Lock-and-key model
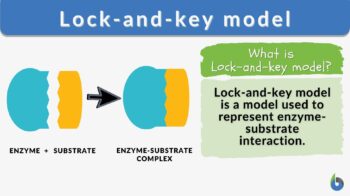
strong>Lock-and-key model n., [lɑk ænd ki ˈmɑdl̩] Definition: a model for enzyme-substrate interaction
Table of Contents

Lock-and-key model Definition
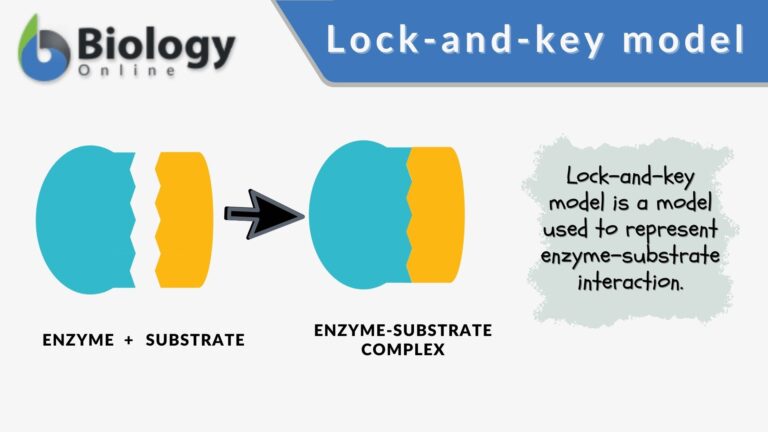
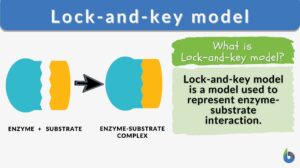
Lock-and-key model is a model for enzyme-substrate interaction suggesting that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. In this model, enzymes are depicted as highly specific. They must bind to specific substrates before they catalyze chemical reactions . The term is a pivotal concept in enzymology to elucidate the intricate interaction between enzymes and substrates at the molecular level. In the lock-and-key model, the enzyme-substrate interaction suggests that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Like a key into a lock , only the correct size and shape of the substrate ( the key ) would fit into the active site ( the keyhole ) of the enzyme ( the lock ).
Compare: Induced fit model See also: enzyme , active site , substrate
Lock-and-key vs. Induced Fit Model
At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model , and the other is the Induced fit model . The lock and key model theory was first postulated by Emil Fischer in 1894. The lock-and-key enzyme action proposes the high specificity of enzymes. However, it does not explain the stabilization of the transition state that the enzymes achieve. The induced fit model (proposed by Daniel Koshland in 1958) suggests that the active site continues to change until the substrate is completely bound to the active site of the enzyme, at which point the final shape and charge are determined. Unlike the lock-and-key model, the induced fit model shows that enzymes are rather flexible structures. Nevertheless, Fischer’s Lock and Key theory laid an important foundation for subsequent research, such as during the refinement of the enzyme-substrate complex mechanism, as ascribed in the induced fit model. The lock-and-key hypothesis has opened ideas where enzyme action is not merely catalytic but incorporates a rather complex process in how they interact with the correct substrates with precision.
Key Components
Components of the lock and key model:
- Enzyme : the enzyme structure is a three-dimensional protein configuration, with an active site from where the substrate binds.
- Substrate : often an organic molecule, a substrate possesses a structural feature that complements the geometry of the enzyme’s active site.
In the lock and key model, both the enzymes and the substrates facilitate the formation of a complex that lowers the activation energy needed for a chemical transformation to occur. Such reduction in the activation energy allows the chemical reaction to proceed at a relatively faster rate, making enzymes crucial in various biological and molecular processes.
Lock-and-key Model Examples
Some of the common examples that are often discussed in the context of the Lock and Key Model are as follows:
- Enzyme lactate dehydrogenase with a specific active site for its substrates, pyruvate and lactate. The complex facilitates the interconversion of pyruvate and lactate during anaerobic respiration
- Enzyme carbonic anhydrase with a specific active site for the substrates carbon dioxide and water. The complex facilitates the hydration of carbon dioxide, forming bicarbonate
- Enzyme lysozyme binding with a bacterial cell wall peptidoglycan, which is a vital immune function
Choose the best answer.
Send Your Results (Optional)

- Aryal, S. and Karki, P. (2023). “Lock and Key Model- Mode of Action of Enzymes”. Microbenotes.com. https://microbenotes.com/lock-and-key-model-mode-of-action-of-enzymes/
- Farhana, A., & Lappin, S. L. (2023, May). Biochemistry, Lactate Dehydrogenase . Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557536/
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on January 11th, 2024
You will also like...
Takahē (Porphyrio hochstetteri)
Meet the colorful takahē, an extremely rare flightless bird. Find out more about its unique features and why they matte..
Homeostasis of Organism Water Regulation
Osmoregulation is the regulation of water concentrations in the bloodstream, effectively controlling the amount of water..

New Zealand’s Unique Geographical History
Explore why New Zealand has such unique flora and fauna, and learn why long periods of geographical isolation. This less..
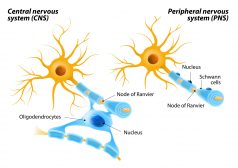
The Central Nervous System
Myelin sheath is essential for a faster conductivity of signals. Know more about this feature of some neurons in the Cen..

Growth Patterns
This tutorial describes the sigmoid curve, annual plant growth, tree growth, human growth, and insect growth as the grow..
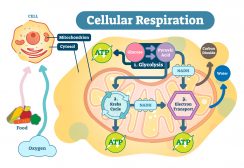
Cell Respiration
Cell respiration is the process of creating ATP. It is "respiration" because it utilizes oxygen. Know the different stag..
Related Articles...
No related articles found
Molecular Recognition: Lock-and-Key, Induced Fit, and Conformational Selection
- Living reference work entry
- First Online: 27 October 2020
- Cite this living reference work entry

