- Patient Care & Health Information
- Diseases & Conditions
- What is a stroke? A Mayo Clinic expert explains
Learn more from neurologist Robert D. Brown, Jr. M.D., M.P.H.
I'm Dr. Robert Brown, neurologist at Mayo Clinic. In this video, we'll cover the basics of a stroke. What is it, who it happens to, the symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. You've likely heard the term stroke before. They affect about 800,000 people in the United States each year. Strokes happen in two ways. In the first, a blocked artery can cut off blood to an area of the brain. And this is known as an ischemic stroke. 85% of strokes are of this type. The second type of stroke happens when a blood vessel can leak or burst. So the blood spills into the brain tissue or surrounding the brain. And this is called a hemorrhagic stroke. Prompt treatment can reduce brain damage and the likelihood of death or disability. So if you or someone you know is experiencing a stroke, you should call 911 and seek emergency medical care right away.
Anyone can have a stroke, but some things put you at higher risk. And some things can lower your risk. If you're 55 and older, if you're African-American, if you're a man, or if you have a family history of strokes or heart attacks, your chances of having a stroke are higher. Being overweight, physically inactive, drinking alcohol heavily, recreational drug use. Those who smoke, have high blood pressure or high cholesterol, have poorly controlled diabetes, suffer from obstructive sleep apnea, or have certain forms of heart disease are at greater risk as well.
Look for these signs and symptoms if you think you or someone you know is having a stroke: Sudden trouble speaking and understanding what others are saying. Paralysis or numbness of the face, arm or leg on one side of the body. Problems seeing in one or both eyes, trouble walking, and a loss of balance. Now many strokes are not associated with headache, but a sudden and severe headache can sometimes occur with some types of stroke. If you notice any of these, even if they come and go or disappear completely, seek emergency medical attention or call 911. Don't wait to see if symptoms stop, for every minute counts.
Once you get to the hospital, your emergency team will review your symptoms and complete a physical exam. They will use several tests to help them figure out what type of stroke you're having and determine the best treatment for the stroke. This could include a CT scan or MRI scan, which are pictures of the brain and arteries, a carotid ultrasound, which is a soundwave test of the carotid arteries which provide blood flow to the front parts of the brain, and blood tests.
Once your doctors can determine if you're having an ischemic or hemorrhagic stroke, they'll be able to figure out the best treatment. If you're suffering an ischemic stroke, it's important to restore blood flow to your brain as quickly as possible, providing the oxygen and other nutrients your brain cells need to survive. To do this, doctors may use an intravenous clot buster medicine, dissolving the clot that is obstructing the blood flow or they may perform an emergency endovascular procedure. This involves advancing a tiny plastic tube called a catheter up into the brain arteries, allowing the blockage in the artery to be removed directly. Unlike ischemic strokes, the goal for treating a hemorrhagic stroke is to control the bleeding and reduce pressure in the brain. Doctors may use emergency medicines to lower the blood pressure, prevent blood vessel spasms, encourage clotting and prevent seizures. Or, if the bleeding is severe, surgery may be performed to remove the blood that is in the brain.
Every stroke is different, and so every person's road to recovery is different. Management of a stroke often involves a care team with several specialties. This may include a neurologist and a physical medicine and rehabilitation physician, among others. Now, in the end, our goal is to help you recover as much function as possible so that you can live independently. A stroke is a life-changing event that can affect you emotionally as much as it can physically. You may feel helpless, frustrated, or depressed. So look for help and support from friends and family. Accept that recovery will take hard work and most of all time. Strive for a new normal and remember to celebrate your progress. If you'd like to learn even more about strokes, watch our other related videos or visit mayoclinic.org. We wish you all the best.
An ischemic stroke occurs when the blood supply to part of the brain is blocked or reduced. This prevents brain tissue from getting oxygen and nutrients. Brain cells begin to die in minutes. Another type of stroke is a hemorrhagic stroke. It occurs when a blood vessel in the brain leaks or bursts and causes bleeding in the brain. The blood increases pressure on brain cells and damages them.
A stroke is a medical emergency. It's crucial to get medical treatment right away. Getting emergency medical help quickly can reduce brain damage and other stroke complications.
The good news is that fewer Americans die of stroke now than in the past. Effective treatments also can help prevent disability from stroke.

Products & Services
- A Book: Future Care
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Products for Independent Living from Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
If you or someone you're with may be having a stroke, pay attention to the time the symptoms began. Some treatments are most effective when given soon after a stroke begins.
Symptoms of stroke include:
- Trouble speaking and understanding what others are saying. A person having a stroke may be confused, slur words or may not be able to understand speech.
- Numbness, weakness or paralysis in the face, arm or leg. This often affects just one side of the body. The person can try to raise both arms over the head. If one arm begins to fall, it may be a sign of a stroke. Also, one side of the mouth may droop when trying to smile.
- Problems seeing in one or both eyes. The person may suddenly have blurred or blackened vision in one or both eyes. Or the person may see double.
- Headache. A sudden, severe headache may be a symptom of a stroke. Vomiting, dizziness and a change in consciousness may occur with the headache.
- Trouble walking. Someone having a stroke may stumble or lose balance or coordination.
When to see a doctor
Seek immediate medical attention if you notice any symptoms of a stroke, even if they seem to come and go or they disappear completely. Think "FAST" and do the following:
- Face. Ask the person to smile. Does one side of the face droop?
- Arms. Ask the person to raise both arms. Does one arm drift downward? Or is one arm unable to rise?
- Speech. Ask the person to repeat a simple phrase. Is the person's speech slurred or different from usual?
- Time. If you see any of these signs, call 911 or emergency medical help right away.
Call 911 or your local emergency number immediately. Don't wait to see if symptoms stop. Every minute counts. The longer a stroke goes untreated, the greater the potential for brain damage and disability.
If you're with someone you suspect is having a stroke, watch the person carefully while waiting for emergency assistance.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
There are two main causes of stroke. An ischemic stroke is caused by a blocked artery in the brain. A hemorrhagic stroke is caused by leaking or bursting of a blood vessel in the brain. Some people may have only a temporary disruption of blood flow to the brain, known as a transient ischemic attack (TIA). A TIA doesn't cause lasting symptoms.
- Ischemic stroke
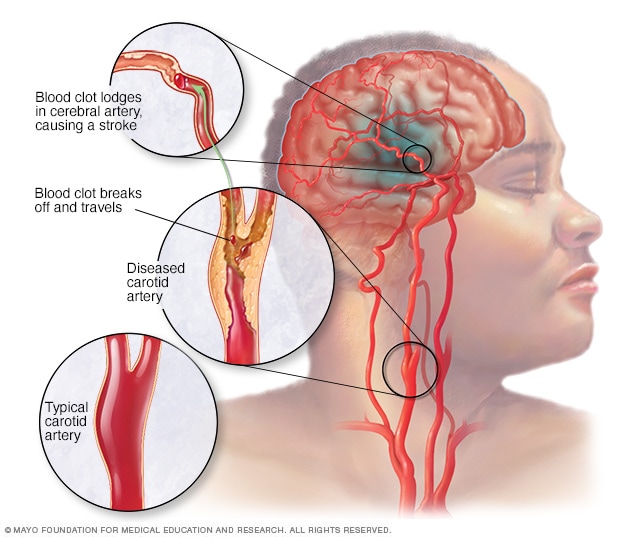
An ischemic stroke occurs when a blood clot, known as a thrombus, blocks or plugs an artery leading to the brain. A blood clot often forms in arteries damaged by a buildup of plaques, known as atherosclerosis. It can occur in the carotid artery of the neck as well as other arteries.
This is the most common type of stroke. It happens when the brain's blood vessels become narrowed or blocked. This causes reduced blood flow, known as ischemia. Blocked or narrowed blood vessels can be caused by fatty deposits that build up in blood vessels. Or they can be caused by blood clots or other debris that travel through the bloodstream, most often from the heart. An ischemic stroke occurs when fatty deposits, blood clots or other debris become lodged in the blood vessels in the brain.
Some early research shows that COVID-19 infection may increase the risk of ischemic stroke, but more study is needed.
Hemorrhagic stroke
Hemorrhagic stroke occurs when a blood vessel in the brain leaks or ruptures. Bleeding inside the brain, known as a brain hemorrhage, can result from many conditions that affect the blood vessels. Factors related to hemorrhagic stroke include:
- High blood pressure that's not under control.
- Overtreatment with blood thinners, also known as anticoagulants.
- Bulges at weak spots in the blood vessel walls, known as aneurysms.
- Head trauma, such as from a car accident.
- Protein deposits in blood vessel walls that lead to weakness in the vessel wall. This is known as cerebral amyloid angiopathy.
- Ischemic stroke that leads to a brain hemorrhage.
A less common cause of bleeding in the brain is the rupture of an arteriovenous malformation (AVM). An AVM is an irregular tangle of thin-walled blood vessels.
Transient ischemic attack
A transient ischemic attack (TIA) is a temporary period of symptoms similar to those of a stroke. But a TIA doesn't cause permanent damage. A TIA is caused by a temporary decrease in blood supply to part of the brain. The decrease may last as little as five minutes. A transient ischemic attack is sometimes known as a ministroke.
A TIA occurs when a blood clot or fatty deposit reduces or blocks blood flow to part of the nervous system.
Seek emergency care even if you think you've had a TIA . It's not possible to tell if you're having a stroke or TIA based only on the symptoms. If you've had a TIA , it means you may have a partially blocked or narrowed artery leading to the brain. Having a TIA increases your risk of having a stroke later.
Risk factors
Many factors can increase the risk of stroke. Potentially treatable stroke risk factors include:
Lifestyle risk factors
- Being overweight or obese.
- Physical inactivity.
- Heavy or binge drinking.
- Use of illegal drugs such as cocaine and methamphetamine.
Medical risk factors
- High blood pressure.
- Cigarette smoking or secondhand smoke exposure.
- High cholesterol.
- Obstructive sleep apnea.
- Cardiovascular disease, including heart failure, heart defects, heart infection or irregular heart rhythm, such as atrial fibrillation.
- Personal or family history of stroke, heart attack or transient ischemic attack.
- COVID-19 infection.
Other factors associated with a higher risk of stroke include:
- Age — People age 55 or older have a higher risk of stroke than do younger people.
- Race or ethnicity — African American and Hispanic people have a higher risk of stroke than do people of other races or ethnicities.
- Sex — Men have a higher risk of stroke than do women. Women are usually older when they have strokes, and they're more likely to die of strokes than are men.
- Hormones — Taking birth control pills or hormone therapies that include estrogen can increase risk.
Complications
A stroke can sometimes cause temporary or permanent disabilities. Complications depend on how long the brain lacks blood flow and which part is affected. Complications may include:
- Loss of muscle movement, known as paralysis. You may become paralyzed on one side of the body. Or you may lose control of certain muscles, such as those on one side of the face or one arm.
- Trouble talking or swallowing. A stroke might affect the muscles in the mouth and throat. This can make it hard to talk clearly, swallow or eat. You also may have trouble with language, including speaking or understanding speech, reading or writing.
- Memory loss or trouble thinking. Many people who have had strokes experience some memory loss. Others may have trouble thinking, reasoning, making judgments and understanding concepts.
- Emotional symptoms. People who have had strokes may have more trouble controlling their emotions. Or they may develop depression.
- Pain. Pain, numbness or other feelings may occur in the parts of the body affected by stroke. If a stroke causes you to lose feeling in the left arm, you may develop a tingling sensation in that arm.
- Changes in behavior and self-care. People who have had strokes may become more withdrawn. They also may need help with grooming and daily chores.
You can take steps to prevent a stroke. It's important to know your stroke risk factors and follow the advice of your healthcare professional about healthy lifestyle strategies. If you've had a stroke, these measures might help prevent another stroke. If you have had a transient ischemic attack (TIA), these steps can help lower your risk of a stroke. The follow-up care you receive in the hospital and afterward also may play a role.
Many stroke prevention strategies are the same as strategies to prevent heart disease. In general, healthy lifestyle recommendations include:
- Control high blood pressure, known as hypertension. This is one of the most important things you can do to reduce your stroke risk. If you've had a stroke, lowering your blood pressure can help prevent a TIA or stroke in the future. Healthy lifestyle changes and medicines often are used to treat high blood pressure.
- Lower the amount of cholesterol and saturated fat in your diet. Eating less cholesterol and fat, especially saturated fats and trans fats, may reduce buildup in the arteries. If you can't control your cholesterol through dietary changes alone, you may need a cholesterol-lowering medicine.
- Quit tobacco use. Smoking raises the risk of stroke for smokers and nonsmokers exposed to secondhand smoke. Quitting lowers your risk of stroke.
- Manage diabetes. Diet, exercise and losing weight can help you keep your blood sugar in a healthy range. If lifestyle factors aren't enough to control blood sugar, you may be prescribed diabetes medicine.
- Maintain a healthy weight. Being overweight contributes to other stroke risk factors, such as high blood pressure, cardiovascular disease and diabetes.
- Eat a diet rich in fruits and vegetables. Eating five or more servings of fruits or vegetables every day may reduce the risk of stroke. The Mediterranean diet, which emphasizes olive oil, fruit, nuts, vegetables and whole grains, may be helpful.
- Exercise regularly. Aerobic exercise reduces the risk of stroke in many ways. Exercise can lower blood pressure, increase the levels of good cholesterol, and improve the overall health of the blood vessels and heart. It also helps you lose weight, control diabetes and reduce stress. Gradually work up to at least 30 minutes of moderate physical activity on most or all days of the week. The American Heart association recommends getting 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous aerobic activity a week. Moderate intensity activities can include walking, jogging, swimming and bicycling.
- Drink alcohol in moderation, if at all. Drinking large amounts of alcohol increases the risk of high blood pressure, ischemic strokes and hemorrhagic strokes. Alcohol also may interact with other medicines you're taking. However, drinking small to moderate amounts of alcohol may help prevent ischemic stroke and decrease the blood's clotting tendency. A small to moderate amount is about one drink a day. Talk to your healthcare professional about what's appropriate for you.
- Treat obstructive sleep apnea (OSA). OSA is a sleep disorder that causes you to stop breathing for short periods several times during sleep. Your healthcare professional may recommend a sleep study if you have symptoms of OSA . Treatment includes a device that delivers positive airway pressure through a mask to keep the airway open while you sleep.
- Don't use illicit drugs. Certain illicit drugs such as cocaine and methamphetamine are established risk factors for a TIA or a stroke.
Preventive medicines
If you have had an ischemic stroke, you may need medicines to help lower your risk of having another stroke. If you have had a TIA , medicines can lower your risk of having a stroke in the future. These medicines may include:
Anti-platelet drugs. Platelets are cells in the blood that form clots. Anti-platelet medicines make these cells less sticky and less likely to clot. The most commonly used anti-platelet medicine is aspirin. Your healthcare professional can recommend the right dose of aspirin for you.
If you've had a TIA or minor stroke, you may take both an aspirin and an anti-platelet medicine such as clopidogrel (Plavix). These medicines may be prescribed for a period of time to reduce the risk of another stroke. If you can't take aspirin, you may be prescribed clopidogrel alone. Ticagrelor (Brilinta) is another anti-platelet medicine that can be used for stroke prevention.
Blooding-thinning medicines, known as anticoagulants. These medicines reduce blood clotting. Heparin is a fast-acting anticoagulant that may be used short-term in the hospital.
Slower acting warfarin (Jantoven) may be used over a longer term. Warfarin is a powerful blood-thinning medicine, so you need to take it exactly as directed and watch for side effects. You also need regular blood tests to monitor warfarin's effects.
Several newer blood-thinning medicines are available to prevent strokes in people who have a high risk. These medicines include dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (Savaysa). They work faster than warfarin and usually don't require regular blood tests or monitoring by your healthcare professional. These medicines also are associated with a lower risk of bleeding complications compared to warfarin.
Stroke care at Mayo Clinic
- Walls RM, et al., eds. Stroke. In: Rosen's Emergency Medicine: Concepts and Clinical Practice. 10th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Sept. 13, 2023.
- Ferri FF. Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Sept. 13, 2023.
- Patients and caregivers. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/public-education/know-stroke/patients-and-caregivers#. Accessed Sept. 13, 2023.
- Stroke. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/stroke. Accessed Sept. 13, 2023.
- Oliveira-Filho J, et al. Initial assessment and management of acute stroke. https://www.uptodate.com/contents/search. Accessed Sept. 13, 2023.
- About stroke. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/healthy_living.htm. Accessed Sept. 13, 2023.
- Effects of stroke. American Stroke Association. https://www.stroke.org/en/about-stroke/effects-of-stroke. Accessed Sept. 13, 2023.
- Rehab therapy after a stroke. American Stroke Association. https://www.stroke.org/en/life-after-stroke/stroke-rehab/rehab-therapy-after-a-stroke. Accessed Sept. 13, 2023.
- Arteriovenous malformations (AVMs). National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/arteriovenous-malformations-avms?search-term=arterial#. Accessed Oct. 2, 2023.
- Cerebral aneurysms. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/cerebral-aneurysms-fact-sheet. Accessed Sept. 13, 2023.
- Transient ischemic attack. Merck Manual Professional Version. https://www.merckmanuals.com/professional/neurologic-disorders/stroke/transient-ischemic-attack-tia?query=transient%20ischemic%20attack#. Accessed Sept. 13, 2023.
- Stroke. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Post-Stroke-Rehabilitation-Fact-Sheet. Accessed Sept. 13, 2023.
- Rose NS, et al. Overview of secondary prevention of ischemic stroke. https://www.uptodate.com/contents/search. Accessed Sept. 13, 2023.
- Prevent stroke: What you can do. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/prevention.htm#print. Accessed Sept. 13, 2023.
- Know your risk for stroke. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/risk_factors.htm#. Accessed Oct. 2, 2023.
- Powers WJ, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke — A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; doi:10.1161/STR.0000000000000211.
- Papadakis MA, et al., eds. Quick Medical Diagnosis & Treatment 2023. McGraw Hill; 2023. https://accessmedicine.mhmedical.com. Accessed Sept. 13, 2023.
