortical hyperexcitability. ( 15 ) Four caffeine-induced psychiatric disorders have been recognized by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition: Including caffeine intoxication, caffeine-induced anxiety, caffeine-induced sleep disorder and caffeine related disorder. ( 16 ) A study of adolescents between 15- and 16-years-of age demonstrated a strong correlation between caffeine intake and violent behavior as well as conduct disorders. ( 17 ) Several reports have suggested that energy drink may contribute to ischemic stroke and lead to epileptic seizures. ( 18 ) Hallucinations might be observed in individuals that consume more than 300 mg of caffeine per day. ( 19 ) High levels of cortisol that follow caffeine intake could explain this. Cortisol enhances the physiological effects of stress resulting in a greater tendency for the subjects to hallucinate. ( 20 )
In vitro studies found that a combination of caffeine, taurine and guarana may promote and enhance apoptosis by reducing both superoxide dismutase and catalase activities on human neuronal SH-SY5Y cells. ( 21 )
Gastrointestinal and metabolic effects
Energy drinks usually contain large amounts of sugar ranging from 21 g to 34 g per oz. The sugar content is mainly in the form of sucrose, glucose or high fructose corn syrup. Therefore, high energy drink intake may increase the risk of obesity and type 2 diabetes. ( 14 ) In addition, the high sugar content in energy drinks may reduce the activity, diversity and gene expression of intestinal bacteria resulting in increased risk of obesity and the metabolic syndrome. ( 22 ) Acute caffeine intake decreases insulin sensitivity, ( 23 ) which could explain the rise in blood glucose levels after energy drink consumption documented in some studies. ( 24 ) Beaudoin et al. demonstrated that caffeine intake reduces insulin sensitivity in a dose dependent manner, with 5.8% increase in insulin for each mg/kg increase in caffeine. ( 25 )
A case has been reported of a woman that presented with jaundice, abdominal pain and highly elevated liver enzymes following energy drink overconsumption. ( 26 ) Huang et al. reported the same finding in a 36-year-old man. ( 27 ) Further studies are needed to determine, which individuals are highly susceptible and the underlying mechanism by which energy drinks cause hepatic injury.
Renal effects
The caffeine in energy drinks has been shown to enhance diuresis. ( 28 ) Therefore, energy drinks should be avoided during prolonged exercise in a hot environment because of the potential for dehydration. Studies have reported that dehydration at a level of 1.5% during prolonged exercise may result in an increase in body temperature, heart rate and perceived rate of exertion. ( 29 )
Caffeine also promotes sodium losses in urine (natriuresis), which effects the plasma volume and results in significant alteration of cardiovascular performance while exercising. ( 30 ) In addition, sodium imbalance during prolonged exercise in a hot environment may reduce isometric force in the legs. ( 31 ) Greene et al reported a case of acute renal insult in a 40-year-old-year man after daily intake of energy drinks for about 2–3 weeks. The serum creatinine was increased fivefold from baseline and returned to normal two days after energy drink consumption was discontinued. ( 32 )
Dental effects
A study in Sweden showed a strong relationship between energy drinks and dental erosion. ( 33 ) Similarly, Marshall et al demonstrated a similar observation in American children. ( 34 ) Energy drinks consumption was associated with about a 2.4-fold increase in dental erosion. This has been attributed to a low pH and the high sugar content of energy drinks. ( 35 ) In addition, Pinto et al found that energy drink intake may lead to cervical dentin hypersensitivity by removing the smear layer of the teeth. ( 36 )
Beneficial effects
The large amount of caffeine in energy drinks provides the consumer with the desirable effects of improved memory, increased alertness and elevated mood. The most widely cited study is the one conducted by Alford et al. ( 37 ) They examined the effects of a market leader energy drink on 36 individuals. Assessments included psychomotor performance (reaction time, concentration and memory), subjective alertness and physical endurance. They showed that the studied energy drink significantly enhanced aerobic endurance (maintaining 65–75% max. heart rate) and aerobic performance (maintaining max. speed) on cycle ergometers. Mental performance included choice reaction, concentration and memory also improved significantly, which indicated increased subjective alertness. ( 37 ) Another study showed that the same brand energy drink significantly increases the upper body muscle endurance during repeated ‘Wingate cycle performance’ in young physically active subjects. However, no change was documented on anaerobic peak or average power. ( 38 ) Hoffman et al also demonstrated that energy drinks caused a significant increase in reaction performance during exercise, but with no effect on anaerobic power performance. ( 39 ) Likewise, Ivy et al in a double-blinded, randomized, crossover study examined the effects of pre-exercise consumption of energy drinks on 12 professional cyclists from both genders. The results showed significant improvement in endurance performance with no change in perceived exertion in the energy drink group compared to the placebo group. ( 40 )