- Norman Tran 4 &
- Todd Holyoak 4
40 Accesses
In the most general sense, molecular recognition is the mechanism by which two or more molecules come together to form a specific complex. These types of molecular interactions are widespread throughout biology and include diverse processes such as enzyme catalysis, antibody–antigen recognition, protein synthesis, receptor–ligand interactions, and transcriptional regulation, to name a few. Because of the universal importance of molecular recognition in biological function, understanding how molecules unambiguously recognize and interact with one another is fundamentally important to appreciating biological systems as a whole.
Introduction
Just as the field biochemistry grew out of the study of biological fermentation, much of the field of molecular recognition grew out of the study of enzyme selectivity (Voet and Voet 2004 ). Early studies led to the conclusion that substrates combine with enzymes at a specific location on each enzyme’s surface. These conclusions generated...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Beach H, Cole R, Gill ML, Loria JP (2005) Conservation of mus-ms enzyme motions in the apo- and substrate-mimicked state. J Am Chem Soc 127(25):9167–9176
Article CAS Google Scholar
Csermely P, Palotai R, Nussinov R (2010) Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem Sci 35(10):539–546
Dixon M, Webb EC (1979) Enzymes. Academic, New York
Google Scholar
Fischer E (1894) The influence of configuration on enzyme activity. Dtsch Chem Ges 27:2984–2993. (Translated from German)
Gerstein M, Lesk AM, Chothia C (1994) Structural mechanisms for domain movements in proteins. Biochemistry 33(22):6739–6749
Greives N, Zhou HX (2014) Both protein dynamics and ligand concentration can shift the binding mechanism between conformational selection and induced fit. Proc Natl Acad Sci U S A 111(28):10197–10202
Hammes GG, Chang YC, Oas TG (2009) Conformational selection or induced fit: a flux description of reaction mechanism. Proc Natl Acad Sci U S A 106(33):13737–13741
Jencks WP (1975) Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol 43:219–410
CAS PubMed Google Scholar
Koshland DE (1958) Application of a theory of enzyme specificity to protein synthesis. Proc Natl Acad Sci U S A 44(2):98–104
Koshland DE Jr (2004) Crazy, but correct. Nature 432(7016):447
Laidler KH (1951) The influence of pressure on the rates of biological reactions. Arch Biochem 30(2):226–236
Monod J, Wyman J, Changeux JP (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118
Sullivan SM, Holyoak T (2008) Enzymes with lid-gated active sites must operate by an induced fit mechanism instead of conformational selection. Proc Natl Acad Sci U S A 105(37):13829–13834
Tsai CJ, Kumar S, Ma B, Nussinov R (1999a) Folding funnels, binding funnels, and protein function. Protein Sci 8(6):1181–1190
Tsai CJ, Ma B, Nussinov R (1999b) Folding and binding cascades: shifts in energy landscapes. Proc Natl Acad Sci U S A 96(18):9970–9972
Voet D, Voet JG (2004) Biochemistry. Wiley, Hoboken
Weikl TR, Paul F (2014) Conformational selection in protein binding and function. Protein Sci 23(11):1508–1518
Yang J, Gao M, Xiong JW, Su ZD, Huang YQ (2019) Features of molecular recognition of intrinsically disordered proteins via coupled folding and binding. Protein Sci 28(11):1952–1965
Download references
Author information
Authors and affiliations.
Department of Biology, University of Waterloo, Waterloo, ON, Canada
Norman Tran & Todd Holyoak
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Todd Holyoak .
Editor information
Editors and affiliations.
University Leicester MRC Centre, Leicester, UK
Gordon Roberts
Dept Biochemistry, University of Oxford, Oxford, UK
Anthony Watts
Rights and permissions
Reprints and permissions
Copyright information
© 2021 European Biophysical Societies' Association (EBSA)
About this entry
Cite this entry.
Tran, N., Holyoak, T. (2021). Molecular Recognition: Lock-and-Key, Induced Fit, and Conformational Selection. In: Roberts, G., Watts, A. (eds) Encyclopedia of Biophysics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35943-9_468-1
Download citation
DOI : https://doi.org/10.1007/978-3-642-35943-9_468-1
Received : 02 September 2020
Accepted : 08 September 2020
Published : 27 October 2020
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-35943-9
Online ISBN : 978-3-642-35943-9
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Limitations and Extensions of the Lock-and-Key Principle: Differences between Gas State, Solution and Solid State Structures
The lock-and-key concept is discussed with respect to necessary extensions. Formation of supramolecular complexes depends not only, and often not even primarily on an optimal geometric fit between host and guest. Induced fit and allosteric interactions have long been known as important modifications. Different binding mechanisms, the medium used and pH effects can exert a major influence on the affinity. Stereoelectronic effects due to lone pair orientation can lead to variation of binding constants by orders of magnitude. Hydrophobic interactions due to high-energy water inside cavities modify the mechanical lock-and-key picture. That optimal affinities are observed if the cavity is only partially filled by the ligand can be in conflict with the lock-and-key principle. In crystals other forces than those between host and guest often dominate, leading to differences between solid state and solution structures. This is exemplified in particular with calixarene complexes, which by X-ray analysis more often than other hosts show guest molecules outside their cavity. In view of this the particular problems with the identification of weak interactions in crystals is discussed.
1. Introduction
After Emil Fischer coined the lock-and-key picture for the reaction between enzymes and substrates [ 1 ], it became a leading concept for the understanding of intermolecular interactions with proteins, and later for the rational design of drugs. With the advent of supramolecular chemistry the idea gained an enormous momentum, as chemists began to synthetize a large variety of host compounds for practically all possible target guest molecules occurring in nature or in the environment. Although few concepts have played a comparatively important role in chemistry, the lock-and-key principle has limitations and extensions, which often are overlooked.
2. Dependence on the Binding Mechanism/Medium, pH and Stereoelectronic Effects
First of all, there are fundamental differences in the function of the lock-and-key principle in the gas state and in solution; the situation in crystals is again quite different and will be discussed in Section 6 and Section 7 . In solution the presence of a geometrically well-fitting cavity in a receptor is not enough for the binding of a substrate: the price for desolvation of the host and guest prior to complex formation must be paid by compensating non-covalent forces between host and guest, although complete desolvation might not be necessary, and desolvation alone can also contribute to a gain in free energy (see Section 5 on hydrophobic effects). Only in fairly rigid molecular containers [ 2 ], the inside binding of substrates may be controlled solely by the size of the portals. Obviously, the penalty for desolvation can be so large that one must change the reaction medium in order to achieve efficient complexation; a well-known example is the design of receptors for recognition of carbohydrates in water [ 3 , 4 ]. Furthermore, the geometric requirements for an optimal binding between host and guest differ enormously with the different non-covalent interactions [ 5 ]. Coulombic forces, with an r −1 dependence of the binding enthalpy on the distance r between interaction atoms or groups, tolerate much more deviation from a perfect geometric fit than for example dispersive interactions, which fall off with r −6 , and hydrogen bond strength depends significantly on orientation of donor and acceptor.
Solvent effects can be more decisive for complexation strength than size matching. Complexation with crown ethers 18C6 and 18C5 shows that not only the absolute binding energies depend on the medium, essentially as linear function of the cation desolvation free energies of the guest metal ions as shown with a variety of solvents [ 6 ]. Also, the differences between 18C6 and 18C5, which binds weaker due to one hydrogen atom protruding into the cavity, are much smaller in water than in other solvents ( Figure 1 and Table 1 ) [ 7 ].

Complexation of potassium ions with crown ethers 18C6 and 18C5; superimposed structures of the K + -complexes (the K + -ion in the 18C5 complex in red); with binding free energies ΔG in kJ/mol, and differences ΔΔG between them [ 7 ]. Adapted with permission from Raevsky, O.A.; Solovev, V.P.; Solotnov, A.F.; Schneider, H.-J.; Rüdiger, V. Conformation of 18-crown-5 and its influence on complexation with alkali and ammonium cations: Why 18-crown-5 binds more than 1000 times weaker than 18C6. J. Org. Chem. 1996 , 61 , 8113–8116. Copyright 1996 American Chemical Society.
Complexation free energies (in kJ/mol) of crown ethers in different solvents, with differences between 18C6 and 18C5.
Stereoelectronics can play a dominating role in complexation strength. A 1.10-diaza-crown ether ( Figure 2 ) binds metal ions much weaker than expected, due to the unfavourable diaxial orientation of the lone pairs (lp) at nitrogen [ 8 ]. Introduction of a methyl groups at the nitrogen atoms enforces a diequatorial lp orientation, and the binding energy increases to ΔG values expected for such ionophores [ 9 ]. The consequences of a different binding mechanism are illustrated in Figure 3 . Here a change in pH alters the inclusion mode of a ligand in the calix[4]arene host, due to a alternatively dominating ion pair or cation-π interaction [ 10 ].

Stereoelectronics: the 1.10-diaza-crown with R = H (diaxial lone pair (lp) orientation, ( A ) binds K + ions with only ΔG = 10 kJ/mol, with R = Me (diequatorial lp orientation; ( B ) ΔG increases to 26 kJ/mol (in methanol) [ 8 ].

Change of inclusion mode with a calix[4]arene host ( n = 4) as function of the pH [ 10 ].
Electron densities can play a larger role than geometric fitting. Molecular clips and tweezers bear a highly negative surface potential inside; the binding of the preferred guest molecules such as, e.g., NAD + is therefore dictated more by Coulombic forces than by exact fitting [ 11 ]. Ancillary ligands such as tetraaza-cyclododecanes can increase the positive charge at bound highly polarizable lanthanide ions, thereby leading to enhanced sensing affinities towards anions [ 12 ]. Cavitands as those shown in Figure 4 exhibit switching between close “vase” and open “kite” conformations as a function of pH, temperature, and of solvent, with the kite preferred in nonpolar solvents [ 13 ].

Cavitands which switch between close “vase” and open “kite” conformations [ 13 ]. Reprinted from [ 13 ] with permission from VCH/Wiley.
3. Induced Fit
An important extension of the lock-and-key principle was introduced early by Koshland, who proposed that conformational changes in an enzyme, induced by the substrate, can strengthen the binding [ 14 ]. With synthetic hosts binding is often only possible by severe conformational distortions of the host, as demonstrated e.g., with metalloporphyrin cages [ 15 ]. In artificial receptors such an induced fit is particularly obvious if the host is flexible and/or too wide for tight fitting. The resorcarene macrocycle in Figure 5 can bind acetylcholine only in a closed conformation; simultaneously two protons are liberated, thus enabling hydrogen bonds between three phenolic units [ 16 ].