- Tsao CW, et al. Heart disease and stroke statistics — 2023 update: A report from the American Heart Association. Circulation. 2023; doi:10.1161/CIR.0000000000001123.
- Grotta JC, et al., eds. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier, 2022. https://www.clinicalkey.com. Accessed Sept. 15, 2023.
- Suppah M, et al. An evidence-based approach to anticoagulation therapy comparing direct oral anticoagulants and vitamin K antagonists in patients with atrial fibrillation and bioprosthetic valves: A systematic review, meta-analysis and network meta-analysis. American Journal of Cardiology. 2023; doi:10.1016/j.amjcard.2023.07.141.
- Tyagi K, et al. Neurological manifestations of SARS-CoV-2: Complexity, mechanism and associated disorders. European Journal of Medical Research. 2023; doi:10.1186/s40001-023-01293-2.
- Siegler JE, et al. Cerebrovascular disease in COVID-19. Viruses. 2023; doi:10.3390/v15071598.
- Lombardo M, et al. Health effects of red wine consumption: A narrative review of an issue that still deserves debate. Nutrients. 2023; doi:10.3390/nu15081921.
- Jim J. Complications of carotid endarterectomy. https://www.uptodate.com/contents/search. Accessed Oct. 2, 2023.
- Van Nimwegan D, et al. Interventions for improving psychosocial well-being after stroke: A systematic review. International Journal of Nursing Studies. 2023; doi:10.1016/j.ijnurstu. 2023.104492 .
- Hasan TF, et al. Diagnosis and management of acute ischemic stroke. Mayo Clinic Proceedings. 2018; doi:10.1016/j.mayocp.2018.02.013.
- Ami TR. Allscripts EPSi. Mayo Clinic. Sept. 4, 2023.
- Barrett KM, et al. Ambulance-based assessment of NIH stroke scale with telemedicine: A feasibility pilot study. Journal of Telemedicine and Telecare. 2017; doi:10.1177/1357633X16648490.
- Sener U, et al. Ischemic stroke in patients with malignancy. Mayo Clinic Proceedings. 2022; doi:10.1016/j.mayocp.2022.09.003.
- Quality check. The Joint Commission. https://www.qualitycheck.org/search/?keyword=mayo%20clinic. Accessed Oct. 4, 2023.
- Quality care you can trust. American Heart Association. https://www.heart.org/en/professional/quality-improvement/hospital-maps. Accessed Oct. 4, 2023.
- Attig JM. Allscripts EPSi. Mayo Clinic. Oct. 9, 2023.
- How much physical activity do you need? American Heart Association. https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-infographic. Accessed Oct. 12, 2023.
- Graff-Radford J (expert opinion). Mayo Clinic. Oct. 11, 2023.
- Healthcare. DNV Healthcare USA, Inc. https://www.dnvhealthcareportal.com/hospitals?search_type=and&q=mayo+clinic&c=&c=20806&c=&c=&prSubmit=Search. Accessed Nov. 1, 2023.
- Brain hemisphere connections
- Cerebral angiogram
- CT scan of brain tissue damaged by stroke
- Stroke rehabilitation
- Strokes FAQ Neurologist Robert D. Brown, Jr. M.D., M.P.H., answers the most frequently asked questions about strokes.
- Typing with Brain Waves
Associated Procedures
- Carotid angioplasty and stenting
- Carotid endarterectomy
- Carotid ultrasound
- Coronary angioplasty and stents
- Echocardiogram
- Home enteral nutrition
News from Mayo Clinic
- Mayo Clinic Minute: How extreme temperatures can increase stroke risk July 20, 2023, 04:30 p.m. CDT
- Mayo Clinic Minute: What women need to know about stroke May 30, 2023, 04:15 p.m. CDT
- From lift off to splash down: An update on Mayo Clinic stem cells in space May 26, 2023, 04:39 p.m. CDT
- Mayo Clinic Minute: How to reduce your stroke risk May 10, 2023, 01:30 p.m. CDT
- A cheeseburger's role in one man's stroke recovery Nov. 11, 2022, 05:30 p.m. CDT
- Florida mom reunites with ICU team after stroke Oct. 28, 2022, 04:30 p.m. CDT
- Mayo Clinic Q&A podcast: World Stroke Day -- know the warning signs, take action Oct. 28, 2022, 12:30 p.m. CDT
- Mayo Clinic Minute: Flu vaccine may reduce risk of stroke Oct. 24, 2022, 04:00 p.m. CDT
- Mayo Clinic experts say time is most critical factor for better stroke outcomes Oct. 20, 2022, 05:58 p.m. CDT
- Sean Bretz reflects on overcoming stroke, becoming a dad Sept. 19, 2022, 04:30 p.m. CDT
- Mayo Clinic in Arizona receives comprehensive stroke center certification May 24, 2022, 04:00 p.m. CDT
- Mayo Clinic Q&A podcast: Don't ignore the warning signs of stroke May 24, 2022, 01:30 p.m. CDT
- Tips to reduce stroke in the diverse communities May 10, 2022, 03:00 p.m. CDT
- Mayo Clinic Minute: African Americans at higher risk of stroke May 06, 2022, 04:30 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Ischemic Stroke
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prognosis |
- Key Points |
Ischemic stroke is sudden neurologic deficits that result from focal cerebral ischemia associated with permanent brain infarction (eg, positive results on diffusion-weighted MRI). Common causes are atherothrombotic occlusion of large arteries; cerebral embolism (embolic infarction); nonthrombotic occlusion of small, deep cerebral arteries (lacunar infarction); and proximal arterial stenosis with hypotension that decreases cerebral blood flow in arterial watershed zones (hemodynamic stroke). No cause is identified in one-third of ischemic strokes at the time of patient discharge; these strokes are categorized as cryptogenic. Diagnosis is clinical, but CT or MRI is done to exclude hemorrhage and confirm the presence and extent of stroke. Thrombolytic therapy may be useful acutely in certain patients. Depending on the cause of stroke, carotid endarterectomy or stenting, antiplatelet medications, or anticoagulants may help reduce risk of subsequent strokes.
Etiology of Ischemic Stroke
The following are the modifiable risk factors that contribute the most to increased risk of ischemic stroke:
Hypertension
Cigarette smoking
Dyslipidemia
Insulin resistance
Abdominal obesity
Obstructive sleep apnea
Excess alcohol consumption
Lack of physical activity
High-risk diet (eg, high in saturated fats, trans fats, and calories)
Psychosocial stress (eg, depression )
Heart disorders (particularly disorders that predispose to emboli, such as acute myocardial infarction , infective endocarditis , and atrial fibrillation )
Carotid artery stenosis
Hypercoagulability
Use of exogenous estrogen
Unmodifiable risk factors include the following:
Prior stroke
Race/ethnicity
Family history of stroke
The most common causes of ischemic stroke can be classified as
Cryptogenic (ie, no clear cardioembolic, lacunar, or atherosclerotic source; the most common classification)
Cardioembolism
Lacunar infarcts.
Large-vessel atherosclerosis (the 4th most common cause)
Cryptogenic stroke
Stroke is classified as cryptogenic when one of the following occurs:
The diagnostic evaluation is incomplete.
No cause is identified despite an extensive evaluation.
There is more than one probable cause (eg, atrial fibrillation and ipsilateral carotid stenosis).
Embolic stroke of undetermined source (ESUS), a subcategory of cryptogenic stroke, is diagnosed when no source has been identified after sufficient diagnostic evaluation has excluded lacunar stroke, major cardioembolic sources, and ipsilateral steno-occlusive vessel disease (> 50% occlusion). Recent evidence suggests that symptomatic nonstenotic carotid disease with 1 ).
Emboli may lodge anywhere in the cerebral arterial tree.
Emboli may originate as cardiac thrombi, especially in the following conditions:
Atrial fibrillation
Rheumatic heart disease (usually mitral stenosis)
Post–myocardial infarction
Vegetations on heart valves in bacterial or marantic endocarditis
Atrial myxoma
Prosthetic heart valves
Mechanical circulatory assist devices (eg, left ventricular assist device, or LVAD [ 2 ])
Other sources include clots that form after open-heart surgery and atheromas in neck arteries or in the aortic arch. Rarely, emboli consist of fat (from fractured long bones), air (in decompression sickness ), or venous clots that pass from the right to the left side of the heart through a patent foramen ovale with shunt (paradoxical emboli). Emboli may dislodge spontaneously or after invasive cardiovascular procedures (eg, catheterization). Rarely, thrombosis of the subclavian artery results in embolic stroke in the vertebral artery or its branches.
Ischemic stroke can also result from lacunar infarcts. These small ( ≤ 1.5 cm) infarcts result from nonatherothrombotic obstruction of small, perforating arteries that supply deep cortical structures; the usual cause is lipohyalinosis (degeneration of the media of small arteries and replacement by lipids and collagen). Emboli may cause lacunar infarcts. Lacunar strokes >1.5 cm in patients without cardiovascular risk factors (eg, hypertension, diabetes, smoking) suggest a central embolic source.
Lacunar infarcts tend to occur in patients with diabetes or poorly controlled hypertension.
Large-vessel atherosclerosis
Large-vessel atherosclerosis can affect intracranial or extracranial arteries.
Atheromas, particularly if ulcerated, predispose to thrombi. Atheromas can occur in any major cerebral artery and are common at areas of turbulent flow, particularly at the carotid bifurcation. Partial or complete thrombotic occlusion occurs most often at the main trunk of the middle cerebral artery and its branches but is also common in the large arteries at the base of the brain, in deep perforating arteries, and in small cortical branches. The basilar artery and the segment of the internal carotid artery between the cavernous sinus and supraclinoid process are often occluded.
Other causes
Less common causes of stroke include vascular inflammation secondary to disorders such as acute or chronic meningitis , vasculitic disorders , and syphilis ; dissection of intracranial arteries or the aorta; hypercoagulability disorders (eg, antiphospholipid syndrome , hyperhomocysteinemia , underlying malignancy); hyperviscosity disorders (eg, polycythemia , thrombocytosis , hemoglobinopathies , plasma cell disorders ); and rare disorders (eg, fibromuscular dysplasia, moyamoya disease, Binswanger disease).
In children, sickle cell disease is a common cause of ischemic stroke.
Any factor that impairs systemic perfusion (eg, carbon monoxide toxicity, severe anemia or hypoxia, polycythemia, hypotension) increases risk of all types of ischemic strokes. A stroke may occur along the borders between territories of arteries (watershed areas); in such areas, blood supply is normally low, particularly if patients have hypotension and/or if major cerebral arteries are stenotic.
Etiology references
1. Ospel JM, Kappelhof M, Ganesh A, et al : Symptomatic non-stenotic carotid disease: current challenges and opportunities for diagnosis and treatment. J Neurointerv Surg jnis-2022-020005, 2023.. doi: 10.1136/jnis-2022-020005 Online ahead of print.
2. Caprio FZ, Sorond FA : Cerebrovascular disease: Primary and secondary stroke Prevention. Med Clin North Am 103 (2):295–308, 2019. doi: 10.1016/j.mcna.2018.10.001 Epub 2018 Nov 28.
Pathophysiology of Ischemic Stroke
Inadequate blood flow in a single brain artery can often be compensated for by an efficient collateral system, particularly between the carotid and vertebral arteries via anastomoses at the circle of Willis and, to a lesser extent, between major arteries supplying the cerebral hemispheres. However, normal variations in the circle of Willis and in the caliber of various collateral vessels, atherosclerosis, and other acquired arterial lesions can interfere with collateral flow, increasing the chance that blockage of one artery will cause brain ischemia.
Some neurons die when perfusion is < 5% of normal for > 5 minutes; however, the extent of damage depends on the severity of ischemia. If it is mild, damage proceeds slowly; thus, even if perfusion is 40% of normal, 3 to 6 hours may elapse before brain tissue is completely lost. However, if severe ischemia persists > 15 to 30 minutes, all of the affected tissue dies (infarction). Damage occurs more rapidly during hyperthermia and more slowly during hypothermia. If tissues are ischemic but not yet irreversibly damaged, promptly restoring blood flow may reduce or reverse injury. For example, intervention may be able to salvage the moderately ischemic areas (penumbras) that often surround areas of severe ischemia; penumbras exist because of collateral flow.
Mechanisms of ischemic injury include
Microvascular thrombosis
Programmed cell death (apoptosis)
Infarction with cell necrosis
Inflammatory mediators (eg, interleukin1-beta, tumor necrosis factor-alpha) contribute to edema and microvascular thrombosis. Edema, if severe or extensive, can increase intracranial pressure.
Many factors may contribute to necrotic cell death; they include loss of adenosine triphosphate (ATP) stores, loss of ionic homeostasis (including intracellular calcium accumulation), lipid peroxidative damage to cell membranes by free radicals (an iron-mediated process), excitatory neurotoxins (eg, glutamate), and intracellular acidosis due to accumulation of lactate.
Symptoms and Signs of Ischemic Stroke
Symptoms and signs of ischemic stroke depend on the part of brain affected. Patterns of neurologic deficits often suggest the affected artery (see table Selected Stroke Syndromes ), but correlation is often inexact.
Deficits may become maximal within several minutes of onset, typically in embolic stroke. Less often, deficits evolve slowly, usually over 24 to 48 hours (called evolving stroke or stroke in evolution), typically in atherothrombotic stroke.
In most evolving strokes, unilateral neurologic dysfunction (often beginning in one arm, then spreading ipsilaterally) extends without causing headache, pain, or fever. Progression is usually stepwise, interrupted by periods of stability.
A stroke is considered submaximal when after it is complete, there is residual function in the affected area, suggesting viable tissue at risk of damage.
Embolic strokes often occur during the day; headache may precede neurologic deficits. Thrombi tend to occur during the night and thus are first noticed on awakening.
Lacunar infarcts may produce one of the classic lacunar syndromes (eg, pure motor hemiparesis, pure sensory hemianesthesia, combined hemiparesis and hemianesthesia, ataxic hemiparesis, dysarthria–clumsy hand syndrome); signs of cortical dysfunction (eg, aphasia) are absent. Multiple lacunar infarcts may result in multi-infarct dementia .
A seizure may occur at stroke onset, more often with embolic than thrombotic stroke. Seizures may also occur months to years later; late seizures result from scarring or hemosiderin deposition at the site of ischemia.
Occasionally, fever develops.
Deterioration during the first 48 to 72 hours after onset of symptoms, particularly progressively impaired consciousness, results more often from cerebral edema than from extension of the infarct. Unless the infarct is large or extensive, function commonly improves within the first few days; further improvement occurs gradually for up to 1 year.
Diagnosis of Ischemic Stroke
Primarily clinical evaluation
Neuroimaging and bedside glucose testing
Evaluation to identify the cause
Diagnosis of ischemic stroke is suggested by sudden neurologic deficits referable to a specific arterial territory. Ischemic stroke must be distinguished from other causes of similar focal deficits (sometimes called stroke mimics, which are non-cerebrovascular disorders that cause focal neurologic signs (eg, hypoglycemia ), such as
Seizures (eg, with postictal paralysis)
CNS infections
Functional neurologic disorders (generally a diagnosis of exclusion)
Migraine (eg, hemiplegic migraine)
Headache, coma or stupor, and vomiting are more likely with hemorrhagic stroke than with an ischemic stroke.
When stroke is suspected, clinicians may use standardized criteria to grade severity and follow changes over time. This approach can be particularly useful as an outcome measure in efficacy studies. The National Institutes of Health Stroke Scale (NIHSS) is a 15-item scale to evaluate the patient's level of consciousness and language function and to identify motor and sensory deficits by asking the patient to answer questions and to perform physical and mental tasks. It is also useful for choosing appropriate treatment and predicting outcome.
Evaluation of ischemic stroke requires assessment of the brain parenchyma, vascular system (including the heart and large arteries), and blood.
Differentiating clinically between the types of stroke is imprecise; however, some clues based on symptom progression, time of onset, and type of deficit can help.
Although diagnosis is clinical, neuroimaging and bedside glucose testing are mandatory.
Distinction between lacunar, embolic, and thrombotic stroke based on history, examination, and neuroimaging is not always reliable, so tests to identify common or treatable causes and risk factors for all of these types of strokes are routinely done. Patients should be evaluated for the following categories of causes and risk factors:
Cardiac (eg, atrial fibrillation, potential structural sources of emboli)
Vascular (eg, critical arterial stenosis detected by vascular imaging)
Blood (eg, diabetes, dyslipidemia, hypercoagulability)
A cause cannot be identified for cryptogenic strokes.
Brain assessment
Neuroimaging with CT or MRI is done first to exclude intracerebral hemorrhage, subdural or epidural hematoma, and a rapidly growing, bleeding, or suddenly symptomatic tumor. CT evidence of even a large anterior circulation ischemic stroke may be subtle during the first few hours; changes may include effacement of sulci or the insular cortical ribbon, loss of the gray-white junction between cortex and white matter, and a dense middle cerebral artery sign. Within 6 to 12 hours of ischemia, medium-sized to large infarcts start to become visible as hypodensities; small infarcts (eg, lacunar infarcts) may be visible only with MRI.
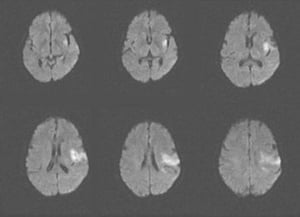
Diffusion-weighted MRI (highly sensitive for early ischemia) can be done immediately after initial CT neuroimaging.
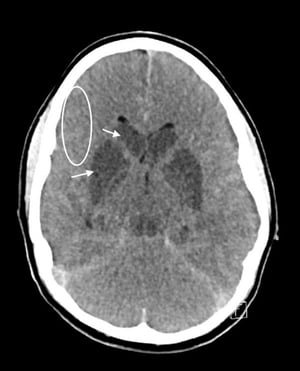
© 2017 Elliot K. Fishman, MD.
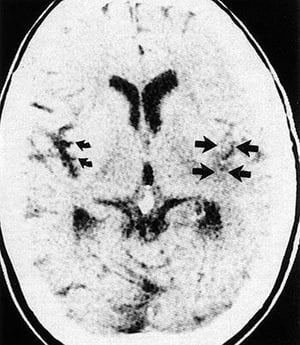
By permission of the publisher. From Geremia G, Greenlee W. In Atlas of Cerebrovascular Disease . Edited by PB Gorelick and MA Sloan. Philadelphia, Current Medicine, 1996.