Walsh et al assessed the effects of energy drinks on time to exhaustion during treadmill exercise. They observed a significant increase in time to exhaustion during a moderate intensity endurance run as well as improvement in perceived feelings of focus, energy and fatigue. ( 41 ) Another study evaluated the ability of caffeinated energy drinks to improve acceleration tolerance and strength under a “G” load. The results showed that energy drinks improved relaxed G tolerance and increased strength but had no effect on acceleration tolerance duration. ( 42 )
The results of a recent study reported that consumption of approximately 3 mg/kg of caffeine in the form of energy drinks significantly improved the physical performance of female volleyball players. ( 43 ) Wesnes et al in a randomized, double blind, placebo controlled, cross over study examined the cognitive and mood effects of energy drinks on 94 subjects. Assessment of cognitive function was performed with a number of automated tests of memory and attention while mood was assessed with various different questionnaires such as the Profile of Mood states (POMS), Bond-Lader and Chalder Fatigue Scales. The results revealed that both cognitive function and mood were significantly improved in partially sleep-deprived individuals who consumed energy drinks. They were able to preserve their initial levels of attention for a period of six hours, whereas the placebo group failed. ( 44 )
A number of studies have examined the behavioral effects of energy drinks containing caffeine, glucose, taurine, and vitamins amongst its components. These studies found improvements in aerobic and anaerobic cycling performance, ( 37 ) attention performance and/or reaction time tasks, ( 37 , 45 ) afternoon driving performance, ( 46 ) and different indices of alertness. ( 37 , 45 , 46 ) Smit and Roger compared the behavioral effects of two tailor-made energy drinks with a still water and no treatment conditions. Both energy drinks contained 75 mg caffeine and the same calorie amount from glucose. In comparison to the water and no treatment groups, both drinks significantly increased reaction time and self-ratings of energetic arousal. However, no changes were observed for either memory or rapid visual information processing. ( 47 )
The combination of caffeine and glucose in energy drinks may show restorative properties. ( 48 ) In one study, a glucose based energy drink was given to 11 tired volunteers being examined in a driving simulator. Significant improvement was observed in lane drifting and reaction times for two hours post consumption. ( 49 ) Another study examined the acute effects of a glucose based energy drink on cognitive function. The results showed that energy drink reduced both reaction times on the behavioral control tasks as well as ratings of mental fatigue, whereas it increased subjective ratings of stimulation. ( 50 )
It is very important to note that although the above-mentioned studies have identified positive effects of energy drinks on exercise performance, other researches have documented no significant effects or detrimental health consequences. Al-fares et al ( 51 ) in a single blind placebo controlled study recently evaluated the effects of energy drinks on exercise performance in 32 untrained healthy females. They found that ingestion of energy drinks before exercise did not enhance the indices of physical performance, which included time to exhaustion, maximum oxygen consumption, blood pressure, heart rate, and capillary oxygen saturation. Similar findings were observed in a double blind, randomized, placebo controlled cross over study of 15 physically active volunteers. The study found no effect of energy drinks on ride time to exhaustion or heart rate. Subjective rating of exertion was also not changed. ( 52 )
A recent study ( 53 ) evaluated the acute effects of energy drinks on exercise performance in 19 professional female volleyball players. The players were recruited in a double blind, randomized, crossover study to determine grip strength, vertical jump and anaerobic power during three sessions. For each performance test, there was no significant change indicating that energy drink had no effect on improving physical performance.
The variability in the results of the above studies is mainly due to methodological differences. Variations in subjects, gender, dose of caffeine, ingredients of energy drinks, and type of placebo used contribute significantly to the inconsistency of the results.
Energy drinks may show positive beneficial effects on exercise performance in various sport activities. However, while energy drinks might benefit performance, possible detrimental health problems have been documented, particularly amongst children and adolescents. Various parts of the body are negatively affected by energy drink consumption. Considering this fact and the increasing popularity of these drinks, caution should be exercised while consuming energy drinks. Overambitious marketing and non-scientific claims should be regulated by governments until independent studies confirm that that these products are safe.