Binding of cholinacetate (Me 3 + N(CH 2 ) 2 OAc) in a resorcarene macrocycle by induced fit (Me groups at + N omitted).
With a calix[6]arene derivative, encapsulation of different charged or neutral species in the hydrophobic cavity is also accompanied by conversion from the 1,3-alternate to the 1,3,5-alternate conformation [ 17 ]. Calix[6]arenes possess a particularly high flexibility; their cavity can by induced fit expand for large ligands or shrink for smaller guest molecules [ 18 ]. Other examples are calix[4]pyrroles which in solution occur in several conformations, but in presence of anions only in the cone conformation ( Figure 6 ); remarkably one finds in crystals mostly the 1,3-alternate form [ 19 , 20 ].

Calix[4]pyrrole in the 1,3-alternate conformation (left side) converts to the cone form by anion binding.
Sometimes a host cavity is only formed by inducing with an added guest the self-assembly of predesigned host parts, leading to so-called capsules [ 21 , 22 , 23 ]. Thus, an assembly of three palladium atoms and two tris -pyridyl ligands is induced by adamantanecarboxylic acid ( Figure 7 a) [ 24 ]; a capsule stabilized by ion pairing forms in presence of e.g., N -methylquinuclidinium cation as guest [ 22 ] ( Figure 7 b); or a steroid as guest induces a host assembly by hydrophobic interactions [ 25 ] ( Figure 7 c).

Self-assembly of predesigned host parts to form capsules, ( a ) with adamantanecarboxylic acid as guest [ 24 ]; ( b ) by ion pairing, with e.g., N -methylquinuclidinium cation as guest [ 22 ]; ( c ) a lipophilic host which self-assembles in presence of a long steroid by hydrophobic interactions [ 25 ].
4. Allosteric Effects
An important extension of the simple lock and key concept is due to allosteric interaction of a second guest component which is not directly acting at the first binding site. A large number of synthetic host guest complexes have been designed which show the typical binding modulation by the presence of a second effector [ 26 , 27 , 28 , 29 ]. This occurs most often, but not necessarily by conformational changes. Figure 8 and Table 2 illustrates the strong influence of an anion as second effector on the binding strength of tetramethylammonium salts in selected calixarenes. NMR analyses verified that the ammonium group is filling the cavity, so that the anion, which forms a strong ion pair with the cation in the apolar solvent chloroform used here, can only bind outside the calix, particularly efficiently with the urea group in the then heterotopic receptor 2 [ 30 ].

Association constants K as (M −1 ) of 1:1 complexes of tetramethylammonium salts Me 4 N + ·X − with hosts 1 and 2 in CDCl 3 , in presence of tosylate, chloride, acetate or trifluoroacetate anions [ 30 ].
Association constants K as (M −1 ) of 1:1 complexes of tetramethylammonium salts Me 4 N + ·X − with hosts 1 and 2 ( Figure 8 ) in CDCl 3 , in presence of tosylate, chloride, acetate or trifluoroacetate anions.
Artificial host compounds can show much stronger allosteric effects than proteins, in which conformational coupling between interacting binding sites is usually much weaker. The example in Figure 9 shows a particularly large ratio K M /K 0 of binding constants with and without second effector; only in the presence of metal ions such as Zn 2+ , a cavity is formed by contraction which binds lipophilic substrates such as dansylamide [ 31 , 32 ].

An example of an allosteric system (L = p -phenyl, M = Zn 2+ , G = dansylamide) in which introduction of metal ions lead to a ratio of binding constants of K M /K 0 >> 100; fluorescence emission occurs only in presence of metal ion [ 31 , 32 ].

5. Hydrophobic Interactions beyond the Lock-and-Key Picture
At first sight it seems that hydrophobic forces, which were traditionally ascribed to an entropy advantage gained by association between lipophilic molecules and subsequent liberation of water molecules, should not lead to particular deviations from the lock-and-key principle: the larger and closer the contact between a host cavity and a guest, the larger will be the number of liberated water molecules. In line with this idea hydrophobic contributions are traditionally evaluated by determination of solvent excluded surfaces. However, there is increasing and recently quantified evidence, that in host guest complexes significant contributions stem from the liberation of high energy water molecules [ 33 , 34 , 35 , 36 ] which in cavities can materialize less than the four hydrogen bonds which exist in bulk water [ 37 ]. Without complexation in a cavity there is only a very small hydrophobic effect, even for saturated compounds [ 38 ]. It has been shown that for essentially closed cavities such as in cucurbiturils the binding free enthalpies with some guest compounds can be completely explained by this non-classical high-energy water effect [ 33 ]. This is particularly so if the host interior offers few non-covalent interactions, as is the case for cucurbiturils, but also for some molecular clips ( Figure 10 ). The higher the number of high-energy water molecules is in a cavity, and the smaller the number of hydrogen bonds of each of these water molecules is, the larger is the energy gain; in accordance to the lock-and-key principle this would be achieved if the fit between host and guest is so perfect that all water molecules are displaced by the guest. However, if the host is large enough to accommodate more water molecules which can develop a satisfactory number of hydrogen bonds the hydrophobic driving force will play a minor role even if there is a perfect fit with a large enough guest which displaces all water molecules. Large hosts such as some cucurbiturils can accommodate a guest molecule and water, which again can exert more or less hydrogen bonds, or even two guest molecules. These possibilities are illustrated in Figure 10 ; complexes with cucurbiturils but also with cyclodextrins or molecular clips exhibit sizeable high-energy water effects [ 33 ]. It has been stressed that also the binding affinity in protein pockets is often not dominated by the lock-and-key principle but by the displacement of free-energetically unfavourable water [ 39 , 40 ].

Host compounds for large hydrophobic binding contributions: cucurbiturils and a molecular clip with four water molecules. Cucur[n]biturils with increasing size: ( a ) Crystal structure of inverted-CB6 with three intracavity water molecules; ( b – d ) Snapshot from molecular dynamics (MD) simulations for ( b ) CB6, ( c ) CB8 and ( d ) CB8·viologen complexes with 4, 14 and six cavity water molecules, respectively. Top : Complexes viewed from the side (CB n atoms in the front removed for clarity); Bottom : Complexes viewed from the top. Reprinted from [ 33 ] with permission from VCH/Wiley.
6. Host and Guest Complexes in the Solid State
In crystals the lattice is stabilized by a multitude of interactions in addition to those between host and guest; the uptake of a guest molecule can lead to a significant change of the solid state structure of the host alone. Metastable different crystalline modifications of the same compound, or polymorphs, are possible in particular if energy differences between molecular conformers and crystal lattice energies are of the same magnitude [ 41 , 42 ]; they are also quite frequent in cocrystals [ 43 ]. Occurrence of polymorphs make the assignment of an optimal host-guest geometry more difficult, but can shed light on the different interaction mechanisms. Isomorphic crystals can show a more unified picture of host and guest complexes, if they offer enough room for ligands, particularly if these are relatively small and if the chemical properties as well as binding mechanisms of different ligands are similar. Such conditions are also typical for complexes with large biomolecules such as proteins, in which the receptor conformation is in addition stabilized by a multitude of interactions. Figure 11 presents an example of a crystal which forms isomorphous structures with a series of linear alcohols [ 44 ]. Interestingly, crystals of inclusion compounds with the guest inside the cavity can often be obtained simply by slow diffusion of guests into the solvent-filled voids of the crystalline sponges [ 45 ], or by exchange of one guest with another one with the complex crystals in the vapour phase [ 46 ].