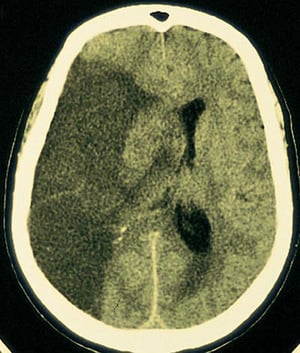
By permission of the publisher. From Furie K, et al: Cerebrovascular disease. In Atlas of Clinical Neurology . Edited by RN Rosenberg. Philadelphia, Current Medicine, 2002.
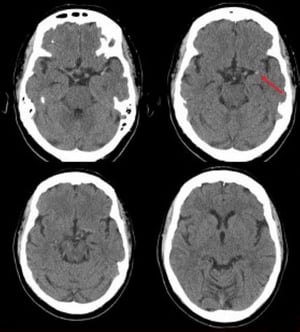
Image courtesy of Ji Y. Chong, MD.
Cardiac causes
For cardiac causes, testing typically includes ECG, telemetry or Holter monitoring, serum troponin, and transthoracic or transesophageal echocardiography. Implantable cardiac monitors are useful for detecting underlying atrial arrhythmias in patients with cryptogenic stroke ( 1 ).
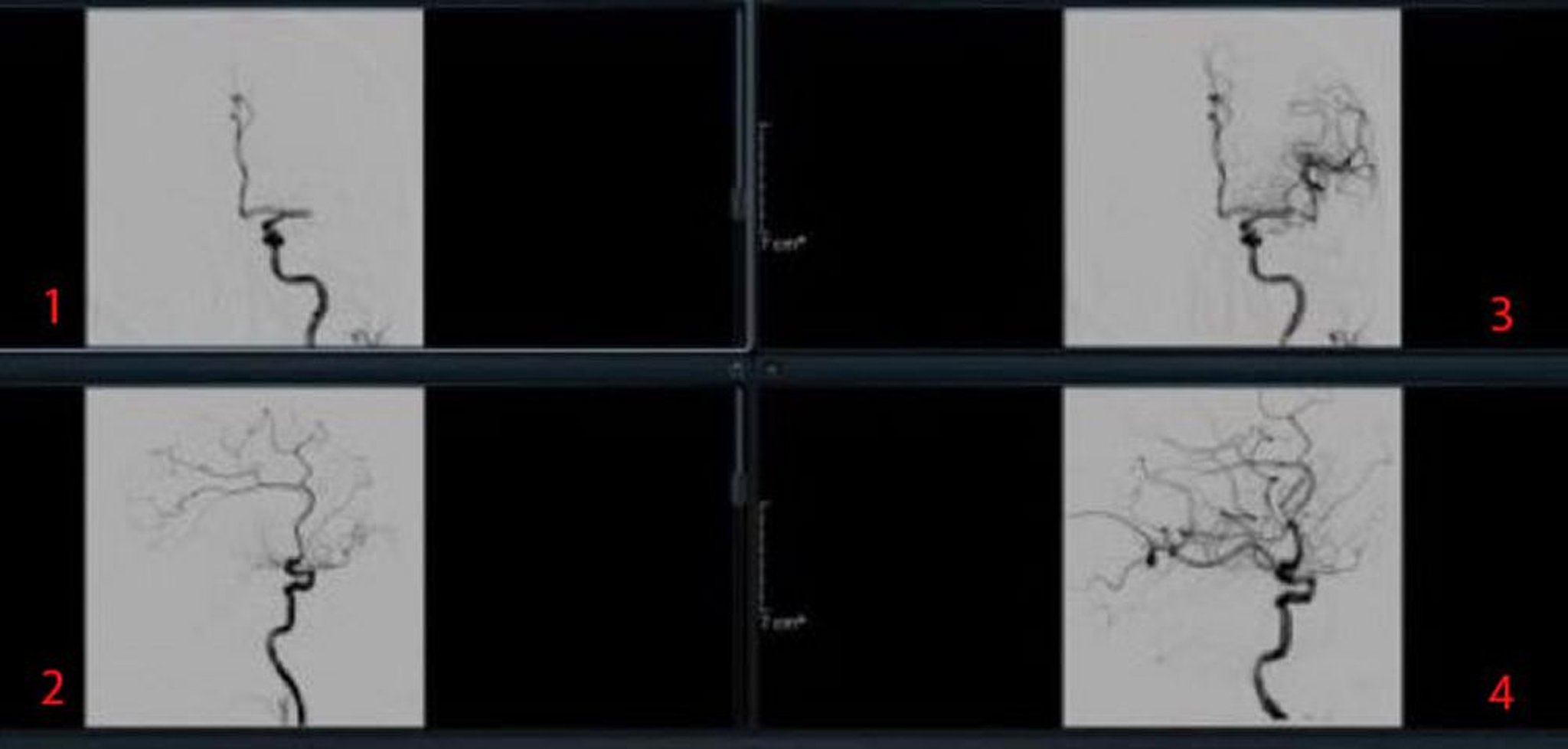
Vascular causes
For vascular causes, testing may include magnetic resonance angiography (MRA), CT angiography (CTA), carotid and transcranial duplex ultrasonography, and conventional angiography. The choice and sequence of testing is individualized, based on clinical findings. MRA, CTA, and carotid ultrasonography all show the anterior circulation; however, MRA and CTA provide better images of the posterior circulation than carotid ultrasonography. In general, CTA is preferred to MRA because motion artifacts are avoided. Usually, CTA or MRA should be done urgently but should not delay treatment with IV tPA if it is indicated.
Blood-related causes
For blood-related causes (eg, t hrombotic disorders ), blood tests are done to assess their contribution and that of other causes. Routine testing typically includes complete blood count (CBC), metabolic panel, prothrombin time/partial thromboplastin time (PT/PTT), fasting blood glucose, hemoglobin A1C, and lipid profile.
Diagnosis reference
1. Sanna T , Diener H-C., Passman RS, et al : Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370:2478–2486, 2014. doi: 10.1056/NEJMoa1313600
Treatment of Ischemic Stroke
General stroke treatments
Acute antihypertensive therapy only in certain circumstances
Sometimes carotid endarterectomy or stenting
Antiplatelet therapy
Sometimes anticoagulation
Long-term control of risk factors
For long-term treatment, rehabilitation
Acute stroke treatment
Guidelines for early management of stroke are available from the American Heart Association and American Stroke Association . Patients with acute ischemic strokes are usually hospitalized.
Supportive measures such as the following may be needed during initial evaluation and stabilization.
Airway support and ventilatory assistance if decreased consciousness or bulbar dysfunction compromises the airway
Supplemental oxygen only if needed to maintain oxygen saturation > 94%
Correction of hyperthermia (temperature > 38° C) by using an antipyretic medication and identifying and treating the cause of hypothermia
Treatment of hypoglycemia (blood glucose
Treatment of hyperglycemia to lower blood glucose to 140 to 180 mg/dL while closely monitoring for hypoglycemia
Perfusion of an ischemic brain area may require a high blood pressure (BP) because autoregulation is lost; thus, BP should not be decreased except in the following cases:
There are signs of other end-organ damage (eg, aortic dissection , acute myocardial infarction , pulmonary edema, hypertensive encephalopathy, retinal hemorrhages, acute renal failure ).
If BP is ≥ 220 mm Hg systolic or ≥ 120 mm Hg diastolic on 2 successive readings 15 minutes apart, lowering BP by 15% in the 24 hours after stroke onset is reasonable.
For patients who are eligible for acute reperfusion therapy, BP is treated to decrease it to
Patients with presumed thrombi or emboli may be treated with one or a combination of the following:
tPA, thrombolysis-in-situ, and/or mechanical thrombectomy
Antiplatelet medications
Anticoagulants
aspirin -induced or nonsteroidal anti-inflammatory drug (NSAID)-induced asthma or urticaria, other hypersensitivity to aspirin
Recombinant tPA ). Some experts recommend using tPA up to 4.5 hours after symptom onset (see Expansion of the Time Window for Treatment of Acute Ischemic Stroke With Intravenous Tissue Plasminogen Activator) ; however, between 3 hours and 4.5 hours after symptom onset, additional exclusion criteria apply (see table ). Thus, tPA must be given within 4.5 hours of symptom onset—a difficult requirement. Because the precise time of symptom onset may not be known, clinicians must start timing from the moment the patient was last observed to be well.
Although tPA can cause fatal or other symptomatic brain hemorrhage, patients treated with tPA strictly according to protocols still have a higher likelihood of functional neurologic recovery. Only clinicians experienced in stroke management should use tPA to treat patients with acute stroke; inexperienced physicians are more likely to violate protocols, resulting in more brain hemorrhages and deaths. When tPA is given incorrectly (eg, when given despite the presence of exclusion criteria), risk of hemorrhage due to tPA is high mainly for patients who have had stroke; risk of brain hemorrhage is very low (about 0.5%; 95% confidence interval of 0 to 2.0% [ 1 ]) for patients who have had a stroke mimic (eg, hemiplegic migraine, certain CNS infections, postictal paralysis, functional neurologic disorders). If experienced clinicians are not available on site, consultation with an expert at a stroke center (including video evaluation of the patient [telemedicine]), if possible, may enable these clinicians to use tPA. Because most poor outcomes result from failure to strictly adhere to the protocol, a checklist of inclusion and exclusion criteria should be used.
Before treatment with tPA, the following are required:
Brain hemorrhage must be excluded by CT
Systolic BP must be
Diastolic BP must be
Blood glucose must be > 50 mg/dL
Dose of tPA is 0.9 mg/kg IV (maximum dose 90 mg); 10% is given by rapid IV injection over 1 minute, and the remainder by constant infusion over 60 minutes. Vital signs are closely monitored for 24 hours after treatment. Any bleeding complications are aggressively managed. Anticoagulants and antiplatelet medications are not used within 24 hours of treatment with tPA.
Recent major surgery or procedure (eg, coronary artery bypass graft, obstetrical delivery, organ biopsy, previous puncture of noncompressible vessels)
Cerebrovascular disease
Recent intracranial hemorrhage
Recent gastrointestinal or genitourinary bleeding
Recent trauma
Hypertension (systolic BP > 175 mm Hg or diastolic BP > 110 mm Hg
Acute pericarditis
Subacute bacterial endocarditis
Hemostatic defects including those due to severe hepatic or renal disease
Significant hepatic dysfunction
Hemorrhagic diabetic retinopathy or other hemorrhagic ophthalmic conditions
Septic thrombophlebitis or occluded arteriovenous cannula at an infected site
Advanced age (> 77 years)
Thrombolysis-in-situ (angiographically directed intra-arterial thrombolysis) of a thrombus or embolus is almost obsolete except when a clot is too distal to be accessed by catheters (eg, distal A2 [anterior cerebral artery distal to the anterior communicating artery]).
Mechanical thrombectomy (angiographically directed intra-arterial removal of a thrombus or embolus by a stent retriever device) is standard of care in large stroke centers for patients with recent large-vessel occlusion in the anterior circulation.
Mechanical thrombectomy had previously been restricted to use within 6 hours of symptom onset in patients with internal carotid artery or middle cerebral artery occlusion. However, at comprehensive stroke centers, clinical and/or imaging findings that suggest a substantial amount of tissue at risk of infarction (penumbra) may justify later treatment. For example, the volume of infarcted tissue and at-risk underperfused tissue (ischemic penumbra) can be identified using perfusion CT or perfusion MRI. A sizeable mismatch between the infarct and at-risk volume identified by diffusion-weighted or perfusion-weighted imaging suggests that substantial penumbra is still potentially salvageable. In the DEFUSE 3 trial, benefit was evident up to 16 hours after symptom onset in patients with a small infarct and a larger penumbra; both findings are based on imaging criteria ( 5 ). In the DAWN trial, benefit was evident up to 24 hours after symptom onset in patients with a large mismatch between infarct volume based on imaging and severity of the clinical deficit based on clinical criteria ( 6 ); this finding suggests that salvageable penumbra is present.
Recently, evidence for the efficacy of mechanical thrombectomy in patients with posterior circulation strokes has been growing ( 7 ).
In the past, clinical trials restricted mechanical thrombectomy to patients with an NIHSS score > 6; however, recent evidence supports the usefulness of mechanical thrombectomy in patients with an NIHSS 8 ).
Current evidence supports the use of mechanical thrombectomy with IV thrombolysis (bridging therapy) for all patients who are otherwise eligible for thrombolysis ( 9 ). It should not be used as an alternative to IV recombinant tPA for patients with acute ischemic stroke if they are eligible for tPA. Devices used to remove thrombi are being improved, and recent models reestablish perfusion in 90 to 100% of patients.
Oral antiplatelet medications are used in acute stroke treatment to reduce the risk of recurrent disabling stroke. The following may be used:
aspirin alone for reducing risk of stroke in the first 90 days and does not increase risk of hemorrhage ( 11 ). However, prolonged (eg, > 3 months) use of clopidogrel plus aspirin is avoided because it has no advantage over aspirin
Anticoagulation
Usually, anticoagulation is avoided in the acute stage because risk of hemorrhage (hemorrhagic transformation) is higher, especially with large infarcts.
Long-term stroke treatment
Supportive care is continued during convalescence:
Controlling hyperglycemia and fever can limit brain damage after stroke, leading to better functional outcomes.
Screening for dysphagia before patients begin eating, drinking, or receiving oral medications can help identify patients at increased risk of aspiration; it should be done by a speech-language pathologist or other trained health care practitioner.
Enteral nutrition if needed should be started within 7 days of admission after an acute stoke.
Intermittent pneumatic compression (IPC) for deep venous thrombosis prophylaxis is recommended for immobile stroke patients without contraindications.
Measures to prevent pressure ulcers are started early.
Physical therapy to help maximize function and prevent sarcopenia and joint contractures
Long-term management also focuses on prevention of recurrent stroke (secondary prevention). Modifiable risk factors (eg, hypertension , diabetes , smoking , alcohol use disorder , dyslipidemia , obesity ) are treated. Reducing systolic BP may be more effective when the target BP is
Depression often occurs after a stroke and may interfere with recovery. Treatment of depression may aid in recovery, Clinicians should ask patients whether they are feeling sad or have lost interest or pleasure in doing formerly enjoyable activities. Clinicians should also ask family members whether they have noticed any signs of depression in the patient.
Extracranial carotid endarterectomy or stenting is indicated for patients with recent nondisabling, submaximal stroke attributed to an ipsilateral carotid obstruction of 70 to 99% of the arterial lumen or to an ulcerated plaque if life expectancy is at least 5 years. In other symptomatic patients (eg, patients with TIAs), endarterectomy or stenting with antiplatelet therapy is indicated for carotid obstruction of ≥ 60% with or without ulceration if life expectancy is at least 5 years. These procedures should be done by surgeons and interventionists who have a successful record with the procedure (ie, morbidity and mortality rate of < 3%) in the hospital where it will be done. If carotid stenosis is asymptomatic, endarterectomy or stenting is beneficial only when done by very experienced surgeons or interventionists, and that benefit is likely to be small. For many patients, carotid stenting with an emboli-protection device (a type of filter) is preferred to endarterectomy, particularly if patients are ≥ 70 years and have a high surgical risk. Carotid endarterectomy and stenting are equally effective for stroke prevention. In the periprocedural period, myocardial infarction is more likely after endarterectomy, and recurrent stroke is more likely after stenting.
Extracranial vertebral angioplasty and/or stenting can be used in certain patients with recurrent symptoms of vertebrobasilar ischemia despite optimal medical treatment and a vertebral artery obstruction of 50 to 99%.
Intracranial major artery angioplasty and/or stenting may be effective in patients when optimal treatment with medications has been ineffective. Key factors to consider are patient characteristics (eg, control of risk factors, adherence to the medication regimen), timing of the procedure (> 3 weeks after the stroke), and the interventionist's experience. Recent evidence indicates that the rate of periprocedural adverse events can be acceptably low after percutaneous transluminal angioplasty and stenting when these factors are considered ( 12 ).
Endovascular closure of a patent foramen ovale plus use of antiplatelet therapy is recommended for patients 13 , 14 ).
Oral antiplatelet medications are used to prevent subsequent noncardioembolic (atherothrombotic, lacunar, cryptogenic) strokes (secondary prevention). The following may be used:
aspirin aspirin
aspirin , if started during acute treatment, is given for only a short time (eg, aspirin aspirin
Oral anticoagulants
Treatment references
1. Tsivgoulis G, Zand R, Katsanos AH, et al : Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke 46 (5):1281–1287, 2015. doi: 10.1161/STROKEAHA.115.009012
2. Menon BK, Singh N, Sylaja, PN Lancet 25;401(10377):618–619, 2023. doi: 10.1016/S0140-6736(22)02633-2 Epub 2023 Feb 9.
3. Frank B, Grotta JC,.Alexandrov AV, et al : Thrombolysis in stroke despite contraindications or warnings? Stroke 44 (3):727–733, 2013. doi: 10.1161/STROKEAHA.112.674622 Epub 2013 Feb 6.
4. Highlights of prescribing information for alteplase . Accessed 6/17/23.
5. Albers GW, Marks MP, Kemp S, et al : Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378 (8):708–718, 2018. doi: 10.1056/NEJMoa1713973 Epub 2018 Jan 24.
6. Nogueira RG, Jadhav AP, Haussen DC, et al : Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378 (1):11–21, 2018. doi: 10.1056/NEJMoa1706442 Epub 2017 Nov 11.
7. Jovin TG, Li C, Wu L, et al : Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022; 387:1373-1384, 2022. doi: 10.1056/NEJMoa2207576
8. Abecassis IJ, Almallouhi E, Chalhoub R, et al : Outcomes after endovascular mechanical thrombectomy for low compared to high National Institutes of Health Stroke Scale (NIHSS): A multicenter study. Clin Neurol Neurosurg 225:107592, 2023. doi: 10.1016/j.clineuro.2023.107592 Epub 2023 Jan 13.