Disclosure of benefit: This work was not supported by any drug or commercial company.
Study says these three diets reduce Alzheimer's disease
by LIZ BONIS, WKRC

CINCINNATI (WKRC) - New research showed people's diet and other activities that challenge the brain may help fight off Alzheimer's disease.
The Alzheimer's Association said three diets appeared to help provide food for thought. But the foods needed to be combined with a few other healthy brain habits.
Events like Brains in Bloom to help those with the disease to socialize is a good mental challenge. Physically challenging activities are helpful too. Both are critical tp keeping memory and thinking strong.
But for the three diets according to the Alzheimer's Association that helped long-term brain health success is the DASH diet, Mediterranean diet and the MIND diet.
DASH diet focused on fruits and vegetables, a plant based diet. It also involved at least three servings of low fat diary foods a day.
Mediterranean diet suggested seafood instead of red meat. It also prefered good fats like olive oil. Fruits and vegetables also go with this diet.
MIND diet is a combo of Dash and Mediterranean diets. It suggested berries, lots of green leafy vegetables and other plant based foods.
A study found that those who eat this way reduced the risk of developing Alzheimer's disease by 53% compared to those who eat more of a traditional high fat or meat based diet.

IMAGES
VIDEO
COMMENTS
Though many prefer diet drinks to regular carbonated sodas for their decreased health risks, in a study published by European Journal of Clinical Nutrition, aspartame, the main ingredient used in diet drinks, was linked to insomnia, headaches, and seizures and in chronic cases blindness, neurotoxicity and memory loss [4,5]. The reason behind is ...
Introduction. Frequent diet (artificially sweetened) and regular (sugar-sweetened) soda consumption have both been associated with an increased risk of cardiovascular events, though the literature on diet soda is less consistent (1, 2).In the Northern Manhattan Study (NOMAS) we observed that participants who drank diet soda daily had an increased rate of vascular events compared to those who ...
Much of the research to date has been focused on SSB in general and conducted in countries ... from 2005-2006 to 2011-2012. The proportion of diet soda drinkers were higher in females (65.8%), non-Hispanic whites (83.7%), middle-aged adults (47.5%), people with family income ≥ $55K (53.5%), and people with higher than high school ...
For example, diet soda consumption has been reported as up to three times higher among adult diabetics in the US than non-diabetics . People diagnosed with heart disease or diabetes may therefore actively opt for artificially sweetened drinks. ... This research was supported in part by the National Heart, Lung, and Blood Institute (grant ...
An association study found that although the consumption of diet soda and AS was high, neither was associated with the ... This review was funded by National Research Competitive Grant (PRIN supporting grant no. F8ZB89) from Ministery for University and Research, Italy (to Professor Claudio Napoli, M.D., Ph.D.); by Research Competitive Grant ...
Results appeared online in the American Journal of Public Health on January 16, 2014. The team found that 11% of healthy-weight, 19% of overweight, and 22% of obese adults drank diet beverages. Diet drinks appeared to help healthy-weight adults maintain their weight. These adults consumed less food and significantly fewer total calories on a ...
Nutraceutical Potential of Diet Drinks: A Critical Review on Components, Health Effects, and Consumer Safety. ... 139 research and review papers (published between 1997 and 2018) were screened out ...
Specific to the four areas of evidence examined by this paper, it is possible to articulate the following: 1) Consumption of diet beverages, or ASBs, is much lower than intake of regular sodas or SSBs 4, 5, 7, 9, 10, 15, 32; ASB intake appears to increase with age in youth 4; ASB intake is more common among girls than boys 7, 9 and among women ...
Background: The purpose of this review paper was to explore the components and their respective health effects and safety aspects regarding the consumption of diet drinks (DDs).Methods: A wide variety of the relevant publications (published before 2018) were identified through searching electronic databases (ScienceDirect, PubMed, SciELO, Google Scholar, Springer Link, and ResearchGate) on the ...
Background/objectives: Non-caloric artificial sweeteners (NAS) are marketed as healthier alternatives to sugar, but the relationship between consumption of NAS and development of diabetes is unclear. This study assessed the associations of diet soda and NAS consumption with (1) early markers of insulin and glucose homeostasis (cross-sectionally) and (2) incident diabetes (over an average of 8 ...
sugar-sweetened soda beverage with one serving of water per day at baseline w as related to a lower incidence of. obesity. A cross-national study of 75 countries found that each 1% rise in soft ...