Example of a crystal of a resorcarene cavitand, containing co-crystalizing trifluorethanol, which forms isomorphous structures with a series of linear alcohols; the refined structure with e.g., n- propanol as ligand shows the relevant electron densities. Reprinted from [ 44 ] with permission of the Royal Society of Chemistry.
The abovementioned similarity between crystals of one receptor with small guest molecules is also the basis of an interesting new method to test selectivities from occupancy factors in a crystal with competing guest molecules [ 44 ]. Thus, isomorphous monoclinic crystals with a resorcarene cavitand and six alcohols were X-rayed without the unnecessary structural refinement; the observed occupancy factors were in close agreement with the relative binding constant ratios of the alcohols. The fully refined structure of the crystal with e.g., n- propanol ( Figure 11 ) shows that the small ligand finds its place without significant distortion of the lattice; comparison with the different alcohols shows an affinity decrease with the increase in the host-guest hydrogen bond distance, which is a function of the alcohol chain length.
7. Intra- and Extra Cavity Complexation in Macrocyclic Receptors/Differences between Solid State, Gas State and Solution Structure
The rather shallow cavity of small calixarenes lead particularly often to extra- (or exo-) cavity complexation, although the simple lock-and-key principle would predict an intra- (or endo-) complex. For complexes between argon and calix[4]arene in the gas state, spectroscopic and quantum-chemical calculations show both orientations, as expected with a preference for the endo-complex ( Figure 12 ) [ 47 , 48 ]. Laser spectroscopic molecular beam experiments and computations of calix[4]arene complexes with a variety of small ligands such as NH 3 , N 2 , CH 4 , and C 2 H 2 indicate also preferred endo complexes, for H 2 O and NH 3 as guest mainly by dipole–dipole interactions, for Ar, N 2 , CH 4 and C 2 H 2 mostly by dispersion forces [ 49 ].

Calix[4]arene complexes with argon; optimized structures of endo-complex and exo-complex. Reprinted from ref. [ 47 , 48 ] with permission of the Royal Society of Chemistry.
That interactions in the solid state are effective also in the gas phase complexes has been aptly discussed by Dalcanale et al. with complexes based on calixarenes or resorcarenes with P=O groups as hydrogen bond acceptors [ 50 ]. Electrospray ionization mass spectrometry (ESI-MS) is a suitable technique to elucidate what happens in the gas state. A major difference is that in the gas phase the outward facing P=O groups are not shielded by neighbouring molecules as in the solid layer, and are therefore amenable to H-bonding with the guest. The complex between the resorcarene cavitand and ethanol ( Figure 13 ) is also a nice example of several supramolecular structures within a crystal, exhibiting hydrogen bonds of EtOH with the two distal P=O groups with a statistical 50% probability; one also observes the synergy of P=O···H–O bonding and CH–π interactions in the cavitand ( Figure 13 a). If as in an isomeric structure ( Figure 13 b) a phenyl group fills the cavity, no C–H···π interaction is possible and also no H-bond to the then outward P=O group; then ethanol is found outside in the crystal lattice. For solid receptor layers, used often for gas detection, the distinction between intracavity vs. extracavity complexation is a particular problem. Location of analytes in the receptor layers can be identified by FT-IR spectroscopy if host and guest diagnostic bands do not overlap due to unspecific adsorption. Unspecific adsorption is characterized by linear adsorption isotherms, in contrast to Langmuir-type isotherms, which deviate significantly from linearity, indicating a specific analyte-layer interaction.

( a ) Resorcarene complexes with ethanol exhibiting two different structures within one crystal (hydrogen bonds of EtOH with the two distal P=O groups with a 50% statistical probability); ( b ) isomeric structure with a phenyl group filling the cavity; ethanol can only bind outside the cavity [ 50 ]. Reprinted from ref. [ 50 ] with permission of the Royal Society of Chemistry.
Complexes with smaller calixarenes show relatively often guest binding outside the cavity, as found e.g., in crystals of the calix[4]arene with toluene; here the guest molecule occupies intermolecular cavities of host channels [ 51 ]. In solution amines in the form of ammonium ions bind to calixarenes or resorcarenes usually as intracavity complexes [ 52 , 53 ], essentially due the cation-π interaction. In the solid state, however, amines bind often to the exo side, or to both sides. Thus, p - tert -butylcalix[4]arene forms with 1,4-butanediamine an inclusion compound with amine side both exo and endo of the cavity [ 54 ]. Both orientations were also found for complexes of amines and calix[6]arene [ 55 ]. In a p-tert -butylcalix[7]arene 1:3 pyridine crystal two pyridines have been found outside the cavity in the crystal lattice, forming a complex/clathrate hybrid [ 56 ]. Crystals of p-tert -butylcalix[8]arene with 8 pyridine molecules in the unit cell show the host macrocycle in an open chairlike conformation, so there is no cavity for the guest molecule [ 57 ].
Metal complexes are frequently bound to the outside of cavities, particularly with the electron-rich outside phenolic parts of calixarenes. For example, p-tert -butylcalix[4]arene coordinates rhodium outside, which allows to bind inside small neutral compounds such as diethylether or small anions such as tetrafluoroborate inside ( Figure 14 ) [ 58 ].

( a ) Crystal structure of a dirhodium p - tert -butylcalix[4]arene complex, with diethylether in the cavity; ( b ) Crystal structure of a triiridinum p-tert- butylcalix[5]arene complex with an encapsulated tetrafluoroborate anion inside [ 58 ]. Reprinted with permission from Staffilani, M.; Hancock, K.S.B.; Steed, J.W.; Holman, K.T.; Atwood, J.L.; Juneja, R.K.; Burkhalter, R.S. Anion binding within the cavity of π-metalated calixarenes J. Am. Chem. Soc. 1997 , 119 , 6324–6335. Copyright 1997 American Chemical Society.
Crystal structures of metal complexes with calix[4]arenes often show metal ions both in- and outside the cavity, e.g., with dinuclear Ti-IV and Ti-III complexes [ 59 ]. Calix[4]bisthiacrowns form with silver an endocyclic disilver complex and with copper exocyclic coordination polymers [ 60 ]. Stacking between the π-surfaces at the outside of 1,3- bis -pyridylmethylcalix[4]arene with different aryl compounds such as perfluoroarene or 1,4-dibromotetrafluorobenzene leads to infinite one-dimensional non-covalent ribbons [ 61 ].
Larger cyclophanes of the type shown in Figure 15 are expected to bind phenyl derivatives in the cavity, as inferred early by Stetter et al . from the formation of a 1:1 crystalline complex with benzene, and from fitting to CPK models [ 62 ]. Later, however, X-ray analysis revealed that the Stetter crystal has the benzene located outside [ 63 ]. Many years later NMR-spectra showed that, in water, benzene in fact does bind within the cavity [ 64 ].

A benzidine-derived cyclophane and its complexation with benzene, expected from Corey–Pauling–Koltun (CPK)-model [ 62 ], and as seen in the crystal by X-ray [ 63 ]; in aqueous solution the benzene is inside [ 64 ]. Adapted from ref. [ 5 ] with permission from Wiley/VCH.
With a complex of europium ion and a (222) cryptand, one can observe the slow movement of the guest out of the cavity to the solution ( Figure 16 ). If one dissolves the solid crystals, which from an earlier X-ray analysis is known to form as expected the inner sphere complex [ 65 ], in water (D 2 O) decomposition occurs into the free metal salt and the protonated ligand. Depending on the pH, two forms of metal complexes with different symmetry appear, as evident from the 1 H-NMR spectra [ 66 ].

Complex of europium ion and a (222) cryptand, crystal structure with the metal ion inside [ 65 ] and structures with the metal in different locations, as observed in solution by NMR spectroscopy [ 66 ]. Partially reprinted from ref. [ 65 ] with permission of the Royal Society of Chemistry.
The triply linked bis -cyclopeptide shown in Figure 17 exhibits remarkable differences between solution and solid state. In aqueous medium the host complexes a sulfate anion with lgK = 6, driven entirely by a gain in entropy. NMR data show that the sulfate is inside the cavity, forming hydrogen bonds to the amide NH groups at the inner surface of the host. In the crystal, however, one finds only water in the cavity, even though the crystals were grown in a solution containing sulfate [ 67 ].