9. Masoud HE, de Havenon A, Castonguay AC, et al : 2022 Brief practice update on intravenous thrombolysis before thrombectomy in patients with large vessel occlusion acute ischemic stroke: A statement from Society of Vascular and Interventional Neurology Guidelines and Practice Standards (GAPS) Committee. Stroke. Vasc Interv Neurol 2 (4) 2022. doi: 10.1161/SVIN.121.000276
10. Zheng-Ming C, CAST (Chinese Acute Stroke Trial) Collaborative Group: Lancet 349 (9065):1641–1649, 1997.
11. Hao Q, Tampi M, O'Donnell M, et al BMJ 363:k5108, 2018. doi: 10.1136/bmj.k5108
12. Alexander MJ, Zauner A, Chaloupka JC, et al : WEAVE Trial: Final results in 152 on-label patients. Stroke 50 (4):889–894, 2019. doi: 10.1161/STROKEAHA.118.023996
13. Powers WJ, Rabinstein AA, Ackerson T, et al :Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50 (12):3331–3332, 2019. doi: 10.1161/STROKEAHA.119.027708 Epub 2019 Oct 30.
14. Kavinsky CJ, Szerlip M, Goldsweig AM, et al : SCAI guidelines for the management of patent foramen ovale. Standards and guidelines 1 (4), 100039, 2022. doi: 10.1016/j.jscai.2022.100039
Prognosis for Ischemic Stroke
Stroke severity and progression are often assessed using standardized measures such as the National Institutes of Health (NIH) Stroke Scale (see table The National Institutes of Health Stroke Scale ); the score on this scale correlates with extent of functional impairment and prognosis. During the first days, progression and outcome can be difficult to predict. Older age, impaired consciousness, aphasia, and brain stem signs suggest a poor prognosis. Early improvement and younger age suggest a favorable prognosis.
About 50% of patients with moderate or severe hemiplegia and most with milder deficits have a clear sensorium and eventually can take care of their basic needs and walk adequately. Complete neurologic recovery occurs in about 10%. Use of the affected limb is usually limited, and most deficits that remain after 12 months are permanent. Patients who have had a stroke are at high risk of subsequent strokes and each tends to worsen neurologic function. About 25% of patients who recover from a first stroke have another stroke within 5 years.
After an ischemic stroke, about 20% of patients die in the hospital; mortality rate increases with age.
Differentiate ischemic stroke from mimics (eg, postictal paralysis, hemiplegic migraine, CNS infections, functional neurologic disorders).
Although clinical differentiation is imprecise, some clues to help differentiate between common types of stroke include symptom progression (maximal deficits within minutes of onset with embolic versus sometimes stepwise or slow onset with thrombotic), time of onset (day with embolic versus night with thrombotic), and type of deficits (eg, specific syndromes and absence of cortical signs with lacunar infarcts).
Test patients for cardiac causes (including atrial fibrillation) and arterial stenosis (with vascular imaging), and do blood tests (eg, for thrombotic, rheumatic, and other disorders) as indicated.
In general, do not aggressively reduce BP soon after acute ischemic stroke.
To determine eligibility for tPA, use a checklist and, when available, consult an expert, either in person or via telemedicine.
To optimize the salvage of penumbral tissue, begin indicated thrombolytic therapy or mechanical thrombectomy as soon as possible ("time is brain").
To prevent future ischemic strokes, control modifiable risk factors and treat, when appropriate, with antiplatelet therapy, statin therapy, and/or endarterectomy or stenting.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Ischemic Stroke
- • A type of stroke that occurs when blood clot or fatty plaque blocks a blood vessel in the brain
- • Symptoms include drooping muscles on one side of face, numbness or weakness on one side of face or in one arm or leg
- • Treatment includes medication, medical procedures, surgical procedures
- • Involves stroke center, stroke telemedicine program, neurology, neurosurgery
- Lacunar Stroke
- Hemorrhagic Stroke
- Cerebrovascular Accident, Stroke
- Intracranial Artery Stenosis
What is an ischemic stroke?
What causes an ischemic stroke, what are the symptoms of an ischemic stroke, what are the risk factors for an ischemic stroke, how is an ischemic stroke diagnosed, how is an ischemic stroke treated, what is the outlook for people who have experienced ischemic stroke, what makes yale unique in its treatment of ischemic stroke.
Everyone has heard of stroke , but many people are not familiar with its symptoms or causes. A stroke occurs either when a blood vessel in the brain bursts, allowing blood to pool in the brain, or when the blood flow to part of the brain is blocked. Either way, affected parts of the brain become damaged or die. An ischemic stroke is the most common type of stroke. It occurs when a blood clot or fatty plaque lodges in a blood vessel within the brain, blocking blood flow. Because brain cells begin to die within minutes of the interruption of blood flow, it’s crucial for an ischemic stroke to be diagnosed and treated as quickly as possible.
In the United States, about 795,000 Americans experience some form of stroke each year, and most of those—about 87%—are ischemic strokes. Strokes are more common among adults aged 65 and older; the risk of stroke increases with age.
People who have one stroke are at higher risk of more strokes in the future; about one-quarter of all strokes occur in people who have had one previously. Some people will recover fully from an ischemic stroke. Others will experience disability afterward, and still others will die from the event. Stroke is the fifth-leading cause of death in the U.S., and a leading cause of disability.
An ischemic stroke is a life-threatening emergency condition. It arises when blood flow to the brain is blocked by a blood clot or a piece of fatty plaque that has broken off from the inside of a blood vessel. When blood can’t reach brain tissue, the tissue is at risk of being damaged or dying. This is why an ischemic stroke may cause brain damage, disability, or death.
In a healthy person, blood flows freely throughout the body, delivering oxygen to different body parts, including the brain. When a clot or piece of plaque disrupts the flow of blood to the brain, function is impaired. The symptoms this can cause depend on the area of the brain starved of blood. Sometimes, the disruption of blood flow can result in difficulty with speech, face or muscle weakness, or loss of coordination. Other times, it may cause cognitive problems. It’s important for ischemic stroke to be diagnosed and treated quickly to unblock blood flow before permanent damage can occur.
An ischemic stroke occurs when blood flow to the brain becomes blocked by a blood clot or a piece of fatty plaque. Some blood clots travel to the brain from the heart. In other circumstances, blood clots or pieces of fatty plaque may travel to the brain from a distant artery. It’s also possible for a piece of fatty plaque to originate in a brain artery, blocking the flow of blood.
In rare instances, clotting disorders or estrogen-containing oral contraceptives may cause blood clots to form, which may increase the risk of clots reaching the brain.
Those experiencing an ischemic stroke may have the following symptoms:
- Drooping muscles on one side of the face
- Numbness on one side of the face or in one arm or leg
- Weakness or paralysis in one arm, leg, or side of the body
- Loss of sensation or abnormal sensations on one side of the body
- Dizziness , balance problems
- Slurred speech
- Difficulty speaking and/or understanding speech
- Vision loss and/or double vision in one or both eyes
- Severe headache
- Memory problems
- Nausea or vomiting
People with the following health conditions may be at increased risk of ischemic stroke:
- High blood pressure
- Atrial fibrillation
- Cardiomyopathy
- Heart-valve disease
- High cholesterol levels
- Narrowing of the carotid artery in the neck
- Insulin resistance
- Sleep apnea
- Heart attack
- Having had recent heart surgery
- An infection of the valves of the heart
- A blood clotting disorder
- A personal or family history of stroke
Additionally, these lifestyle habits may increase the risk of ischemic stroke:
- Excessive alcohol intake
- Physical inactivity
- Eating a high-calorie diet that’s high in saturated fats and/or trans fats
- Using cocaine or amphetamines
A stroke is a life-threatening emergency that is typically diagnosed in the emergency department. If you or a loved one is experiencing stroke symptoms, call 911 immediately.
Doctors can make a diagnosis after learning about your medical history, performing a neurological exam, and running diagnostic tests. Because time is of the essence for stroke treatment, it’s important for the diagnosis to be made quickly.
Doctors may rely on your relative for details about your medical history if you are experiencing confusion or having difficulty speaking. You or your loved one should discuss any history of high blood pressure, high cholesterol, diabetes, or a previous stroke. Lifestyle habits, including smoking and alcohol intake, should also be disclosed.
In the emergency room, a dedicated stroke team will perform a rapid neurological assessment of your speech, facial muscles, strength and sensation in your arms and legs, and coordination and balance to see if you are having a stroke.
A diagnosis may be obtained from these diagnostic tests:
- An imaging test , such as a CT scan or MRI, to rule out other conditions, including hemorrhagic stroke, and diagnose the problem
- A blood sugar test , because low blood sugar levels may cause symptoms that are similar to a stroke
- CT angiography , which shows images of the blood vessels in the brain, which can be used to pinpoint the location of a blockage
- CT perfusion, to determine how much brain tissue is permanently damaged and how much can be saved
One or more of the following treatments will be administered as quickly as possible to restore blood flow to the brain:
- Tissue plasminogen activator (tPA) drugs, such as alteplase or tenecteplase, which are given intravenously within 3 hours (and for some patients up to 4.5 hours) of stroke onset to break apart a clot that is blocking blood flow within the brain. This is sometimes called thrombolytic therapy. Research has shown that the earlier a patient receives tPA, the more likely they are to have better outcomes.
- Thrombectomy, a surgical catheter-based procedure during which a blood clot that is blocking blood flow within a large artery in the brain is removed. Similar to tPA, the earlier a blocked artery is opened, the better the chances are of recovery.
As you recover from a stroke, medications may be prescribed to lower the risk of another one. The type of medication prescribed varies, based on the type of stroke you had. Possibilities include:
- Cholesterol-lowering drugs
- Medication to decrease blood pressure
- Antiplatelet therapy
- Anticoagulant medications
Lifestyle changes may also be recommended, including:
- Quitting smoking
- Consuming less alcohol
- Following a low-sodium Mediterranean diet
- Getting regular physical activity
- Losing weight and/or maintaining a healthy weight
To reduce the risk of additional strokes, doctors may recommend:
- Carotid endarterectomy, a surgical procedure during which some of the fatty plaque from the interior of the carotid artery will be removed.
- Stenting, a minimally invasive procedure during which a catheter is used to insert a mesh-wire, tube-shaped stent that helps to hold the carotid artery open, preventing future blockages.
People who seek immediate treatment in an emergency department for stroke symptoms are more likely to have better outcomes than those who avoid or delay treatment. Many people experience some degree of disability after an ischemic stroke, including muscle weakness, lack of coordination, difficulty with speech or swallowing, or cognitive symptoms. These symptoms can improve with aggressive physical, occupational, and speech therapies. The window for meaningful recovery is about six months but can be longer for some patients.
“The Comprehensive Stroke Center at Yale provides expertise in the management of both ischemic and hemorrhagic strokes,” says Yale Medicine stroke specialist Hardik Amin, MD. “We have a stroke team ready 24-7 that can perform state-of-the-art imaging in the emergency room and provide rapid medical and surgical treatments to maximize the chance of recovery.”
Our team of highly experienced stroke neurologists and neurosurgeons, dedicated trainees, specialized nurse practitioners, and nurse navigators provide comprehensive care for stroke patients from the moment they arrive at the emergency room, through their hospital stay, and when they are seen in our follow up clinics, he adds.
“By pairing a thoughtful, individualized approach with state-of-the-art imaging and diagnostic testing, we work to understand the cause of each patient’s stroke and how to lower the risk of future events. Our physical, occupational, and speech therapists provide detailed patient evaluations to set patients on a path to reaching their full rehabilitation potential,” he says. “We also participate in national clinical trials to help further our understanding of stroke causes and treatments.”
Ischemic Stroke
- Diagnosis |
- Treatment |
- Prognosis |
An ischemic stroke is death of an area of brain tissue (cerebral infarction) resulting from an inadequate supply of blood and oxygen to the brain due to blockage of an artery.
Ischemic stroke usually results when an artery to the brain is blocked, often by a blood clot and/or a fatty deposit due to atherosclerosis.
Symptoms occur suddenly and may include muscle weakness, paralysis, lost or abnormal sensation on one side of the body, difficulty speaking, confusion, problems with vision, dizziness, and loss of balance and coordination.
Diagnosis is usually based on symptoms and results of a physical examination and brain imaging.
Other imaging tests (computed tomography and magnetic resonance imaging) and blood tests are done to identify the cause of the stroke.
Treatment may include medications to break up blood clots or to make blood less likely to clot and procedures to physically remove blood clots, followed by rehabilitation.
About one third of people recover all or most of normal function after an ischemic stroke.
Preventive measures include control of risk factors, medications to make blood less likely to clot, and sometimes surgery or angioplasty to open blocked arteries.
(See also Overview of Stroke .)
Causes of Ischemic Stroke
An ischemic stroke typically results from blockage of an artery that supplies blood to the brain, most commonly a branch of one of the internal carotid arteries. As a result, brain cells are deprived of blood. Most brain cells die if they are deprived of blood for 4.5 hours.
Supplying the Brain With Blood
Common causes.
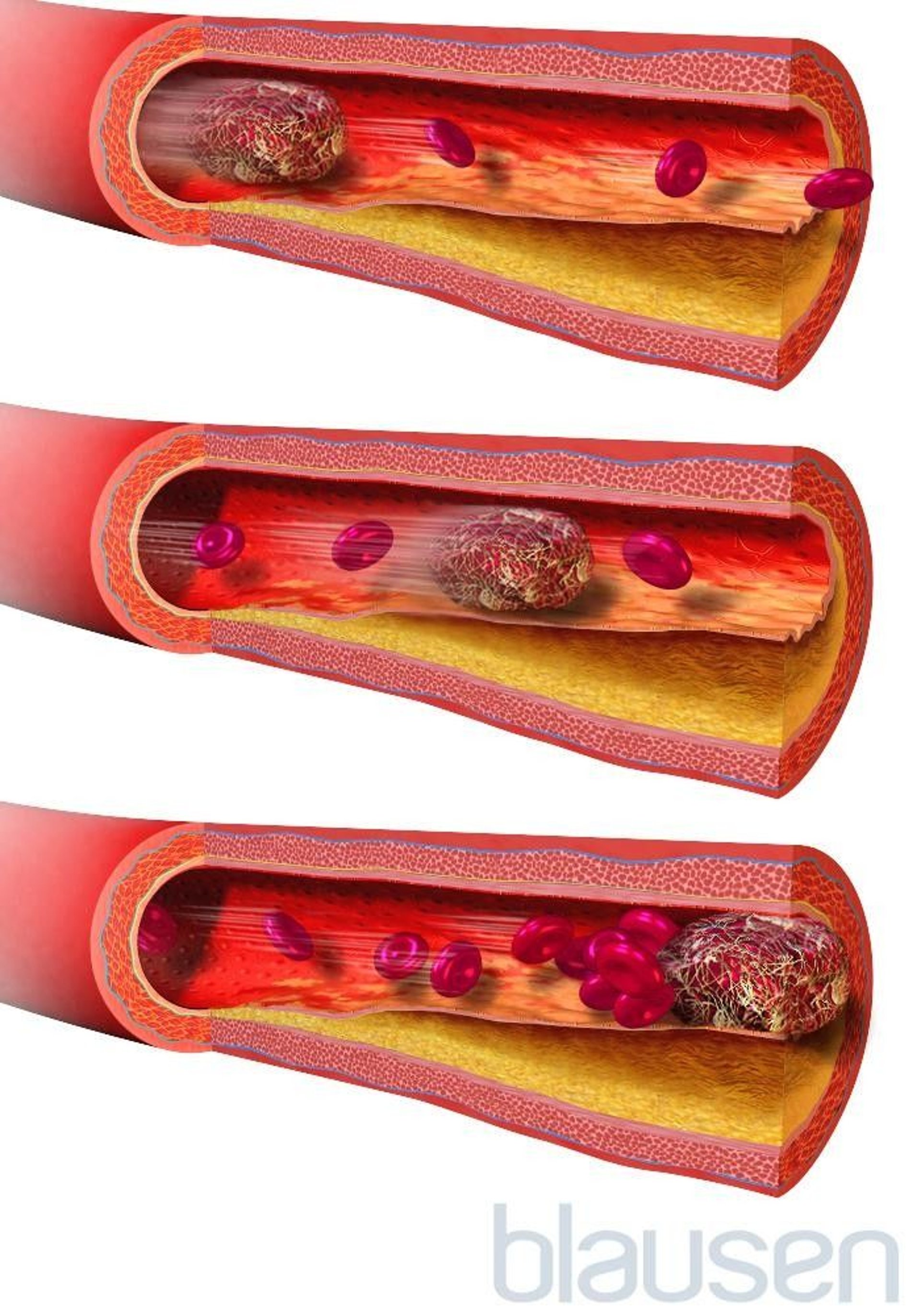
Commonly, blockages are blood clots (thrombi) or pieces of fatty deposits (atheromas, or plaques) due to atherosclerosis . Such blockages often occur in the following ways:
By forming in and blocking an artery: An atheroma in the wall of an artery may continue to accumulate fatty material and become large enough to block the artery. Even if the artery is not completely blocked, the atheroma narrows the artery and slows blood flow through it, like a clogged pipe slows the flow of water. Slow-moving blood is more likely to clot. A large clot can block enough blood flowing through the narrowed artery that brain cells supplied by that artery die. Or if an atheroma splits open (ruptures), the material in it can trigger formation of a blood clot that can block the artery (see figure How Atherosclerosis Develops ).
By traveling from another artery to an artery in the brain: A piece of an atheroma or a blood clot in the wall of an artery can break off and travel through the bloodstream (becoming an embolus). The embolus may then lodge in an artery that supplies the brain and block blood flow there. (Embolism refers to blockage of arteries by materials that travel through the bloodstream to another part of the body.) Such blockages are more likely to occur where arteries are already narrowed by fatty deposits.
By traveling from the heart to the brain: Blood clots may form in the heart or on a heart valve, particularly artificial valves and valves that have been damaged by infection of the heart's lining ( endocarditis ). These clots may break off and travel as emboli and block an artery to the brain. Strokes due to such blood clots are most common among people who have recently had heart surgery, who have had a heart attack, or who have a heart valve disorder or an abnormal heart rhythm (arrhythmia), especially a fast, irregular heart rhythm called atrial fibrillation .