The proportion indicating diet sodas were healthier than sodas was slightly lower among females than males (18·4 % and 22·4 %, respectively; P < 0·001), and appeared to decline slightly with increasing age (P < 0·001). The proportion reporting diet sodas were less healthy than soda appeared to increase with greater disadvantage (P < 0·001).
Specific research linking frequent diet soda consumption to adverse cardiovascular effects includes an analysis of seven large studies, with a total of 308,420 participants, conducted by scientists in Great Britain. A link to an increased risk of long-term weight gain was found when analyzing data from the San Antonio Heart Study, a 10-year ...
Soft drinks include carbonated drinks, still and juice drinks, dilutables, fruit juices, bottled waters, sports and energy drinks (British Soft Drinks Association Annual Report 2016).According to the British Soft Drinks Association Annual Report (), the overall consumption of soft drinks in the UK has increased slightly from 2010 to 2015 by 0.2%.. In 2015; 13.3 billion litres of soft drinks ...
The association between soft drinks and dental caries was not observed for diet soft drinks.86 Also, 5 studies reported that soft drink consumption was positively associated with urinary or kidney stones, but 2 studies reported no association (average r = 0.05).76, 77, 92 - 96 Two of the 5 studies that found positive associations76, 77 ...
These were calculated based on aspartame and other NNS intake during this period, from the sum of diet sodas, other diet drinks, and sweetener packets, retrospectively recalled by biological mothers. c NNS: non-nutritive sweetener: ≥1 serving/day of any NNS denotes either ≥1 packet/day of any NNS, ≥1 DS/day, or ≥1 other diet drink/day.
Abstract. Soft drink is the most consumable drinks worldwide although of their serious impact on human health, it affects many body systems such as locomotor system, gastrointestinal system, and ...
Diet was assessed at phases 3, 5, 7 and 9 using a 127-item machine-readable semi-quantitative food frequency questionnaire (FFQ) which originates from the tool used in the US Nurses' Health ...
Without water, humans can survive only for days. Water comprises from 75% body weight in infants to 55% in the elderly and is essential for cellular homeostasis and life. 1 Nevertheless, there are many unanswered questions about this most essential component of our body and our diet. This review attempts to provide some sense of our current ...
Energy Drinks: A Contemporary Issues Paper Curr Sports Med Rep. 2018 Feb;17(2):65-72. doi: 10.1249/JSR ... tea, soft drinks/sodas, juices, or flavored water), and sports drinks. The research about energy drinks safety and efficacy is often contradictory, given the disparate protocols and types of products consumed: this makes it difficult to ...
Research shows that diet beverage drinkers consume significantly more calories from food than regular soda drinkers. These extra food calories can add up to a higher number on your bathroom scale . In addition, some studies indicate sugar substitutes like aspartame, sucralose and saccharin, which are commonly found in diet drinks, can throw off ...
coffee contains around 100mg of caffeine, tea has 50mg and a can of cola has 30mg. Many energy drinks do not clearly label the exact caffeine content per serving, but. some products contain as ...
IJRTI1811010 International Journal for Research Trends and Innovation (www.ijrti.org) 55 The Contents of Soft Drinks &Their Impacts on Health 1 ... Diet Pepsi, Coca- Cola, Diet Coke, Thumps Up etc. Cola drinks account for nearly 61-62% of the total soft drinks market in India. Non-Cola products account for 36% the total soft drink market Major ...
Inhabitants of the United States consume sugar, particularly in the form of sugar-sweetened beverages (SSBs), at an alarming rate. SSBs are a leading cause of dental cavities,1 obesity, and type II diabetes.2 SSBs include soft drinks, sports drinks, energy drinks, fruit drinks, flavored milk, and other beverages that contain added caloric sweeteners. In the United States, SSB consumption has ...
Energy drinks are aggressively marketed in places popular with teens and young adults. Approximately, two thirds of energy drink consumers are 13-35 years old, and boys are two thirds of the market. In the U.S., energy drinks are the second most common dietary supplement used by young people; about 30% consume energy drinks on a regular basis.
New research showed people's diet and other activities that challenge the brain may help fight off Alzheimer's disease. Fri, 26 Apr 2024 09:52:57 GMT (1714125177369) ...