A triply linked bis-cyclopeptide complexing in aqueous solution with high efficiency sulfate ions inside the cavity; in the crystal (right side) only water, no sulfate, is found inside [ 67 ]. Adapted from ref. [ 67 ] with permission from Wiley/VCH.
Cyclodextrin complexes are prone to differ in the solid and solution state, since the hydrophobic effect as important driving force is missing in crystals, and the inside of cyclodextrins offers only C–H bonds for non-covalent interaction, in contrast to the outside and rim. Hydrophilic compounds are said to generally bind with cyclodextrins preferentially outside the cavity [ 68 ]; earlier publications suggested similar possibilities [ 69 ]. Open chain analogues of cyclodextrins often show even more efficient chromatographic enantiorecognition of e.g., drugs [ 70 , 71 , 72 ]. However there are until now not enough conclusive spectroscopic studies for related cyclodextrin complexes in the solid and solution state.
8. Cavity Filling Factors—Conflict with the Lock-and-Key Principle?
Cyclophanes, cavitands and capsules have been shown to bind all kind of organic ligands, particularly those of an aromatic nature, in solution inside the cavity as long as the host leaves enough room for the guest molecule [ 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 ]. However, it has been noted early that there are deviations from the simple lock-and-key picture. Collet et al. found that water-soluble derivatives of cryptophanes, such as 2 in Figure 18 , bind ammonium guest molecules in water not as expected as a function of the size match, but preferred a loose association with smaller ligands [ 81 ]. Similarly, fluorophores composed of smaller phenyl-parts and larger naphthyl-parts bind in water to cyclodextrins, not with the better fitting larger naphthyl part but with the seemingly too small phenyl entity [ 82 ].

Calix[4]arene-carceplex 1 , cryptophane 2 ( n = 2), and carceplex 3 , with volume of the internal cavity, in [Å 3 ] [ 85 ].
Collet et al. showed already in 1993 [ 83 , 84 ] for cryptophanes such as 2 in Figure 18 , that e.g., chloroform binds better than methane, although methane fits geometrically as well in the cavity; they calculated for CHCl 3 in 2 an occupancy factor or packing coefficient (PC) of 0.886, corresponding to a very closely packed crystal; they also observed that the measured free enthalpy and entropy of complexation is comparable with the heat and entropy of crystallization of organic compounds. In contrast, for methane, PC amounts to only 0.348, which is consistent with later systematic evaluations by Rebek et al. [ 85 ] Analyses of many supramolecular complexes in solution, comprising in particular container- and capsule-type hosts have led Rebek et al. to the important generalization, that optimal binding occurs if 55% ± 9% of the space available in a cavity is occupied [ 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 ]. This is in the range of the packing density of organic liquids with a packing coefficient (PC) 0.51 to 0.63. Binding in hosts such as those in Figure 18 is indeed only observed if the PC is between 0.43 and 0.63. Larger packing coefficients of up to 0.70 can be reached if the complex is particularly stabilized by non-covalent interactions; in crystals and the interior of globular proteins the reported PC amounts to 0.66 to 0.77 [ 85 ].
That only a part of the available space is used for filling a cavity seems at first sight to be in conflict with the traditional lock-and-key principle. However, thermal motions, and the vibrational and translatory freedom of movement of host and guest require additional space. Moreover, a complete geometric match between host and guest molecules without any empty space between the complementary van der Waals surfaces can barely exist in the interaction between molecules of different shape and nature, characterized by corners and dimples. The exact calculation of the volume enclosed by the van der Waals surface is also therefore difficult, different methods can lead to variations of up to e.g., 15% [ 96 ]. Molecular dynamics (MD) simulation at 300 K predict e.g., that the volume in cavitands such as in Figure 18 vary over a range of 10% [ 85 ].
Polycyclic aromatic hydrocarbons (PAHs) with high binding affinities resulting from stacking and C–H···π interactions show larger deviation from Rebeks 55% filling factor [ 97 ]. Deviation from the optimal occupation rule was also observed e.g., with deep-cavity cavitand complexes in water [ 98 ]. A crystalline cryptophane complex with xenon exhibits an unusually large packing coefficient of 0.82 instead of 0.55 ± 0.09, with very short Xe···C contacts [ 99 ].
Complexes of an octanuclear cubic coordination cage ( Figure 19 ) in water with a series of aliphatic cyclic ketones show a linear relation between the guest’s surface and the binding ΔG as long as a 55% occupancy is reached [ 100 ]. Whether a crystal contains a guest molecule inside a host cavity can also depend on the preparation mode. With the complex shown in Figure 19 growing crystals from solvents containing excess guest afforded only the empty cage, whereas immersing preformed crystals of the cage in the neat guest cycloundecanone yielded the crystal with the entrapped guest [ 100 ].

Host cage [Co 8 L 12 ](BF 4 ) 16 , complex with cycloundecanone, with a 55% occupancy of the cavity space, Co atoms in green [ 100 ]. With permission from Turega, S.; Cullen, W.; Whitehead, M.; Hunter, C.A.; Ward, M.D. Mapping the internal recognition surface of an octanuclear coordination cage using guest libraries J. Am. Chem. Soc. 2014 , 136 , 8475–8483. Copyright 2014 American Chemical Society.
9. Problems with Identification of Weak Interactions in Crystals
Crystallography has been the most important source for metrical details also of intermolecular bonds [ 101 , 102 ]. The availability of nearly half a million crystal structures in the Cambridge Structural Data Base (CSD) allows identification of the most significant non-covalent interactions also in supramolecular complexes with respect to their geometry [ 103 ]. The combination with computational approaches has led to often reliable generalizations also for weak interactions, although it has been stated that “experimentally found crystal structures of a given compound need not be those of minimal free energy” and that “the choice of relevant intermolecular bonds is sometimes arbitrary” [ 104 ]. This is different in solution or in the gas state: as long there is the commonly observed rapid exchange all occurring structures will reflect the dominating free energies.
That purely statistical evaluations with data bases such as the CSD can be misleading is obvious from the recent controversy about hydrogen bonds with organic fluorine as acceptor. Dunitz et al. found in 5947 crystal structures containing organic fluorine only 37%, or 0.6% with short CF···HX (X = O, N) distances, and therefore concluded in 2004 “Organic Fluorine Hardly Ever Makes Hydrogen Bonds” [ 105 ]. Other crystallographers did find evidence for hydrogen bonds with fluorine; e.g., Mehta and Sen [ 106 ] found with fluorinated polycyclitols H···F distances 2.55 Å and C–H···F angles around 150°; Desiraju et al. [ 107 ] found in layers of polyfluoro-substituted benzenes often 2.23–2.35 Å and C–H···F angles 150–175 Å; some researchers consider 2.41–2.78 Å H···F distances as still typical [ 108 ]. For other halogens (Cl, Br, I) crystal structures seemed to be in agreement with their possibility to act as hydrogen bond acceptor.
For solution and the gas state, all available evidence clearly speaks for fluorine as in fact a much better acceptor than other halogen derivatives [ 109 ], which in view of the electronegativity differences is of course expected in the framework of Pauling’s description of hydrogen bonds. In particular, measurements of equilibrium constants between compounds with a large range of donors and halogen acceptors in solvents such as CCl 4 or CHCl 3 furnished interaction free energies [ 109 ], systematically decreasing from e.g., 7.5 kJ/mol for fluoroalkanes RF to 4.7 kJ/mol for iodoalkanes RI (tested with 1-haloadamantanes with 4-fluorophenol in CCl 4 ), with a systematical dependence on the substitution fragment for all halides [ 110 ]. For binding of fluoro derivatives to proteins, which is important in view of the 20% fluorine occurrence of all drugs, there is also clear indication of relatively strong hydrogen bonding with organic fluorine [ 111 ].
Obviously, the chances to find a significant number of hits in crystals of the thousands of fluorine containing compounds which have been prepared for all kind of reasons amounts to a lottery. The search for weak non-covalent forces in crystals is more promising if no other strong interactions are dominating the lattice: this is the case for example in pure hydrocarbons with e.g., one or more fluorine atoms, or if ones compares similar structures with many of the weak interactions one is looking for. Also, the search in protein databases is more promising, as generally protein complexes are more preselected—nobody will go to the expense of a solid state protein X-ray or NMR analysis if there is no prior evidence or at least hope that e.g., a fluorine generates a particular effect.
10. Conclusions
The lock-and-key principle is still a valuable starting point for the understanding and the design of natural and synthetic supramolecular complexes. Recent examples show the importance of the lock-and-key principle and induced fit also for selectivity in enzymatic reactions [ 112 , 113 ]; how it can apply to the stabilization of transition states has been demonstrated with the bowl-to-bowl inversion of the non-planar corannulene by complexation with a tetracationic cyclophane, accompanied by an induced fit [ 114 ]. As illustrated in Figure 20 only the flat transition state structure of the substrate, not its ground state fits into the host cavity, which leads to a calculated rate increase of inversion by a factor of 10.