Clogs and Clots: Causes of Ischemic Stroke
Blood clots in a brain artery do not always cause a stroke. If the clot breaks up spontaneously within less than 15 to 30 minutes, brain cells do not die and people's symptoms resolve. Such events are called transient ischemic attacks (TIAs).
If an artery narrows very gradually, other arteries (called collateral arteries—see figure Supplying the Brain With Blood ) sometimes enlarge to supply blood to the parts of the brain normally supplied by the clogged artery. Thus, if a clot occurs in an artery that has developed collateral arteries, people may not have symptoms.
The most common causes of ischemic stroke can be classified as
Cryptogenic stroke
Embolic stroke, lacunar infarction.
Large-vessel atherosclerosis (the 4th most common cause)
Stroke is classified as cryptogenic when no clear cause is identified despite a complete evaluation.
Blood clots can form in the heart, especially in people who have or have had the following:
Atrial fibrillation
Rheumatic heart disease (usually mitral stenosis )
Heart attack
Endocarditis
Atrial myxoma (a tumor)
Prosthetic heart valves
Mechanical circulatory assist devices (such as a left ventricular assist device )
Tiny pieces of these blood clots can break off and travel to small arteries in the brain (as emboli).
Lacunar infarction refers to tiny ischemic strokes, typically no larger than about a third of an inch (1 centimeter). In lacunar infarction, one of the small arteries deep in the brain becomes blocked when part of its wall deteriorates and is replaced by a mixture of fat and connective tissue—a disorder called lipohyalinosis. Lipohyalinosis is different from atherosclerosis, but both disorders can cause arteries to be blocked.
Lacunar infarction can also occur when tiny pieces of fatty material that has been deposited in arteries (atheromas or atherosclerotic plaques ) break off and travel to small arteries in the brain.
Lacunar infarction tends to occur in older people with diabetes or poorly controlled high blood pressure. Only a small part of the brain is damaged in lacunar infarction, and the prognosis is usually good. However, over time, many small lacunar infarcts may develop and cause problems, including problems with thinking and other mental functions (cognitive impairment).
Large-vessel atherosclerosis
In large-vessel atherosclerosis , atherosclerotic plaques develop in the walls of large arteries, such as those that supply the brain (cerebral arteries).
The plaques can gradually enlarge and cause the artery to narrow. As a result, tissues supplied by the artery may not receive enough blood and oxygen. Plaques tend to split open (rupture). Then material inside the plaque is exposed to the bloodstream. The material triggers the formation of blood clots (called thromboses). These blood clots can suddenly block all blood flow through an artery. Sometimes the blood clots break off, travel through the bloodstream and block an artery that supplies blood to the brain (called emboli). Both thromboses and emboli can cause a stroke by blocking the blood supply to an area of the brain.
Other causes
Several conditions besides rupture of an atheroma can trigger or promote the formation of blood clots, increasing the risk of blockage by a blood clot. They include the following:
Blood disorders: Some disorders, such as an excess of red blood cells ( polycythemia ), antiphospholipid syndrome , and a high homocysteine level in the blood ( hyperhomocysteinemia ), make blood more likely to clot. In children, sickle cell disease can cause ischemic stroke.
Oral contraceptives : Taking oral contraceptives, particularly those with a high estrogen dose, increases the risk of blood clots.
An ischemic stroke can also result from any disorder that reduces the amount of blood supplied to the brain. For example,
An ischemic stroke can occur if inflammation of blood vessels ( vasculitis ) or infection (such as herpes simplex , meningitis , or syphilis ) narrows blood vessels that supply the brain.
In atrial fibrillation , the heart does not contract normally, and blood can stagnate and clot. A clot may break loose, then travel to an artery in the brain, and block it.
Sometimes the layers of the walls of an artery that carries blood to the brain (such as arteries in the neck) separate from each other (called dissection) and interfere with blood flow to the brain.
Rarely, a stroke results from a general decrease in blood flow, as occurs when people lose a lot of blood, become severely dehydrated, or have very low blood pressure. This type of stroke often occurs when arteries supplying the brain are narrowed but had not previously caused any symptoms and had not been detected.
Occasionally, an ischemic stroke occurs when blood flow to the brain is normal but the blood does not contain enough oxygen. Disorders that reduce the oxygen content of blood include a severe deficiency of red blood cells ( anemia ), suffocation, and carbon monoxide poisoning . Usually, brain damage in such cases is widespread (diffuse), and coma results.
Sometimes a blood clot in a leg vein ( deep venous thrombosis ) or, rarely, small pieces of fat from the marrow of a broken leg bone move into the bloodstream. Usually, these blood clots and pieces of fat travel to the heart and block an artery in the lungs (called pulmonary embolism ). However, some people have an abnormal opening between the right and left upper chambers of the heart (called a patent foramen ovale). In such people, the blood clots and pieces of fat may go through the opening and thus bypass the lungs and enter the aorta (the largest artery in the body). If they travel to arteries in the brain, a stroke can result.
Risk factors
Some risk factors for ischemic stroke can be controlled or modified to some extent—for example, by treating the disorder that increases risk.
The major modifiable risk factors for ischemic stroke are
Narrowing (stenosis) of a carotid artery in the neck
High cholesterol levels
Coronary artery disease
High blood pressure
Insulin resistance (an inadequate response to insulin ), which occurs in type 2 diabetes
Cigarette smoking
Obesity , particularly if the excess weight is around the abdomen
Obstructive sleep apnea
Consumption of too much alcohol
Lack of physical activity
An unhealthy diet (such as one that is high in saturated fats , trans fats, and calories)
Depression or other mental stresses
Heart disorders that increase the risk of blood clots forming in the heart, breaking off, and traveling through the blood vessels as emboli (such a heart attack or an abnormal heart rhythm called atrial fibrillation )
Infective endocarditis (infection of the heart's lining and usually of the heart valves)
Use of or amphetamines
Inflammation of blood vessels ( vasculitis )
Clotting disorders that result in excessive clotting
Use of estrogen therapy, including oral contraceptives
Risk factors that cannot be modified include
Having had a stroke previously
Being older
Having relatives who have had a stroke
Symptoms of Ischemic Stroke
Usually, symptoms of an ischemic stroke occur suddenly and are often most severe a few minutes after they start because most ischemic strokes begin suddenly, develop rapidly, and cause death of brain tissue within minutes to hours. Then, most strokes become stable, causing little or no further damage. Strokes that remain stable for 2 to 3 days are called completed strokes. Sudden blockage by an embolus is most likely to cause this kind of stroke.
In about 10 to 15% of strokes, damage continues to occur and symptoms continue to worsen for up to 2 days, as a steadily enlarging area of brain tissue dies. Such strokes are called evolving strokes. In some people, symptoms affect one arm, then spread to other areas on the same side of the body. The progression of symptoms and damage usually occurs in steps, interrupted by somewhat stable periods. During these periods, the area temporarily stops enlarging or some improvement occurs. Such strokes are usually due to the formation of clots in a narrowed artery.
Strokes caused by an embolus often occur during the day, and a headache may be the first symptom. Strokes caused by a blood clot in a narrowed artery often occur at night and are first noticed when the person wakes up.
Many different symptoms can occur, depending on which artery is blocked and thus which part of the brain is deprived of blood and oxygen (see Brain Dysfunction by Location ).
When the arteries that branch from the internal carotid artery (which carry blood along the front of the neck to the brain) are affected, the following are most common:
Blindness in one eye
Loss of vision on either the left side or the right side of both eyes
Abnormal sensations, weakness, or paralysis in one arm or leg or on one side of the body
When the arteries that branch from the vertebral arteries (which carry blood along the back of the neck to the brain) are affected, the following are most common:
Dizziness and vertigo
Double vision or loss of vision in both eyes
Generalized weakness on one or both sides of the body
Many other symptoms, such as difficulty speaking (for example, slurred speech), impaired consciousness (such as confusion), loss of coordination, and urinary incontinence, can occur.
Severe strokes may lead to stupor or coma . In addition, strokes, even milder ones, can cause depression or an inability to control emotions. For example, people may cry or laugh inappropriately.
Some people have a seizure when the stroke begins. Seizures may also occur months to years later. Late seizures result from scarring or materials that are deposited from blood in the damaged brain tissue.
Occasionally, fever develops. It may be caused by the stroke or another disorder.
If symptoms, particularly impaired consciousness, worsen during the first 2 to 3 days, the cause is often swelling due to excess fluid (edema) in the brain. In large strokes, the swelling in the brain is typically at its worst about 3 days after the stroke begins. Symptoms usually lessen within a few days, as the fluid is absorbed. Nonetheless, the swelling is particularly dangerous because the skull does not expand. The resulting increase in pressure can cause the brain to shift, further impairing brain function, even if the area directly damaged by the stroke does not enlarge. If the pressure becomes very high, the brain may be forced sideways and downward in the skull, through the rigid structures that separate the brain into compartments. The resulting disorder is called herniation , which can be fatal.
Complications of stroke
Strokes can lead to other problems (complications):
If swallowing is difficult, people may not eat enough and become malnourished and dehydrated.
Food, saliva, or vomit may be inhaled (aspirated) into the lungs, resulting in aspiration pneumonia .
Being in one position too long can result in pressure sores and lead to muscle loss, deconditioning, urinary tract infections, and permanent shortening of muscles (contractures).
Not being able to move the legs can result in the formation of blood clots in deep veins of the legs and groin ( deep vein thrombosis ).
Clots in the deep veins of the legs can break off, travel through the bloodstream, and block an artery to a lung (a disorder called pulmonary embolism ).
People may have difficulty sleeping.
The losses and problems resulting from the stroke may make people depressed.
Diagnosis of Ischemic Stroke
A doctor's evaluation
Computed tomography and sometimes magnetic resonance imaging
Laboratory tests, including those to measure blood sugar
Doctors can usually diagnose an ischemic stroke based on the history of events and results of a physical examination. Doctors can usually identify which artery in the brain is blocked based on symptoms. For example, weakness or paralysis of the left leg suggests blockage of the artery supplying the area on the right side of the brain that controls the left leg’s muscle movements.
Doctors often use a standardized set of questions and commands to determine how severe the stroke is, how well people are functioning, and how symptoms are changing over time. These test helps doctors evaluate the person's level of consciousness, ability to answer questions, ability to obey simple commands, vision, arm and leg function, and speech.
When Specific Areas of the Brain Are Damaged
Doctors measure the blood sugar level. A low blood sugar level ( hypoglycemia ) can cause similar symptoms.
Computed tomography (CT) is usually done next. CT helps distinguish an ischemic stroke from a hemorrhagic stroke, a brain tumor, an abscess, and other structural abnormalities. However, during the first hours after some strokes, the CT scan may be normal or show only subtle changes. As a result, diagnosis may be delayed. So if available, diffusion-weighted magnetic resonance imaging (MRI), which can detect ischemic strokes within minutes of their start, may be done next.
As soon as possible, doctors may also do other imaging tests ( CT angiography or magnetic resonance angiography ) to check for blockages in large arteries. Prompt treatment of these blockages can sometimes limit the amount of brain damage caused by the stroke.
Tests to identify the cause
Identifying the precise cause of an ischemic stroke is important. If the blockage is a blood clot, another stroke may occur unless the underlying disorder is corrected. For example, if blood clots result from an abnormal heart rhythm, treating that disorder can prevent new clots from forming and causing another stroke.
Tests for causes may include the following:
Electrocardiography (ECG) to look for abnormal heart rhythms
Continuous ECG monitoring (done at home or in the hospital) to record the heart rate and rhythm continuously for 24 hours (or more), which may detect abnormal heart rhythms that occur unpredictably or briefly
Echocardiography to check the heart for blood clots, pumping or structural abnormalities, and valve disorders
Imaging tests— color Doppler ultrasonography , magnetic resonance angiography , CT angiography (CT done after a contrast agent is injected into a vein), or cerebral angiography (done using a catheter inserted into an artery to inject the contrast agent)—to determine whether arteries, especially the internal carotid arteries, are blocked or narrowed
Blood tests to check for anemia , polycythemia , blood clotting disorders , vasculitis , and some infections (such as heart valve infections and syphilis ) and for risk factors such as high cholesterol levels or diabetes
Urine drug screen for cocaine and amphetamines
Imaging tests enable doctors to determine how narrowed the carotid arteries are and thus to estimate the risk of a subsequent stroke or transient ischemic attack (TIA). Such information helps determine which treatments are needed.
For cerebral angiography, a thin, flexible tube (catheter) is inserted into an artery, usually in the groin, and threaded through the aorta to an artery in the neck. Then, a substance that can be seen on x-rays (radiopaque contrast agent) is injected to outline the artery. Thus, this test is more invasive than other tests that provide images of the brain’s blood supply. However, it provides more information. Cerebral angiography is done before any endovascular procedure that uses a catheter to treat a blocked or narrowed arteries. Cerebral angiography is also done when vasculitis is suspected.
Because CT angiography is less invasive, it has largely replaced cerebral angiography done with a catheter. The exceptions are when endovascular procedures are planned. These procedures involve using instruments threaded through a catheter to physically remove a clot ( mechanical thrombectomy ), to widen a narrowed artery (angioplasty), and/or to place a tube made of wire mesh (a stent) to keep the artery open.
Treatment of Ischemic Stroke
Measures to support vital functions, such as breathing
Medications to break up blood clots or make blood less likely to clot
Sometimes surgery to remove a blockage or angioplasty with a stent
Measures to manage problems that stroke can cause, such as difficulty swallowing
Measures to prevent blood clots in the legs
Rehabilitation
When a stroke occurs, minutes matter. The longer blood flow to the brain is reduced or stopped, the more brain damage there will be. People who have any symptom suggesting an ischemic stroke should immediately call 911 and go to an emergency department.
Treatment to remove or break up clots is most effective when done as soon as possible. For some medications (thrombolytic therapy) to be effective, they must be started within 4.5 hours of when the stroke began. Procedures to remove clots through a catheter (mechanical thrombectomy) can be effective up to 6 hours after a stroke began and sometimes even later. Starting treatment as soon as possible is crucial because the earlier blood flow is restored to the brain, the less brain damage there is and the better are the chances for recovery. Thus, doctors try to rapidly determine when the stroke began and confirm that the stroke is an ischemic stroke, not a hemorrhagic stroke, which is treated differently.
Generally, doctors do not immediately treat high blood pressure unless it is very high (over 220/120 mm Hg) because when arteries are narrowed, blood pressure must be higher than normal to push enough blood through them to the brain. However, very high blood pressure can injure the heart, kidneys, and eyes and must be lowered.
Specific treatment of stroke may include medications to break up blood clots (thrombolytic therapy) and medications to make blood less likely to clot (antiplatelet medications and anticoagulants), followed by rehabilitation. At some specialized centers, blood clots are physically removed from arteries in the brain (mechanical thrombectomy). Or angioplasty is done to widen the artery. For angioplasty, a catheter with a balloon at its tip is threaded into the narrowed artery (see figure Understanding Percutaneous Coronary Intervention (PCI) ). The balloon is then inflated for several seconds to widen the artery. To keep the artery open, doctors insert a tube made of wire mesh (a stent) into the artery.
Thrombolytic (fibrinolytic) medications
Because tPA can cause bleeding in the brain and elsewhere, it usually should not be given to people with certain conditions, such as the following:
Bleeding within the brain or a very large area of dead brain tissue detected by CT or MRI
A suspected hemorrhagic stroke , even if CT does not detect evidence of one
A tendency to bleed (indicated by a low platelet count or abnormal results of other blood tests)
Internal bleeding (hemorrhage)
A recent head injury (within the past 3 months)
A brain disorder that may increase the risk of bleeding, such as some cancers, an arteriovenous malformation (an abnormal connection between arteries and veins), or a cerebral aneurysm (a bulge in the wall of an artery)
Blood pressure that remains high after treatment with an antihypertensive medication
Brain or spinal surgery within the past 3 months
A tendency to bleed or bruise easily
Before tPA is given, CT is done to rule out bleeding in the brain. To be effective and safe, tPA, given intravenously, must be started within 3 hours of the beginning of an ischemic stroke. Some experts recommend using tPA up to 4.5 hours after an ischemic stroke begins.
But when tPA is given between 3 and 4.5 hours, additional conditions may prohibit its use. These conditions include
Being over age 80
Taking an anticoagulant by mouth (regardless of its effect on clotting)
Having a severe stroke that resulted in substantial loss of function
Having a history of both stroke and diabetes mellitus
After 4.5 hours, giving tPA intravenously increases the risk of bleeding.
Pinpointing when the stroke began may be difficult. So doctors assume that the stroke began the last time a person was known to be well. For example, if a person awakens with symptoms of a stroke, doctors assume the stroke began when the person was last seen awake and well. Thus, tPA can be used in only some people who have had a stroke. If advanced imaging identifies undamaged brain tissue, people may be given tPA even if doctors cannot determine when the stroke began—for example, if people wake up and have had a stroke sometime during the night.
Mechanical thrombectomy
For mechanical thrombectomy, doctors use a device to physically remove the blood clot in large cerebral arteries. This procedure is often done when people have had a severe stroke. New evidence suggests that mechanical thrombectomy can effectively treat people who have a stroke, regardless of its severity.
Mechanical thrombectomy is traditionally done within 6 hours of when symptoms began. However, the procedure can be done up to 24 hours after symptoms began if imaging tests show undamaged brain tissue. Thus, at some stroke centers, doctors are starting to use a special type of CT or MRI ( perfusion imaging ) and other imaging tests to determine how much a stroke has progressed, rather than going strictly by time. These tests can show how much blood flow has been reduced and indicate how much brain tissue may be saved. This approach (based on brain tissue status, not time) is especially useful when doctors are unsure of when the stroke began—for example, when people wake up in the morning and have symptoms of a stroke. If imaging tests show that blood flow is only somewhat reduced, treatment with mechanical thrombectomy up to 24 hours after symptoms start may still be able to save brain tissue. But if blood flow has been greatly reduced or has stopped, treatment after only 1 hour may be unable to save any brain tissue.
Different types of devices can be used. For example, the stent retriever may be used. It resembles a tiny wire cage. It can be attached to a catheter, which is inserted through an incision, often in the groin, and threaded to the clot. The cage is opened up, then closed around the clot, which is drawn out through a larger catheter. If done within 6 hours of the stroke's start, mechanical thrombectomy with a stent retriever can dramatically improve outcomes in people with a large blockage. Devices can restore blood flow in 90 to 100% of people.
Mechanical thrombectomy is done only in stroke centers.
Antiplatelet medications and anticoagulants
aspirin alone for reducing the risk of another stroke, but only if given within 24 hours after stroke symptoms began. It is given only for first 3 weeks after the stroke and reduces the risk of recurrence only for the first 3 months after a stroke. After that, the combination has no advantage over aspirin alone. Also, taking clopidogrel plus aspirin for more than 3 weeks increases the risk of bleeding by a small amount. However, the combination is sometimes given for 3 months in certain circumstances—for example, when people have a partial blockage of a large artery.
If people have been given a thrombolytic medication, doctors usually wait at least 24 hours before antiplatelet medications or anticoagulants are started because these medications add to the already increased risk of bleeding in the brain. Anticoagulants are not given to people who have uncontrolled high blood pressure or who have had a hemorrhagic stroke.
Carotid artery surgery
Once an ischemic stroke is completed, surgical removal of fatty deposits (atheromas, or plaques) due to atherosclerosis or clots in an internal carotid artery may be done (see figure Supplying the Brain With Blood ). This procedure, called carotid endarterectomy, can help if all of the following are present:
The stroke resulted from narrowing of a carotid artery by more than 70% (more than 60% in people who have been having transient ischemic attacks).
Some brain tissue supplied by the affected artery still functions after the stroke.
The person’s life expectancy is at least 5 years.
In such people, carotid endarterectomy may reduce the risk of subsequent strokes. This procedure also reestablishes the blood supply to the affected area, but it cannot restore lost function because some brain tissue is dead.
For carotid endarterectomy, a general anesthetic is used. The surgeon makes an incision in the neck over the area of the artery that contains the blockage, then an incision in the artery. The blockage is removed, and the incisions are closed. For a few days afterwards, the neck may hurt, and swallowing may be difficult. Most people stay in the hospital 1 or 2 days. Heavy lifting should be avoided for about 3 weeks. After several weeks, people can resume their usual activities.
Carotid endarterectomy can trigger a stroke because the operation may dislodge clots or other material that can then travel through the bloodstream and block an artery. However, after the operation, the risk of stroke is lower than it is when medications are used, and this risk is lower for several years. The procedure can result in a heart attack because people who have this procedure often have risk factors for coronary artery disease .
People should find a surgeon who is experienced doing this operation and who has a low rate of serious complications (such as heart attack, stroke, and death) after the operation. If people cannot find such a surgeon, the risks of endarterectomy may outweigh its expected benefits.
Carotid artery angioplasty and stenting
If endarterectomy is too risky or cannot be done because of the artery's anatomy, a less invasive procedure (carotid artery angioplasty) can be done to widen the artery.
For this procedure, a local anesthetic is given. Then a catheter with an umbrella filter at its tip is inserted through a small incision into a large artery near the groin or in the arm, and the catheter is threaded to the internal carotid artery in the neck. A substance that can be seen on x-rays (radiopaque contrast agent) is injected, and x-rays are taken so that the narrowed area can be located. Doctors use the catheter to widen the carotid artery, then insert a tube made of wire mesh (a stent) into the artery. Once in place, the stent is expanded to help keep the artery open. The filter catches any debris that may break off during the procedure.
After the stent is placed, the catheter and the filter at its tip are removed. People remain awake for the procedure, which usually takes 1 to 2 hours.
Placement of a stent appears to be as safe and as effective in preventing strokes and death as endarterectomy . For younger people and people who do not have risk factors for heart or blood vessel disorders (such as high blood pressure , high cholesterol levels , diabetes , and smoking ), carotid endarterectomy is usually done.
A similar procedure can be done for other types of large blocked arteries (see figure Understanding Percutaneous Coronary Intervention (PCI) ).
Long-term treatment of strokes
Long-term treatment of stroke includes measures to do the following:
Control problems that can make the effects of stroke worse
Prevent or treat problems caused by strokes
Prevent future strokes
Treat any disorders that are also present
During the recovery period, high blood sugar (hyperglycemia) and fever can make brain damage worse after a stroke. Lowering them limits the damage and results in better functioning.
Before people who have had a stroke start to eat, drink, or take medications by mouth, they are checked for problems with swallowing. Problems with swallowing can lead to aspiration pneumonia . Measures to prevent this problem are started early. If problems are detected, a therapist can teach people how to swallow safely. Sometimes people need to be fed through a tube ( tube feeding ).
If people cannot move on their own or have difficulty moving, they are at risk of developing blood clots in their legs ( deep vein thrombosis ) and pressure sores
Measures to prevent pressure sores are started early. For example, staff members periodically change the person's position in bed to help prevent pressure sores from forming. They also regularly inspect the skin for any sign of pressure sores.
Controlling or treating risk factors for stroke (such as high blood pressure, diabetes, smoking, consumption of too much alcohol, high cholesterol levels, and obesity) can help prevent future strokes.
Statins atherosclerosis ). Such therapy can help prevent strokes from recurring.
Antiplatelet medications , taken by mouth, may be used to reduce the risk of blood clots and thus help prevent strokes due to atherosclerosis. One of the following can be used:
aspirin alone, but only for the first 3 months after a stroke. After that, the combination has no advantage over aspirin alone. Also, taking clopidogrel plus aspirin
Anticoagulants
warfarin warfarin .
People who have atrial fibrillation or a heart valve disorder
If other disorders such as heart failure , abnormal heart rhythms, and lung infections are present, they must be treated.
Because a stroke often causes mood changes, especially depression , family members or friends should inform the doctor if the person seems depressed. Depression can be treated with antidepressants and psychotherapy .
Prognosis for Ischemic Stroke
The sooner a stroke is treated with a medication that breaks up blood clots (thrombolytic medication), the less severe brain damage is likely to be and the better the chances for recovery.
During the first few days after an ischemic stroke, doctors usually cannot predict whether a person will improve or worsen. Younger people and people who start improving quickly are likely to recover more fully.
About 50% of people with one-sided paralysis and most of those with less severe symptoms recover some function by the time they leave the hospital, and they can eventually take care of their basic needs. They can think clearly and walk adequately, although use of the affected arm or leg may be limited. Use of an arm is more often limited than use of a leg.
About 10% of people who have an ischemic stroke recover all normal function.
Some people are physically and mentally devastated and unable to move, speak, or eat normally.
About 20% of people who have an ischemic stroke die within 28 days. The proportion is higher among older people. About 25% of people who recover from a first stroke have another stroke within 5 years. Subsequent strokes impair function further.
Most impairments still present after 12 months are permanent.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.

Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
CALCULATORS
Related topics.
INTRODUCTION
Patient assessment and management during the acute phase (first few hours) of an ischemic stroke will be reviewed here. Use of thrombolytic therapy, endovascular thrombectomy, treatment of patients not eligible for reperfusion therapy, the clinical diagnosis of various types of stroke, and the subacute and long-term assessment of patients who have had a stroke are discussed separately. (See "Approach to reperfusion therapy for acute ischemic stroke" and "Early antithrombotic treatment of acute ischemic stroke and transient ischemic attack" and "Clinical diagnosis of stroke subtypes" and "Overview of the evaluation of stroke" .)
INITIAL ASSESSMENT
In addition, patients suffering a stroke may present with other serious medical conditions. Thus, the initial evaluation requires a rapid but broad assessment.
The goals in the initial phase include:

- See All Locations
- Primary Care
- Urgent Care Facilities
- Emergency Rooms
- Surgery Centers
- Medical Offices
- Imaging Facilities
- Browse All Specialties
- Diabetes & Endocrinology
- Digestive & Liver Diseases
- Ear, Nose & Throat
- General Surgery
- Neurology & Neurosurgery
- Obstetrics & Gynecology
- Orthopaedics
- Pain Medicine
- Pediatrics at Guerin Children’s
- Urgent Care
- Medical Records Request
- Insurance & Billing
- Pay Your Bill
- Advanced Healthcare Directive
- Initiate a Request
- Help Paying Your Bill
Ischemic Stroke
Ischemic strokes occur when blood supply is cut off to part of the brain. This type of stroke accounts for the majority of all strokes .
The blocked blood flow in an ischemic stroke may be caused by a blood clot or by atherosclerosis, a disease which causes narrowing of the arteries over time. Ischemic strokes can be caused by a blockage anywhere along the arteries feeding the brain.
Immediate emergency treatment is critical to surviving a stroke with the least amount of damage to the brain and ability to function.
Most ischemic strokes occur rapidly, over minutes to hours, and immediate medical care is vital. If you notice one or more of these signs in another person or in yourself, do not wait to seek help. Call 9-1-1 immediately.
The signs of a stroke are:
- Sudden numbness or weakness of the face, arm or leg, especially on one side of the body
- Sudden confusion
- Sudden trouble speaking
- Sudden trouble seeing in one or both eyes
- Sudden trouble walking
- Sudden dizziness, loss of balance or coordination
- Sudden, severe headache with no known cause
The effects of an acute ischemic stroke may cause additional symptoms in women including:
- Face, arm or leg pain
- Hiccups or nausea
- Chest pain or palpitations
- Shortness of breath
Not all symptoms occur with every stroke, and sometimes they go away and return.
Some patients experience symptoms that clear up within only a few minutes, which may be a sign of a transient ischemic attack (TIA) . This is known to be one of the early warning signs of a stroke.
Ischemic strokes occur when blood supply is cut off to part of the brain by a blood clot or narrowing of the arteries.
Blood clots may be caused by an irregular heartbeat such as arrhythmia , problems with the heart valve, infection of the heart muscle, hardening of the arteries, blood-clotting disorders, inflammation of the blood vessels, or a heart attack .
A less common cause of ischemic stroke occurs when blood pressure becomes too low (hypotension), reducing blood flow to the brain. This usually occurs with narrowed or diseased arteries. Low blood pressure can result from a heart attack, large loss of blood or severe infection. Each of these conditions affects the flow of blood through the heart and blood vessels and increases the risk of stroke.
Strokes can happen to a person of any age, including children. However, the older a person is, the higher their risk of stroke. Strokes are more common in men, but more women die from them. A family history of stroke, or a personal history of stroke or heart attack, also increase the risk of stroke. Research also has shown African-Americans are at higher risk of stroke than Caucasians.
The top preventable risk factor for stroke is smoking. Quitting smoking is a far more powerful way to prevent stroke than any other pill or procedure.
Uncontrolled high blood pressure , diabetes , coronary artery disease and high blood cholesterol are all risk factors for stroke.
In people younger than 50, the more common causes of stroke also include migraine, drug abuse, consumption of "energy" drinks or herbal supplements, and arterial dissection, which occurs when a small tear forms in the innermost lining of the artery wall allowing blood to leak into the space between the inner and outer layers of the vessel.
Diagnosis of an ischemic stroke usually is based on a detailed history of events and a physical examination. The Stroke Program at Cedars-Sinai accesses myriad diagnostic services to produce a detailed diagnosis, and allow for the best possible course of treatment.
In general, if a stroke is suspected, imaging tests, including magnetic resonance imaging (MRI) and computed tomography (CT) scans, will be done to produce a detailed picture of the brain.
Further testing may include:
- Electrical activity tests, including electroencephalogram (EEG) and evoked potential tests
- Blood flow tests, including angiography and echocardiography
Some diagnostic tests may be done to see if other conditions are present, check the person's overall health and see if the patient's blood clots too easily.
People who have symptoms of a stroke need emergency medical care. Immediate medical attention may prevent life-threatening complications, more widespread brain damage, and is critical for the recovery.
If emergency treatment is sought for ischemic stroke within the first three hours after symptoms begin, the patient may receive a medication to dissolve the clot, such as tissue plasminogen (tPA), which can increase the chances of a full recovery. A surgical procedure, known as thrombectomy, also may reverse stroke symptoms. Medication may be given to treat brain swelling or pressure that can occur after a stroke. Many effects of a stroke require oxygen or an intravenous line to provide the patient with fluids and nourishment.
Removing blood vessel blockages after a small stroke or transient ischemic attack (TIA) may reduce the risk of future strokes. In this case, carotid artery stenting or treating aneurysms or arteriovenous malformations may be recommended. Additional treatments of acute ischemic strokes vary according to the underlying cause.
The goals of treatment are to prevent life-threatening complications that may occur soon after stroke symptoms develop, prevent future strokes, reduce disability, prevent long-term complications and help the patient get back as much normal functioning as possible through rehabilitation.
Choose a doctor and schedule an appointment.
Get the care you need from world-class medical providers working with advanced technology.
Cedars-Sinai has a range of comprehensive treatment options.
(1-800-233-2771)
Available 7 days a week, 6 am - 9 pm PT
Expert Care for Life™ Starts Here
Looking for a physician.
Stroke Helpline
Monday to Friday: 9 am to 5 pm
Saturday: 10 am to 1 pm
Sunday: Closed
Supporter Relations
Saturday: Closed
- Professionals
Ischaemic stroke
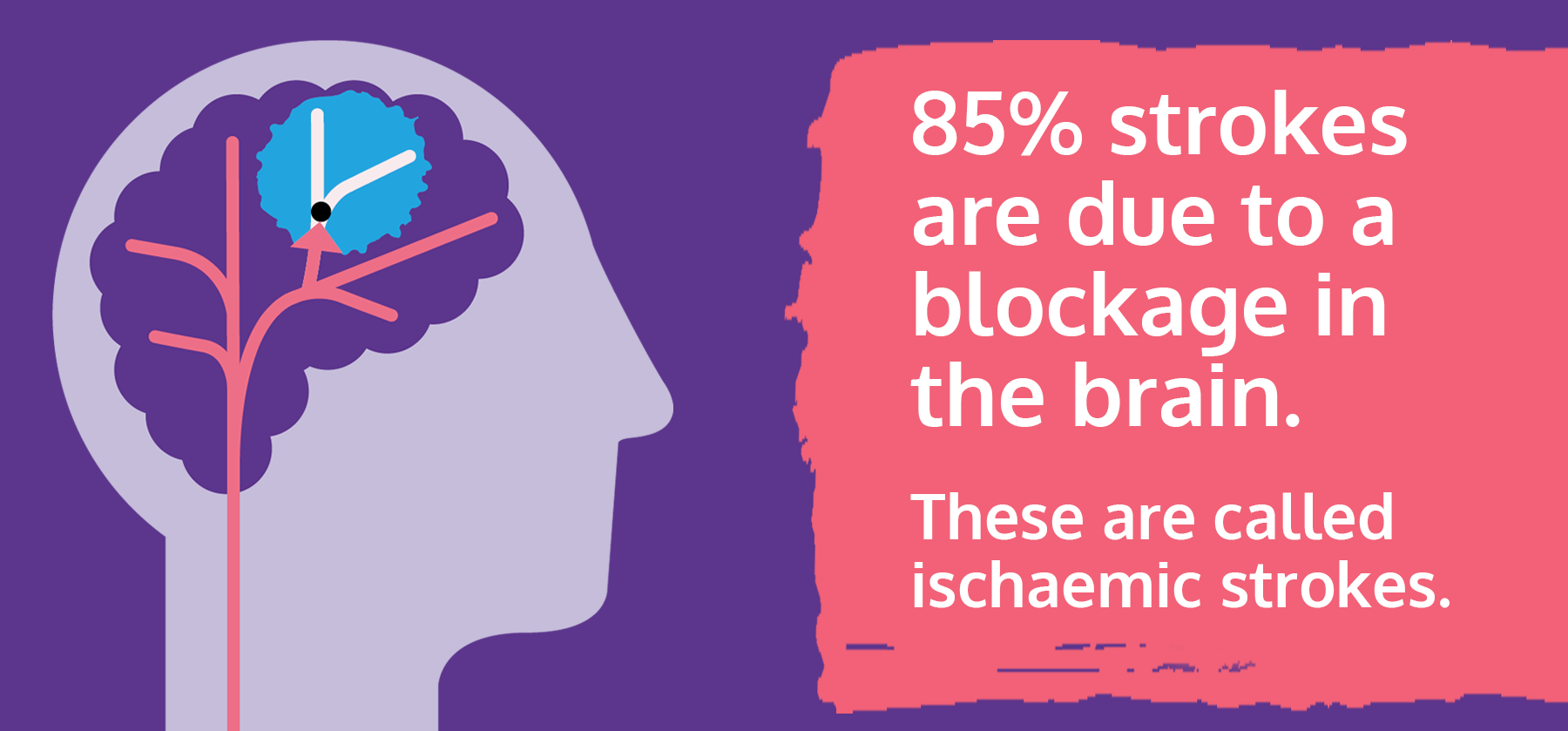
This webpage is about strokes due to a blockage in the blood supply to the brain, also known as ischaemic stroke.
The information on this page can be accessed in the following formats:
- Download this information as a pdf or large print document.
- Order a printed copy from our shop
- To request a braille copy, email [email protected]
On this page
What is an ischaemic stroke? What happens when you have an ischaemic stroke? What causes an ischaemic stroke? How is an ischaemic stroke diagnosed? Other checks and tests How is an ischaemic stroke treated? Treatments to break up or remove clots Your care in the first 24 hours after a stroke What effects can a stroke have? Will I be able to make a full recovery? Support after leaving hospital Will I have another stroke? Driving
What is an ischaemic stroke?
An ischaemic stroke happens when a blockage cuts off the blood supply to part of your brain, killing brain cells. Damage to brain cells can affect how the body works. It can also change how you think and feel.
It's the most common type of stroke, and around 85% of strokes in the UK are ischaemic strokes. The other 15% of strokes are due to bleeding in or around the brain, known as haemorrhagic stroke .
A transient ischaemic attack (TIA or mini-stroke) is the same as a stroke but the symptoms only last for a short amount of time. It is a major warning sign of a stroke and should always be taken seriously. For more information about the signs of stroke and TIA turn to 'Spotting the signs of stroke' near the end of this webpage.