Corannulene ( a ) fits to a tetracationic cyclophane host ( b ) only in the flat transition state structure of the substrate, not its ground state, leading to faster inversion of the corannulene [ 114 ]. Reprinted from ref. [ 114 ] with permission from Nature Publishing Group.
As demonstrated in this review the lock-and-key principle underlies important modifications. Optimal geometric fit may be a prerequisite, but high binding affinities depend often on a whole range of other factors, as discussed above. The possible self-inclusion of side groups is also a limitation of the simple lock-and-key concept, as are associations between several host molecules, in which one part of the host is inserted in the cavity of another one. Both interferences depend on the surrounding medium, and can in particular differ in the solid state. Typically, complexes in which the ligand occupies not the cavity of a host but are located outside are more often found in crystals than in solution. Statistical evidence from the analysis of not pre-selected crystal structure databases can be misleading with respect to the identification of very weak interactions. Structures of supramolecular complexes in solution can be evaluated by spectroscopic methods, preferably by NMR spectroscopy. The most often used Nuclear Overhauser Effect (NOE) provide intermolecular distances, but may reflect complexes which exhibit very short distances, and yet are less populated. In contrast to NOE data chemical shifts reflect usually the mixture of all conformers present in solution, according to their stability. Although the accuracy of structure elucidation based on chemical shifts cannot compete with crystallography they can be a useful and time-saving approach for the characterization of host–guest complexes. Both semiempirical and quantum-chemical calculations have been developed for this purpose [ 115 , 116 , 117 , 118 ], recently with emphasis on protein structures [ 119 , 120 , 121 ].
Acknowledgments
The author sincerely thanks Professor Stefan Kubik, Kaiserslautern, for valuable suggestions. He also remembers with gratitude the coworkers whose contributions are mentioned in the references.
Conflicts of Interest
The authors declare no conflict of interest.
- Subscriber Services
- For Authors
- Publications
- Archaeology
- Art & Architecture
- Bilingual dictionaries
- Classical studies
- Encyclopedias
- English Dictionaries and Thesauri
- Language reference
- Linguistics
- Media studies
- Medicine and health
- Names studies
- Performing arts
- Science and technology
- Social sciences
- Society and culture
- Overview Pages
- Subject Reference
- English Dictionaries
- Bilingual Dictionaries
Recently viewed (0)
- Save Search
- Share This Facebook LinkedIn Twitter
Related Content
Related overviews.
enzyme-substrate complex
induced-fit model
'lock-and-key theory' can also refer to...
Lock-and-key theory, more like this.
Show all results sharing these subjects:
- Life Sciences
Quick Reference
A theory to explain the mechanism of enzymatic reactions, in which it is proposed that the enzyme and substrate(s) bind temporarily to form an enzyme–substrate complex. The binding site on the enzyme is known as the ‘active site’ and is structurally complementary to the substrate(s). Thus the enzyme and substrate(s) are said to fit together as do a lock and a key.
From: lock-and-key theory in A Dictionary of Zoology »
Subjects: Science and technology — Life Sciences
Related content in Oxford Reference
Reference entries, lock‐and‐key model, lock and key model.
View all reference entries »
View all related items in Oxford Reference »
Search for: 'lock-and-key theory' in Oxford Reference »
- Oxford University Press
PRINTED FROM OXFORD REFERENCE (www.oxfordreference.com). (c) Copyright Oxford University Press, 2023. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use (for details see Privacy Policy and Legal Notice ).
date: 21 May 2024
- Cookie Policy
- Privacy Policy
- Legal Notice
- Accessibility
- [66.249.64.20|195.190.12.77]
- 195.190.12.77
Character limit 500 /500
RLO: Lock and Key Hypothesis
Introduction.
- Lock and Key
- Drug-target interactions
- Drug development
Have you ever wondered why drug actions are so specific? - why drugs can be targeted at one specific biochemical function within the body? This specificity lies in the interaction between the drug molecule and the molecule within the body that it targets.
Let's look at a cell. Click on the various molecules that can act as drug targets to learn a little more about each.
© 2004 School of Nursing and Academic Division of Midwifery , University of Nottingham
Developer: Ahmad Athamneh
Content author: Richard Windle
RLO released: 2nd June, 2004
Page last updated: 23 May, 2007
Blog The Education Hub
https://educationhub.blog.gov.uk/2024/05/16/new-rshe-guidance-what-it-means-for-sex-education-lessons-in-schools/
New RSHE guidance: What it means for sex education lessons in schools

R elationships, Sex and Health Education (RSHE) is a subject taught at both primary and secondary school.
In 2020, Relationships and Sex Education was made compulsory for all secondary school pupils in England and Health Education compulsory for all pupils in state-funded schools.
Last year, the Prime Minister and Education Secretary brought forward the first review of the curriculum following reports of pupils being taught inappropriate content in RSHE in some schools.
The review was informed by the advice of an independent panel of experts. The results of the review and updated guidance for consultation has now been published.
We are now asking for views from parents, schools and others before the guidance is finalised. You can find the consultation here .
What is new in the updated curriculum?
Following the panel’s advice, w e’re introducing age limits, to ensure children aren’t being taught about sensitive and complex subjects before they are ready to fully understand them.
We are also making clear that the concept of gender identity – the sense a person may have of their own gender, whether male, female or a number of other categories – is highly contested and should not be taught. This is in line with the cautious approach taken in our gu idance on gender questioning children.
Along with other factors, teaching this theory in the classroom could prompt some children to start to question their gender when they may not have done so otherwise, and is a complex theory for children to understand.
The facts about biological sex and gender reassignment will still be taught.
The guidance for schools also contains a new section on transparency with parents, making it absolutely clear that parents have a legal right to know what their children are being taught in RSHE and can request to see teaching materials.
In addition, we’re seeking views on adding several new subjects to the curriculum, and more detail on others. These include:
- Suicide prevention
- Sexual harassment and sexual violence
- L oneliness
- The prevalence of 'deepfakes’
- Healthy behaviours during pregnancy, as well as miscarriage
- Illegal online behaviours including drug and knife supply
- The dangers of vaping
- Menstrual and gynaecological health including endometriosis, polycystic ovary syndrome (PCOS) and heavy menstrual bleeding.
What are the age limits?
In primary school, we’ve set out that subjects such as the risks about online gaming, social media and scams should not be taught before year 3.
Puberty shouldn’t be taught before year 4, whilst sex education shouldn’t be taught before year 5, in line with what pupils learn about conception and birth as part of the national curriculum for science.
In secondary school, issues regarding sexual harassment shouldn’t be taught before year 7, direct references to suicide before year 8 and any explicit discussion of sexual activity before year 9.
Do schools have to follow the guidance?
Following the consultation, the guidance will be statutory, which means schools must follow it unless there are exceptional circumstances.
There is some flexibility w ithin the age ratings, as schools will sometimes need to respond to questions from pupils about age-restricted content, if they come up earlier within their school community.
In these circumstances, schools are instructed to make sure that teaching is limited to the essential facts without going into unnecessary details, and parents should be informed.
When will schools start teaching this?
School s will be able to use the guidance as soon as we publish the final version later this year.
However, schools will need time to make changes to their curriculum, so we will allow an implementation period before the guidance comes into force.
What can parents do with these resources once they have been shared?
This guidance has openness with parents at its heart. Parents are not able to veto curriculum content, but they should be able to see what their children are being taught, which gives them the opportunity to raise issues or concerns through the school’s own processes, if they want to.
Parents can also share copyrighted materials they have received from their school more widely under certain circumstances.
If they are not able to understand materials without assistance, parents can share the materials with translators to help them understand the content, on the basis that the material is not shared further.
Copyrighted material can also be shared under the law for so-called ‘fair dealing’ - for the purposes of quotation, criticism or review, which could include sharing for the purpose of making a complaint about the material.
This could consist of sharing with friends, families, faith leaders, lawyers, school organisations, governing bodies and trustees, local authorities, Ofsted and the media. In each case, the sharing of the material must be proportionate and accompanied by an acknowledgment of the author and its ownership.
Under the same principle, parents can also share relevant extracts of materials with the general public, but except in cases where the material is very small, it is unlikely that it would be lawful to share the entirety of the material.
These principles would apply to any material which is being made available for teaching in schools, even if that material was provided subject to confidentiality restrictions.
Do all children have to learn RSHE?
Parents still have the right to withdraw their child from sex education, but not from the essential content covered in relationships educatio n.
You may also be interested in:
- Education Secretary's letter to parents: You have the right to see RSHE lesson material
- Sex education: What is RSHE and can parents access curriculum materials?
- What do children and young people learn in relationship, sex and health education
Tags: age ratings , Gender , Relationships and Sex Education , RSHE , sex ed , Sex education
Sharing and comments
Share this page, related content and links, about the education hub.
The Education Hub is a site for parents, pupils, education professionals and the media that captures all you need to know about the education system. You’ll find accessible, straightforward information on popular topics, Q&As, interviews, case studies, and more.
Please note that for media enquiries, journalists should call our central Newsdesk on 020 7783 8300. This media-only line operates from Monday to Friday, 8am to 7pm. Outside of these hours the number will divert to the duty media officer.
Members of the public should call our general enquiries line on 0370 000 2288.
Sign up and manage updates
Follow us on social media, search by date, comments and moderation policy.
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
Illustrate the lock and key hypothesis of enzyme action.
Enzymes: a catalyst is a material that acts to initiate a chemical reaction, and enzymes are specialized compounds that catalyze biological events. when the substrates are attached to the enzyme's active site, the enzyme catalyzes the reaction, and the chemical process begins. the active site is the enzyme's specific place where it is linked with the substrate. the substrate's attachment to the enzyme causes changes in the distribution of electrons in the substrate's chemical bonds. this eventually leads to reactions that aid in the creation of products. products are released from the enzyme surface in order to recycle the enzyme for use in a subsequent reaction step. the active site has a distinct geometric form that contrasts with the geometric shape of a substrate fragment. this obviously indicates that the enzymes can only react with one or a few related molecules. lock and key model: a lock and key analogy may be used to describe the fundamental action of a single substrate enzyme. in this case, the enzyme is the lock, and the substrate is the key. only the correct size key, which is the substrate, enters the keyhole, which is the active site of the lock, which is the enzyme. other keys that are too tiny, too big, or have wrongly positioned teeth do not fit into the lock. only the right-shaped key can open the lock..