What happens when you have an ischaemic stroke?
If you have an ischaemic stroke, you will be given specialist care and treatment, including medication to reduce your risk of another stroke. Afterwards, you will have support for your recovery including medical treatment and rehabilitation therapy.
The effects of your stroke depend on where the stroke was in your brain, and the amount of damage. For more information, see 'Effects of stroke' later on this page. You can also find comprehensive information about all the effects of stroke on our dedicated webpage .
What causes an ischaemic stroke?
There are a number of reasons why blockages can form and cause an ischaemic stroke.
Atherosclerosis (narrowed arteries)
Atherosclerosis is where fatty deposits build up on the inside walls of the blood vessels carrying blood away from the heart (arteries). These deposits are called plaques or atheromas.
Atheromas can build up in the large arteries in your neck leading to the brain, making them narrower and stiffer. Atheromas can break down or become inflamed. When this happens a clot forms around the atheroma, which can block the blood vessel. It may break off and move through the bloodstream into the brain, causing a stroke.
What causes atherosclerosis?
Some things can make you more likely to have a build-up of fatty materials in your blood vessels. These include:
- Medical conditions including high blood pressure, high cholesterol and diabetes.
- Lifestyle factors such as smoking and being overweight.
After a stroke, you should get advice about treating your medical conditions, and making healthy lifestyle changes. For more information about things you can do to stay healthy after a stroke, visit our reduce your risk webpage .
Small vessel disease
Small vessel disease means having damage to the tiny blood vessels deep inside the brain. The blood vessels become narrowed which reduces blood flow, and makes a stroke more likely. It can also lead to many small strokes, as well as increasing the risk of bleeding in the brain. Small vessel disease can be diagnosed on a brain scan, where it looks like scars in the brain structure. It can affect your thinking ability and your mood, and it's linked to cognitive decline and dementia.
What causes small vessel disease?
High blood pressure is a common cause of small vessel disease. If you have high blood pressure, you will be offered treatment and advice for healthy lifestyle changes you can make to reduce your blood pressure. Read more on our stroke and high blood pressure webpage .
Heart conditions
- Atrial fibrillation (irregular heartbeat)
Atrial fibrillation (AF) means your heartbeat is irregular and may be abnormally fast. Because the heart doesn't empty itself of blood at each heartbeat, a clot can form in the blood left behind. If this clot travels through the bloodstream to the brain, it causes a stroke.
AF often has no symptoms, but it can cause palpitations (feeling as if your heart is racing or skipping a beat). For more information about symptoms and diagnosing AF visit our atrial fibrillation webpage .
Patent foramen ovale (PFO, or hole in the heart)
All babies in the womb have an opening between the right and left side of their heart, known as the 'foramen ovale'. This gap is needed while the baby is connected to the mother's blood supply. After birth, the baby's blood circulation changes, and this gap usually closes up. However, in as many as one in four people, the gap stays open. This is known as a 'patent' (open) foramen ovale, or PFO. It's sometimes referred to as a 'hole in the heart'.
A PFO may be a risk for stroke, if a blood clot forms in the heart and travels up to the brain. PFO does not always cause problems, and may not need to be treated.
In children, surgery is sometimes be used to close the PFO. If you have a stroke, you will be assessed to decide if a PFO could have been a reason for your stroke, and what treatment you need. Treatment options include blood thinning medication to reduce the risk of clots, or surgery to close the PFO. Your doctor will talk to you about the best treatment for you.
Other heart conditions
Other heart problems, such as a recent heart attack or a mechanical heart valve, can also make a stroke more likely.
Arterial dissection (damage to the artery)
Arterial dissection is when the lining of an artery (a blood vessel leading away from the heart) gets torn. It can happen after an injury, but it can also happen with no obvious cause. Blood builds up in the damaged area, and a clot can form. If this clot restricts the flow of blood to your brain, or moves up into your brain, it can cause a stroke.
Other causes
Sometimes stroke can be associated with other health conditions such as inherited blood clotting disorders or heart infections. Your medical team will investigate these too.
How is an ischaemic stroke diagnosed?
If someone has any signs of a stroke, it's time to call 999 immediately.
Ambulance paramedics are trained in stroke. They assess the person and take them to the right type of hospital for the treatment they need. This could be a hospital with a specialist stroke unit or a hyper-acute stroke unit. A stroke unit has an inter-disciplinary team of trained professionals who are experienced in stroke care.
The important thing when a stroke happens is time. The faster someone can get to a specialist stroke unit, the better their chances of reducing damage to the brain.
Once you're admitted to hospital, you have tests and checks to confirm if you have had a stroke, and what type of stroke it is.
If you have a suspected stroke, a brain scan should be carried out urgently, and if possible within one hour of arriving at hospital. A brain scan can help doctors decide if you are suitable for an emergency treatment such as clot-busting treatment (thrombolysis) and mechanical clot removal (thrombectomy).
A computed tomography (CT) scan or a magnetic resonance imaging (MRI) scan is used to produce pictures of your brain. Doctors use scans to rule out other causes of your symptoms, and see how much of your brain has been affected. It also helps them decide how best to treat you, as treatments are different depending on the cause and timing of your stroke.
Some types of scan involve an injection to highlight the blood vessels of the neck and brain more clearly, known as computed tomography angiography (CTA) or magnetic resonance angiography (MRA).
Other checks and tests
Your blood pressure is checked, and you have blood tests for health conditions linked to stroke, such as diabetes and high cholesterol.
You may have other tests to check for conditions that could have contributed to your stroke. These include an electrocardiogram (ECG), which checks for an irregular heartbeat, or a Doppler ultrasound scan to check for narrowing of the blood vessels in your neck.
How is an ischaemic stroke treated?
The main treatments aiming to break up or remove clots from the brain are usually only available within a few hours of a stroke. But there is also a range of other types of care, including medication to reduce your blood pressure and reduce your risk of another stroke. You will be monitored for signs of complications and given any treatment you need. You will be assessed to find out how the stroke has affected you, and what help you need with your recovery.
Treatments to break up or remove clots
The two ways of treating clots in the brain are:
- Thrombolysis (clot-busting medication)
- Thrombectomy (mechanical clot removal)
Thrombolysis (clot-busting treatment)
Thrombolysis uses a clot-busting medicine to break up clots in the brain. This helps to save more of the brain by allowing blood to return to the brain cells more quickly. Fewer brain cells die, and the impact of the stroke can be reduced.
Thrombolysis needs to be given within four and a half hours of stroke symptoms starting. In some circumstances doctors may decide that it could still be of benefit beyond four and a half hours.
Who can have thrombolysis?
This treatment is only suitable in around 12% of strokes, as there are guidelines for who can and can't have it, to make sure it's safe and effective.
To have thrombolysis, the person needs to reach hospital within the time limits for treatment (usually four and a half hours after symptoms begin). If they don't know when symptoms began, perhaps because the stroke happened while they were asleep, this may rule out thrombolysis.
Other reasons why thrombolysis can't be given include:
- Your stroke was due to bleeding in the brain, not a clot.
- Your stroke is very mild.
- You have a bleeding disorder.
- You have recently had brain surgery.
- You have had another stroke or head injury within the past three months.
- Your current medication is not compatible with the clot-busting medication (alteplase).
If you are able to have thrombolysis, your medical team will explain the treatment to you. You do not have to sign any paperwork–a verbal agreement is enough. If you are unable to give your consent, either because of the effects of your stroke or another reason, the medical team will seek permission from your next of kin or another family member.
Time is critical so if it isn't immediately possible to talk to your family, the medical staff will make the decision based on what they feel is in your best interests.
How it works
Thrombolysis uses a drug called alteplase, or recombinant tissue plasminogen activator (rt-PA). You are given alteplase through a small tube into a vein in your arm. During this procedure, which takes around one hour, the medical team will closely monitor your blood pressure, body temperature, breathing and blood sugar levels to ensure that they remain stable.
Risks of thrombolysis
Despite its benefits, there is a risk that thrombolysis can cause bleeding in the brain. Within seven days of having thrombolysis, about one in 25 people treated will have bleeding in the brain, and this can be fatal in about one in 40 cases.
Doctors carefully balance the risk to the patient against the potential benefit of the treatment. So someone may not be eligible for thrombolysis if they have conditions like internal bleeding or head injury, an aneurysm or uncontrolled high blood pressure.
Thrombectomy (clot removal)
Thrombectomy involves pulling the blood clot out of your brain using a clot retrieval device. This is done by inserting a wire into a blood vessel in your groin, moving it up to your brain, and pulling the blood clot out.
Like thrombolysis, thrombectomy can help reduce brain damage by restoring blood flow in the brain. This means that fewer brain cells die, lowering the chance of serious disability.
This procedure can be given to around 10% of people with ischaemic stroke. It is only used when the clot is in a large blood vessel in the brain. It should be carried out as soon as possible after the stroke and within six hours at the latest. However it can be done up to 24 hours after the stroke, if doctors think it will benefit the person. It's often used in combination with thrombolysis (clot-busting medication).
What happens if the clot is not treated?
Clot removal and clot-busting treatment are effective at reducing disability after stroke, but only around 10-15% of people are able to have them. These treatments are given on top of the standard stroke care, which includes tests, medication and therapy.
Without removal or clot-busting treatment, the blood clot usually breaks up naturally within a few days or weeks. You are assessed to find out how the stroke is affecting you. You will be supported to recover by specialist doctors, nurses and therapists working in a team to give you expert care. You will also be given treatments to reduce your risk of another stroke, such as blood-thinning medications and pills for high blood pressure.
Surgery: decompressive hemicraniectomy
When the brain is injured the tissues can swell, just like a bruise. If there is a lot of swelling, it can put pressure on other areas of your brain, causing further damage.
In a very small number of cases an operation may be needed to relieve pressure on your brain. A decompressive hemicraniectomy involves opening up a section of your skull to allow the brain to swell outwards and relieve some of the pressure.
Treatments to reduce the risk of another stroke
Most people who have an ischaemic stroke will be given blood-thinning medication to help prevent clots from forming. For most people this will be a daily dose of aspirin followed by clopidogrel. If you receive thrombolysis, you normally have to wait at least 24 hours before you can begin taking aspirin.
How long will I need to take blood thinning medication?
Most people will need to take blood-thinning medication for life. There are two main types of blood-thinning medication, known as antiplatelets and anticoagulants. Many people need antiplatelets such as aspirin and clopidogrel People with heart conditions like atrial fibrillation are more likely to have an anticoagulant such apixaban, dabigatran, edoxaban, rivaroxaban or warfarin. Find out more about blood-thinning medications on our dedicated webpage .
Surgery for narrowed arteries in the neck (carotid artery disease)
Around 15% of ischaemic strokes are due to narrowed arteries in the neck, known as carotid artery disease. This is diagnosed using specialist ultrasound scans of your neck. Carotid artery disease is due to atherosclerosis, the build-up of fatty materials in your arteries.
Carotid artery disease is sometimes treated using a surgical procedure. This means either removing the artery lining, or inserting a mesh cylinder (stent) to keep the artery open. You'll be assessed to decide on the best treatment to help reduce your risk of a stroke, which might include medication instead of surgery.
For more information, read our carotid artery disease webpage .
Your care in the first 24 hours after a stroke
The team on the stroke unit continue to monitor you closely for at least 24 hours to ensure you remain stable. You should have a swallowing test within four hours of being in hospital, to make sure it's safe for you to eat and drink, or take medicine by mouth.
You may see some signs of recovery from your stroke early on, but if you're still showing lasting effects after 24 hours, you will have a full assessment with all the professionals on the stroke team. The team can include physiotherapist, speech and language therapist, occupational therapist, dietitian, orthoptist and a psychologist.
After 24 hours, you will be supported to get up, or walk around if it is safe for you to do so.
If you're not able to move about very much, the way you are positioned is very important to help you avoid problems with breathing, chest infections (pneumonia), shoulder pain or pressure sores. The members of your stroke team should work with you to find the best position for you to sit or lie down, and help you to move at regular intervals.
As soon as you are well enough, your doctor should talk to you about what may have caused your stroke and things you can do to reduce the risk of it happening again. This could mean taking medication, or making changes to your lifestyle, or both.
What effects can a stroke have?
The effects of stroke depend on the size and location of the damaged area in your brain. For some people the effects of a stroke may be relatively minor and may not last long, while others may be left with long-term effects or a disability.
- The effects of stroke include:
- Movement and balance problems
- Communication problems
- Problems with memory, concentration and thinking (cognition).
- Problems with vision.
- Problems with swallowing.
- Continence problems.
You can read more about all the effects of stroke in our next steps after a stroke webpage .
Emotional changes
Stroke can have a powerful emotional effect on you and the people around you. Many people have emotional changes after a stroke, including anxiety and depression. A stroke can change how people see themselves. Stroke usually comes as a big shock, and many people say they have lost some of their confidence.
Help is available with emotional problems, so if you feel low or anxious, or think you may be depressed, visit your GP.
Will I be able to make a full recovery?
Everyone recovers differently. Some people recover fully. Other people will have health problems or a disability. The fastest recovery takes place in the first few months. After that progress can be slower, but people can continue to improve for months or years after a stroke.
Rehabilitation
You should receive rehabilitation soon after your stroke. It may begin in hospital and should carry on at home if you need it. Rehabilitation is part of your recovery. It means trying to restore function to as near normal as possible, and helping you adapt to disability.
During rehabilitation, the therapist assesses you and designs treatment tailored to your needs. Depending on the type of therapy, you may have exercises to practise. You may work towards building up stamina, or learn new ways of doing things.
Neuroplasticity
Although brain cells that have been severely damaged or have died can't grow back, the brain can re-wire itself, allowing you to relearn things like walking, speech and swallowing. This is called neuroplasticity . Neuroplasticity is the process that happens in the brain when you do rehabilitation therapy. By repeating the therapy activities, your brain starts to form new connections, allowing you to improve.
Support after leaving hospital
When you are able to leave hospital, the discharge process should ensure that you get all the support you need. You and your family will be involved in planning your discharge. The discharge plan covers:
- Rehabilitation.
- Medical treatment.
- Care at home.
- Equipment you may need.
Early supported discharge
Some people can leave hospital soon after a stroke and have their treatment and therapy at home. You need to be able to move from a bed to a chair, and have a safe home environment to go to.
Post-stroke review
Around six months after you leave hospital, you should get a review of your progress. This makes sure you are getting the right support if your needs have changed, including rehabilitation. The review is sometimes carried out by a stroke specialist nurse or other stroke professional. In some areas, you may see a Stroke Association Coordinator . If a review does not take place, contact your GP.
Who will support me?
- Your GP coordinates your care after leaving hospital, and can help with your medical problems or support needs.
- You might need support from therapists, such as physiotherapists, occupational therapists, speech and language therapists and psychologists.
- You might have a community stroke nurse.
- You may have a social worker.
- Depending on where you live, you may have help from a Stroke Association Coordinator.
For more information about support and life after stroke including accommodation, money and benefits, and information for carers, visit our life after stroke page .
Will I have another stroke?
One of the biggest worries for many people is whether they will have another stroke. This can be part of the emotional impact of stroke on you, your family and friends. But it can help to know that when you have a stroke, one of the main aims of your hospital team is to stop you having another stroke.
Brain scans and other test and checks find out what caused your stroke and allow doctors to target your treatment. After an ischaemic stroke, you will be given medicine to avoid blood clots forming. If you have a health condition linked to stroke such as high blood pressure, you will be given any treatment and advice that you need to help you avoid another stroke.
Having a stroke or TIA means that you are at greater risk of having another stroke. The risk is highest in the days and weeks after a stroke, which is why doctors work so hard to reduce your risk early on.
In the months and years after a stroke, you could help to keep your risks low by following the treatments for your health conditions, and making healthy lifestyle changes.
When you have a stroke, doctors check you for any health conditions linked to stroke. These health conditions include:
- High blood pressure
- High cholesterol
One of the best ways to reduce your risk is to carry on with any treatment you are given. If you have any questions about your medication, speak to your GP or pharmacist. Never stop taking your medication without talking to your GP first.
You should also be given advice about other ways of reducing your risk of a stroke. Some people need to lose weight, be more active, give up smoking or drink less alcohol.
Ask your GP what you can do to reduce your risk of another stroke. Read our how to reduce your risk of a stroke webpage .
Read more about the symptoms of stroke and how to spot the signs of stroke here .
By law, you must not drive for a month after a stroke or TIA. You might need to tell the DVLA (or DVA if you are in Northern Ireland) about your stroke. Depending what kind of stroke you had and the kind of driving licence you hold, you might not be able to drive for a longer period or may have to stop driving. To find out what you should do, read our driving after stroke webpage .
Useful links

- Ischemic stroke
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Frank Gaillard had no recorded disclosures.
At the time the article was last revised Brooke Cao had no financial relationships to ineligible companies to disclose.
- Cerebral ischaemia
- Cerebral infarction
- Ischaemic strokes
- Ischemic strokes
- Ischaemic infarction
- Ischemic infarction
- Non hemorrhagic infarction
- Non haemorrhagic infarction
- Non hemorrhagic infarct
- Non haemorrhagic infarct
- Cerebral ischemia
- Ischaemic CVA
Ischemic stroke is an episode of neurological dysfunction due to focal infarction in the central nervous system attributed to arterial thrombosis, embolization, or critical hypoperfusion. While ischemic stroke is formally defined to include brain, spinal cord , and retinal infarcts 1 , in common usage, it mainly refers to cerebral infarction, which is the focus of this article.
On this page:
Terminology, epidemiology, clinical presentation, radiographic features, treatment and prognosis.
- Related articles
- Cases and figures
- Imaging differential diagnosis
The term " stroke " is a clinical determination, whereas "infarction" is fundamentally a pathologic term 1 . Bridging these terms, ischemic stroke is the subtype of stroke that requires both a clinical neurologic deficit and evidence of CNS infarction (cell death attributable to ischemia). The evidence of infarction may be based on imaging, pathology, and/or persistent neurologic symptoms, with other causes excluded. If there is imaging or pathologic evidence of an infarct but no attributable clinical symptoms, then it is called a "silent CNS infarction."