Key and lock hypothesis of enzyme action was given by
The 'lock and key' model of enzyme action illustrates that a particular enzyme molecule


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

U.S. Department of Commerce
- Press Releases
Was this page helpful?
Biden-harris administration announces preliminary terms with polar semiconductor to establish an independent american foundry, office of public affairs.
Proposed Investment of Approximately $120 Million From CHIPS and Science Act Would Build on Minnesota’s Decades-Long History in the Semiconductor Industry and Create over 160 Jobs
Today, the Biden-Harris Administration announced that the U.S. Department of Commerce and Polar Semiconductor (Polar) have reached a non-binding preliminary memorandum of terms (PMT) to provide the company with up to $120 million in proposed federal incentives under the CHIPS and Science Act. This proposed funding would catalyze investment from private and state sources to expand Polar's manufacturing facility and introduce new technology capabilities in Bloomington, Minnesota. The expansion and modernization would enable Polar to double its U.S. production capacity of sensor and power chips within two years. Additionally, this proposed investment would bring in more U.S. private capital, which would transform Polar from a majority foreign-owned in-house manufacturer to a majority U.S.-owned commercial foundry, expanding opportunities for U.S. chip designers to innovate and produce technologies domestically.
President Biden signed the CHIPS and Science Act into law in August 2022, with the goal of strengthening U.S. supply chains, creating good-paying jobs, and advancing U.S. competitiveness. Shortages of power and sensor chips were among the most acute bottlenecks during the COVID-19 pandemic, disrupting critical industries served by Polar, including automotive, healthcare, aerospace, and defense. Because of the Biden-Harris Administration’s proposed investment in Polar, the U.S. will have an expanded and stable domestic supply of these essential semiconductor technologies, bolstering U.S. supply chain resilience as a result. Additionally, this investment can help strengthen our national security by securing a domestic chip supply for defense purposes.
This proposed CHIPS funding supports an investment of more than $525 million, catalyzing contributions from the company, state and local entities, and private investors. This collaborative funding approach would not have been possible without the CHIPS and Science Act.
“Thanks to President Biden’s leadership, with this announcement we are making taxpayer dollars go as far as possible to create jobs, secure our supply chains, and bolster manufacturing in Minnesota,” said U.S. Secretary of Commerce Gina Raimondo . “This proposed investment in Polar will crowd in private capital, which will help make Polar a U.S.-based, independent foundry. They will be able to expand their customer base and create a stable domestic supply of critical chips, made in America’s heartland.”
Through Polar’s semiconductor manufacturing operations, the Biden-Harris Administration’s proposed investment would create over 160 manufacturing and construction jobs in Minnesota. For the purposes of this project, Polar and construction partner Mortenson have committed to utilizing a Project Labor Agreement (PLA) to support its construction workforce. Polar plays a leading role in the Minnesota CHIPS Coalition Workforce Partnership, a coalition of employers, education and training providers, labor organizations and others that seeks to improve workforce development programs to ensure a stronger talent pipeline for immediate and future workforce needs. As part of this announcement, Polar has committed to expanding access to quality, affordable child care for its facility workers.
Additionally, Polar is committed to climate and environmental responsibility as a top priority in its operations. Recently, Polar transitioned its semiconductor fab operation to rely entirely on clean and renewable energy sources to fulfill its electric power needs. Polar also highly prioritizes water conservation efforts including reclamation of wastewater for manufacturing processes and water collection for irrigation.
“When President Biden signed the CHIPS and Science Act, he put a stake in the ground about the importance of semiconductor manufacturing in the United States,” said Assistant to the President for Science and Technology and Director of the White House Office of Science and Technology Policy Arati Prabhakar . “This is where the President’s leadership changes communities and changes lives. This proposed federal investment will catalyze $525 million from private companies and the state of Minnesota to create a healthy supply of made-in-Minnesota semiconductors that are essential to producing cars, electrical grids, defense systems, and more.”
“The future of the semiconductor industry is being built right here in the United States, and Polar will be part of that innovation boom. The company’s technology plays a critical role in high-voltage applications across the aerospace, automotive, and defense sectors and this proposed investment would enable new capabilities to manufacture the next generation of semiconductors,” said Under Secretary of Commerce for Standards and Technology and National Institute of Standards and Technology Director Laurie E. Locascio .
“We are very pleased to announce this historic investment in Minnesota semiconductor manufacturing. Our expanded manufacturing facility will allow us to increase capacity and branch into innovative technologies to serve new customers and markets,” said Surya Iyer, President and Chief Operating Officer of Polar Semiconductor . “Polar and its employees are grateful to the U.S. Department of Commerce and the State of Minnesota for their commitment to the future of American semiconductor manufacturing and appreciate the strong collaboration with the CHIPS Program Office, Minnesota Department of Employment and Economic Development (“DEED”), and the City of Bloomington, Minnesota, throughout this process. Polar is also pleased to welcome a significant equity investment from Niobrara Capital and Prysm Capital, which will allow the Company to become U.S.-owned, and for the continued support of our long-term partners, Sanken Electric and Allegro MicroSystems.”
Polar has received state and federal support for its proposed expansion, including $75 million from the Minnesota Department of Employment and Economic Development (DEED). The company has also indicated that it plans to claim the Department of the Treasury’s Investment Tax Credit, which is expected to be up to 25% of qualified capital expenditures.
As explained in its first Notice of Funding Opportunity , the Department may offer applicants a PMT on a non-binding basis after satisfactory completion of the merit review of a full application. The PMT outlines key terms for a potential CHIPS incentives award, including the amount and form of the award. The award amounts are subject to due diligence and negotiation of award documents and are conditional on the achievement of certain milestones. After the PMT is signed, the Department begins a comprehensive due diligence process on the proposed projects and continues negotiating or refining certain terms with the applicant. The terms contained in any final award documents may differ from the terms of the PMT being announced today.
About CHIPS for America
The Department has received more than 650 statements of interest, more than 200 pre-applications and full applications for NOFO 1, and more than 160 small supplier concept plans for NOFO 2. The Department is continuing to conduct rigorous evaluation of applications to determine which projects will advance U.S. national and economic security, attract more private capital, and deliver other economic benefits to the country. The announcement with Polar Semiconductor is the eighth PMT announcement the Department of Commerce has made under the CHIPS and Science Act, with additional PMT announcements expected to follow throughout 2024.CHIPS for America is part of President Biden’s economic plan to invest in America, stimulate private sector investment, create good-paying jobs, make more in the United States, and revitalize communities left behind. CHIPS for America includes the CHIPS Program Office, responsible for manufacturing incentives, and the CHIPS Research and Development Office, responsible for R&D programs, that both sit within the National Institute of Standards and Technology (NIST) at the Department of Commerce. NIST promotes U.S. innovation and industrial competitiveness by advancing measurement science, standards, and technology in ways that enhance economic security and improve our quality of life. NIST is uniquely positioned to successfully administer the CHIPS for America program because of the bureau’s strong relationships with U.S. industries, its deep understanding of the semiconductor ecosystem, and its reputation as fair and trusted. Visit https://www.chips.gov to learn more.

IMAGES
VIDEO
COMMENTS
The key-lock hypothesis (see above The nature of enzyme-catalyzed reactions) does not fully account for enzymatic action; i.e., certain properties of enzymes cannot be accounted for by the simple relationship between enzyme and substrate proposed by the key-lock hypothesis. A theory called the induced-fit theory retains… Read More
Lock-and-key vs. Induced Fit Model. At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model, and the other is the Induced fit model.The lock and key model theory was first postulated by Emil Fischer in 1894.The lock-and-key enzyme action proposes the high specificity of enzymes.
The "keyhole-lock-key" model proposed by Prokop et al. suggests that complementarity exists between the substrate ligand and the enzyme-substrate channel, ... However, the identification of TR EDs and synthetic analogs has proven this theory incomplete. Synthetic GC1, for example, lacks iodine and halogens but functions as a TRβ agonist ...
In 1894, Emil Fisher discovered that glycolytic enzymes are able to distinguish between sugar stereoisomers. Based upon that discovery, he formulated the lock-and-key hypothesis (Fischer 1894), which proposed that enzymes recognize their substrates just as a lock receives a key.That is, only in the case of exact geometric complementarity between the substrate (key) and enzyme (lock) is the ...
In 1894, Emil Fischer proposed the lock and key theory, which states that enzymes have a specific shape that directly correlates to the shape of the substrate. This model was accepted for a long ...
The Induced Fit Model Builds upon the Lock-and-Key Hypothesis. This lock-and-key model served the biochemical community well for over 50 years. However, while this model adequately explained how substrates that are too large to fit within the confines of the active site would fail to act as substrates, it did not explain how small substrates, for instance water, often acted as non-substrates ...
1 Emil Fischer's Lock-and-Key Hypothesis after 100 Years- Friedrich Cramer Towards a Supracellular Chemistry 1 2 Molecular Recognition in Biology: Models for Analysis of Protein-Ligand Interactions 25 Doron Lancet, Amnon Horovitz and Ephraim Katchalski-Katzir 3 New Biocatalysts via Chemical Modification Ian M. Bell and Donald Hilvert 73
This theory of induced fit extends the lock-and-key principle that Emil Fischer proposed exactly 100 years ago. The new theory proposed by D. E. Koshland, Jr. in 1958 allows one to explain regulation and cooperative effects, and adds some new specificity principles as well.
The Lock-and-Key Hypothesis In 1894, Emil Fisher discovered that glycolytic enzymes are able to distinguish between sugar stereoisomers. Based upon that discovery, he for-mulated the lock-and-key hypothesis (Fischer 1894), which proposed that enzymes recognize their substrates just as a lock receives a key. That
Abstract. Published 100 years after Emil Fischer first proposed the lock-and-key principle, this volume provides a complete review of the subject to date and offers suggestions for futher research ...
Summary This chapter contains sections titled: Introduction Classical Locks Catenanes Safety Locks Magnetic Locks Carbohydrate-Lectin Interactions: ... Emil Fischer's Lock-and-Key Hypothesis after 100 years—Towards a Supracellular Chemistry. Friedrich Cramer, Friedrich Cramer. Max-Planck-Institut, für Experimentelle Medizin, Göttingen, Germany.
Figure 1. Illustration of 'Lock and Key' (top), Induced fit (middle) and Combination Lock (bottom) model of protein-ligand binding interaction. But, enzymes show conformational flexibility and, on that basis, Daniel Koshland proposed a modification to the 'lock and key' model. Koshland's suggestion was that active sites of enzymes are ...
An important extension of the lock-and-key principle was introduced early by Koshland, who proposed that conformational changes in an enzyme, induced by the substrate, can strengthen the binding . With synthetic hosts binding is often only possible by severe conformational distortions of the host, as demonstrated e.g., with metalloporphyrin ...
Lock and Key Model. A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme's mode of action. Fischer's theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another ...
The surface of every cell is covered with a thick layer of sugar molecules, some of them very complex. Here, we can apply the lock and key analogy once again. The sugar structures are the keys. As shown in the schematic drawing of a cell membrane in Fig. 6, these sugar keys protrude from the membrane surface and serve as recognition elements.
The lock-and-key theory proposed 100 years ago is expanded by this induced fit theory. The new theory put forth by D. E. Koshland, Jr. in 1958 explains regulatory and cooperative effects and also introduces some new specificity concepts. According to this hypothesis, an enzyme's active site is not architecturally ideal for substrate binding ...
The lock-and-key analogy in Emil Fischer's program on sugar fermentation, 1890-1907 Download; XML; The making of the lock-and-key model of the antibody-antigen relationship, 1886-1930 Download; XML; Lock-and-key foundations for molecular biology:: Linus Pauling and the Caltech group, 1930-1960 Download; XML
"lock-and-key theory" published on by null. A theory to explain the mechanism of enzymatic reactions, in which it is proposed that the enzyme and substrate(s) bind temporarily to form an enzyme-substrate complex. The binding site on the enzyme is known as the 'active site' and is structurally complementary to the substrate(s).
induced-fit theory, model proposing that the binding of a substrate or some other molecule to an enzyme causes a change in the shape of the enzyme so as to enhance or inhibit its activity. Induced-fit theory retains the key-lock idea of a fit of the substrate at the active site but postulates in addition that the substrate must do more than simply fit into the already preformed shape of an ...
In its 145-year history the lock-and-key hypothesis has provoked many confinnation and refutation attempts. It is remarkable that explicit tests were attempted as early as the late nineteenth ...
Universities' Collaboration in eLearning (UCEL) RLO: Lock and Key Hypothesis. Introduction. Lock and Key. Drug-target interactions. Drug development. Activity. Resources.
The lock and key hypothesis: The theory was postulated by Emil Fisher in 1898. According to the hypothesis, like a lock can be open by its key only, a substance possessing specific composition only can combine with the specific active site found in the specific enzyme's surface.
Relationships, Sex and Heath Education (RSHE) is a subject taught at both primary and secondary school. In 2020, Relationships and Sex Education was made compulsory for all secondary school pupils in England, and Health Education compulsory for all pupils in state-funded schools. Last year, the Prime Minister and Education Secretary brought ...
In the proposed rule, while describing the roles and responsibilities of signal employees, FRA stated that signal maintainers are tasked with inspecting and testing signal systems and performing minor and emergency repairs as needed. AAR and ASLRRA objected to this statement, asserting that FRA did not explain what was meant by "minor repairs."
In this case, the enzyme is the lock, and the substrate is the key. Only the correct size key, which is the substrate, enters the keyhole, which is the active site of the lock, which is the enzyme. Other keys that are too tiny, too big, or have wrongly positioned teeth do not fit into the lock. Only the right-shaped key can open the lock.
Proposed Investment of Approximately $120 Million From CHIPS and Science Act Would Build on Minnesota's Decades-Long History in the Semiconductor Industry and Create over 160 Jobs ... Secure .gov websites use HTTPS A lock (A locked padlock) or https: ... The PMT outlines key terms for a potential CHIPS incentives award, including the amount ...
Key testimony: The final witness was Robert Costello, who was once an informal legal adviser to Michael D. Cohen, Mr. Trump's one-time fixer who paid the porn star, Stormy Daniels, $130,000 to ...