Stroke is the second most common cause of morbidity worldwide (after myocardial infarction) and is the leading cause of acquired disability 2 .
Risk factors for ischemic stroke largely mirror the risk factors for atherosclerosis and include age, gender, family history, smoking, hypertension , hypercholesterolemia , and diabetes mellitus .
An ischemic stroke typically presents with rapid onset neurological deficit, which is determined by the area of the brain that is involved. The symptoms often evolve over hours and may worsen or improve, depending on the fate of the ischemic penumbra.
The vascular territory affected will determine the exact symptoms and clinical behavior of the lesion:
anterior circulation infarct
- anterior cerebral artery infarct
middle cerebral artery infarct
lacunar infarct
- striatocapsular infarct
posterior circulation infarct
posterior cerebral artery infarct
- cerebellar infarct
- brainstem infarct
Interruption of blood flow through an intracranial artery leads to deprivation of oxygen and glucose in the supplied vascular territory. This initiates a cascade of events at a cellular level which, if circulation is not re-established in time, will lead to cell death, mostly through liquefactive necrosis.
The mechanism of vessel obstruction is important in addressing therapeutic maneuvers to both attempt to reverse or minimize the effects and to prevent future infarcts. Popular and simple etiological classifications of ischemic stroke include the TOAST classification and ASCOD classification 20 .
Examples of etiologies include:
cardiac embolism (e.g. atrial fibrillation , ventricular aneurysm , endocarditis )
paradoxical embolism
artery-to-artery (atherosclerotic) embolism (e.g. internal carotid artery stenosis , intracranial atherosclerotic disease )
fat embolism
air embolism
perforator thrombosis: lacunar infarct
acute plaque rupture with overlying thrombosis
arterial dissection
Global cerebral hypoxia (e.g. as is seen in drowning or asphyxiation) is usually considered separately.
In many institutions with active stroke services which provide reperfusion therapies, a so-called code stroke aimed at expediting diagnosis and treatment of patients will include a non-contrast CT brain, CT perfusion and CT angiography .
Aging ischemic strokes can be important in a number of clinical and medicolegal settings. Both CT and MRI can help in determining when a stroke occurred as imaging features evolve in a reasonably predictable fashion. There is substantial heterogeneity in the terminology denoting time from onset. For the purposes of this article, the following definitions are used 10 :
early hyperacute: 0 to 6 hours
late hyperacute: 6 to 24 hours
acute: 24 hours to 1 week
subacute: 1 to 3 weeks
chronic: more than 3 weeks
The above definition of hyperacute as 0-24 hours and acute as 1-7 days was affirmed by the international Stroke Recovery and Rehabilitation Roundtable 19 . However, this group defined subacute as 1 week to 6 months (with 3 months dividing early and late subacute phases) and chronic as older than 6 months 19 .
Video - acute infarction
Non-contrast CT of the brain remains the mainstay of imaging in the setting of an acute stroke. It is fast, inexpensive and readily available. Its main limitation, however, is the limited sensitivity in the acute setting. Detection depends on the territory, the experience of the interpreting radiologist and of course the time of the scan from the onset of symptoms. Whether tissue is supplied by end arteries (e.g. lenticulostriate arteries ) or has collateral supply (much of the cerebral cortex) will influence how quickly cytotoxic edema develops 6 . For example, detection of MCA territory infarct has been shown to be approximately 60-70% in the first 6 hours 3 , although changes in the deep grey matter nuclei (especially lentiform nucleus) can be visible within 1 hour of occlusion in up to 60% of patients 6 .
The goals of CT in the acute setting are:
exclude intracranial hemorrhage, which would preclude thrombolysis
look for any "early" features of ischemia
exclude other intracranial pathologies that may mimic a stroke, such as a tumor
Non-contrast CT has also been used historically to exclude patients from receiving thrombolysis based on the extent of hypoattenuation at presentation. This criterion has, however, been removed from the 2018 American Heart Association guidelines 18 . Nonetheless, finding large areas of established infarction on acute non-contrast CT continues to play an important role in patient selection and management.
The earliest CT sign visible is the hyperdense vessel sign , representing direct visualization of the intravascular thrombus/embolus and as such is visible immediately 7,21 . Although this can be seen in any vessel, it is most often observed in the middle cerebral artery (see hyperdense middle cerebral artery sign and middle cerebral artery dot sign ) 21 . It may be of therapeutic and prognostic value to differentiate this hyperdense 'regular' thromboembolic focus from a calcified cerebral embolus . In very rare instances of fat macroembolism, a hypodense vessel sign may be seen instead 22 .
Early hyperacute
Within the first few hours, a number of signs are visible depending on the site of occlusion and the presence of collateral flow. Early features include:
loss of grey-white matter differentiation, and hypoattenuation of deep nuclei:
lentiform nucleus changes are seen as early as 1 hour after occlusion, visible in 75% of patients at 3 hours 6
cortical hypodensity with associated parenchymal swelling with resultant gyral effacement
cortex which has poor collateral supply (e.g. insular ribbon) is more vulnerable 6
Visualization of loss of grey-white matter differentiation is aided by the use of a stroke window which has a narrow width and slightly lower center than routine brain window (width = 8, center = 32 HU) 18 .
The hypoattenuation and swelling become more marked with time, resulting in a significant mass effect. This is a major cause of secondary damage in large infarcts.
As time goes on, the swelling starts to subside and small amounts of cortical petechial hemorrhages (not to be confused with hemorrhagic transformation ) result in elevation of the attenuation of the cortex. This is known as the CT fogging phenomenon 5 . Imaging a stroke at this time can be misleading as the affected cortex will appear near normal.
Later still the residual swelling passes, and gliosis sets in eventually appearing as a region of low density with a negative mass effect. Cortical mineralization can also sometimes be seen appearing hyperdense.
Video - stroke evolution
CT perfusion
CT perfusion has emerged as a critical tool in selecting patients for reperfusion therapy as well as increasing the accurate diagnosis of ischemic stroke among non-expert readers four-fold compared to routine non-contrast CT 9 .
It allows both the core of the infarct (that part destined to never recover regardless of reperfusion) to be identified as well as the surrounding penumbra (the region which although ischemic has yet to go on to infarct and can be potentially salvaged). CT perfusion may also demonstrate early evidence of associated crossed cerebellar diaschisis .
The key to interpretation is understanding a number of perfusion parameters:
cerebral blood volume (CBV)
cerebral blood flow (CBF)
mean transit time (MTT)
time to peak (TTP)
Areas that demonstrate matched defects in CBV and MTT represent the unsalvageable infarct core, whereas areas that have prolonged MTT but preserved CBV are considered to be the ischemic penumbra 9 .
These factors will be discussed further separately. See CT perfusion .
CT angiography
may identify thrombus within an intracranial vessel, and may guide intra-arterial thrombolysis or clot retrieval
it is useful to determine whether the affected occluded vessel constitutes a large vessel occlusion (LVO) or middle vessel occlusion (MeVO)
evaluation of the carotid and vertebral arteries in the neck
establishing stroke etiology (e.g. atherosclerosis , dissection , web )
assess endovascular access and potential limitation for endovascular treatment (e.g. tortuosity, stenosis)
maybe necessary prior to thrombolysis in pediatric stroke cases
some guidelines only advise that children with an arterial thrombus benefit from thrombolysis
assess collateral vessels using single-phase CTA
Multiphase or delayed CT angiography
Multiphase or delayed CT angiography is showing benefit either replacing CT perfusion or as an additional 4th step in the stroke CT protocol as it guides patient selection for endovascular therapy by assessing collateral blood flow in ischemic and infarct tissue.
MRI is more time consuming and less available than CT but has significantly higher sensitivity and specificity in the diagnosis of acute ischemic infarction in the first few hours after onset.
Within minutes of arterial occlusion, DWI demonstrates increased signal and reduced ADC values 4,10 . This correlates well with infarct core (for a detailed discussion of DWI and ADC in stroke see diffusion-weighted MRI in acute stroke ). At this stage, the affected parenchyma appears normal on other sequences, although changes in flow will be detected (occlusion on MRA) and the thromboembolism may be detected (e.g. the susceptibility vessel sign on SWI ). Slow or stagnant flow in vessels may also be detected as a loss of normal flow void and high signal on T2/FLAIR and T1 C+ (intravascular enhancement), and presence of a the prominent vessel sign on SWI may indicate poor collateralisation 23 .
If infarction is incomplete then cortical contrast enhancement may be seen as early as 2 to 4 hours 10 .
In a minority of cases, DWI may be normal (please refer to DWI-negative acute ischemic stroke for more details).
Late hyperacute
Generally, after 6 hours, high T2 signal will be detected, initially more easily seen on FLAIR than conventional fast spin-echo T2 10 . This change continues to increase over the next day or two.
T1 hypointensity is only seen after 16 hours 10 and persists.
During the first week, the infarcted parenchyma continues to demonstrate high DWI signal and low ADC signal, although by the end of the first week ADC values have started to increase. The infarct remains hyperintense on T2 and FLAIR, with T2 signal progressively increasing during the first 4 days. T1 signal remains low, although some cortical intrinsic high T1 signal may be seen as early as 3 days after infarction 10 . After day 5 the cortex usually demonstrates contrast enhancement on T1 C+ 10 . Less common patterns of enhancement include arterial enhancement, encountered in approximately half of strokes and becomes evident after 3 days, and meningeal enhancement which is uncommon and is usually seen between 2 and 6 days 10 .
Hemorrhage, most easily seen on susceptibility-weighted imaging (SWI), is not a good indicator of age. Although most commonly seen after 12 hours and within the first few days, it may occur earlier or as late as 5 days 10 .
ADC demonstrates pseudonormalization typically occurring between 10-15 days 10 . As ADC values continue to rise, infarcted tissue progressively gets brighter than normal parenchyma. In contrast, DWI remains elevated due to persistent high T2/FLAIR signal ( T2 shine through ), unless hemorrhage ( T2 blackout ) or cystic encephalomalacia 10 . T2 fogging is also encountered typically between 1 and 5 weeks, most commonly around week 2 10,11 . Cortical enhancement is usually present throughout the subacute period.
T1 weighted sequences continue to show hypointensity throughout the area of infarct with cortical intrinsic high T1 signal due to the liquefactive necrosis and influx of monocytes as a response. The terms "cortical laminar necrosis" or "pseudolaminar necrosis" are occasionally, but incorrectly, used to describe this appearance in the context of thromboembolic stroke, but should be restricted to use in cases of isolated cortical necrosis. See the article on cortical laminar necrosis for a fuller discussion of this.
T1 signal remains low with intrinsic high T1 in the cortex if cortical necrosis is present 10 . T2 signal is high. Cortical contrast enhancement usually persists for 2 to 4 months 10 . Importantly if parenchymal enhancement persists for more than 12 weeks the presence of an underlying lesion should be considered 10 .
ADC values are high. DWI signal is variable, but as time goes on signal progressively decreases.
Transcranial Doppler ultrasound
Often described as an emerging application of point-of-care ultrasonography , use of transcranial Doppler (TCD) sonography has been utilized for the diagnosis of intracranial vessel occlusion, as well as the differentiation between ischemic and hemorrhagic stroke 14 .
In the context of a CT negative for intracerebral hemorrhage and a clinically suspicious patient presentation, diagnostic criteria for occlusion of an isolated vessel are as follows 12 ;
complete absence of color flow Doppler signals
absence of pulsed-wave Doppler signals
concurrent adequate visualization of surrounding parenchyma and vessels
color flow and pulsed wave Doppler signals must be demonstrated adequately in the remainder of the circle of Willis 16
Sonographic monitoring of the complications of ischemic stroke is also possible, including the detection of;
hemorrhagic transformation 16
midline shift 15
elevated intracranial pressure (ICP)
measurement of the optic nerve sheath diameter ( ONSD ) in common use as an intracranial pressure surrogate
Acute treatment focuses on prompt application of reperfusion therapies:
intravenous or (rarely) intra-arterial thrombolysis (e.g. alteplase, tenecteplase)
endovascular clot retrieval for large vessel occlusions - response graded with TICI
Neurosurgical intervention can also be pursued in certain cases, to allow patients to survive the period of maximal swelling by performing decompressive craniectomies (with or without duroplasty), particularly in younger patients with either large MCA infarcts or posterior fossa infarcts.
Additionally, supportive care should be provided, including caring for patients in dedicated inpatient stroke units and attempting to prevent the numerous complications which are encountered by patients with neurological impairment from stroke.
Complications
hemorrhagic transformation
non-neurological complications: e.g. aspiration pneumonia , pressure ulcers , venous thromboembolism , etc.
Quiz questions
- 1. Sacco R, Kasner S, Broderick J et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064-89. doi:10.1161/STR.0b013e318296aeca - Pubmed
- 2. Tomandl B, Klotz E, Handschu R et al. Comprehensive Imaging of Ischemic Stroke with Multisection CT. Radiographics. 2003;23(3):565-92. doi:10.1148/rg.233025036 - Pubmed
- 3. Lev M, Farkas J, Gemmete J et al. Acute Stroke: Improved Nonenhanced CT Detection--Benefits of Soft-Copy Interpretation by Using Variable Window Width and Center Level Settings. Radiology. 1999;213(1):150-5. doi:10.1148/radiology.213.1.r99oc10150 - Pubmed
- 4. Srinivasan A, Goyal M, Al Azri F, Lum C. State-Of-The-Art Imaging of Acute Stroke. Radiographics. 2006;26 Suppl 1(suppl_1):S75-95. doi:10.1148/rg.26si065501 - Pubmed
- 5. Becker H, Desch H, Hacker H, Pencz A. CT Fogging Effect with Ischemic Cerebral Infarcts. Neuroradiology. 1979;18(4):185-92. doi:10.1007/BF00345723 - Pubmed
- 6. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of Early CT Signs in the Deep Middle Cerebral Artery Territories with Angiographically Confirmed Site of Arterial Occlusion. AJNR Am J Neuroradiol. 2001;22(4):654-9. PMC7976034 - Pubmed
- 7. Pressman B, Tourje E, Thompson J. An Early CT Sign of Ischemic Infarction: Increased Density in a Cerebral Artery. AJR Am J Roentgenol. 1987;149(3):583-6. doi:10.2214/ajr.149.3.583 - Pubmed
- 8. Allmendinger A, Tang E, Lui Y, Spektor V. Imaging of Stroke: Part 1, Perfusion CT--Overview of Imaging Technique, Interpretation Pearls, and Common Pitfalls. AJR Am J Roentgenol. 2012;198(1):52-62. doi:10.2214/AJR.10.7255 - Pubmed
- 9. Hopyan J, Ciarallo A, Dowlatshahi D et al. Certainty of Stroke Diagnosis: Incremental Benefit with CT Perfusion over Noncontrast CT and CT Angiography. Radiology. 2010;255(1):142-53. doi:10.1148/radiol.09091021 - Pubmed
- 10. Allen L, Hasso A, Handwerker J, Farid H. Sequence-Specific MR Imaging Findings That Are Useful in Dating Ischemic Stroke. Radiographics. 2012;32(5):1285-97; discussion 1297. doi:10.1148/rg.325115760 - Pubmed
- 11. O'Brien P, Sellar R, Wardlaw J. Fogging on T2-Weighted MR After Acute Ischaemic Stroke: How Often Might This Occur and What Are the Implications? Neuroradiology. 2004;46(8):635-41. doi:10.1007/s00234-004-1230-2 - Pubmed
- 12. Nedelmann M, Stolz E, Gerriets T et al. Consensus Recommendations for Transcranial Color-Coded Duplex Sonography for the Assessment of Intracranial Arteries in Clinical Trials on Acute Stroke. Stroke. 2009;40(10):3238-44. doi:10.1161/STROKEAHA.109.555169 - Pubmed
- 13. Kaps M, Damian M, Teschendorf U, Dorndorf W. Transcranial Doppler Ultrasound Findings in Middle Cerebral Artery Occlusion. Stroke. 1990;21(4):532-7. doi:10.1161/01.str.21.4.532 - Pubmed
- 14. Mäurer M, Shambal S, Berg D et al. Differentiation Between Intracerebral Hemorrhage and Ischemic Stroke by Transcranial Color-Coded Duplex-Sonography. Stroke. 1998;29(12):2563-7. doi:10.1161/01.str.29.12.2563 - Pubmed
- 15. Lau V & Arntfield R. Point-Of-Care Transcranial Doppler by Intensivists. Crit Ultrasound J. 2017;9(1):21. doi:10.1186/s13089-017-0077-9 - Pubmed
- 16. Blanco P & Blaivas M. Applications of Transcranial Color-Coded Sonography in the Emergency Department. J Ultrasound Med. 2017;36(6):1251-66. doi:10.7863/ultra.16.04050 - Pubmed
- 17. Nael K, Sakai Y, Khatri P, Prestigiacomo C, Puig J, Vagal A. Imaging-Based Selection for Endovascular Treatment in Stroke. Radiographics. 2019;39(6):1696-713. doi:10.1148/rg.2019190030 - Pubmed
- 18. Potter C, Vagal A, Goyal M, Nunez D, Leslie-Mazwi T, Lev M. CT for Treatment Selection in Acute Ischemic Stroke: A Code Stroke Primer. Radiographics. 2019;39(6):1717-38. doi:10.1148/rg.2019190142 - Pubmed
- 19. Bernhardt J, Hayward K, Kwakkel G et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Int J Stroke. 2017;12(5):444-50. doi:10.1177/1747493017711816 - Pubmed
- 20. Adams H, Bendixen B, Kappelle L et al. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi:10.1161/01.str.24.1.35 - Pubmed
- 21. Chieng J, Singh D, Chawla A, Peh W. The Hyperdense Vessel Sign in Cerebral Computed Tomography: Pearls and Pitfalls. Singapore Med J. 2020;61(5):230-7. doi:10.11622/smedj.2020074 - Pubmed
- 22. Lee T, Bartlett E, Fox A, Symons S. The Hypodense Artery Sign. AJNR Am J Neuroradiol. 2005;26(8):2027-9. PMC8148856 - Pubmed
- 23. Lee H, Roh H, Lee S et al. Collateral Estimation by Susceptibility-Weighted Imaging and Prediction of Functional Outcomes After Acute Anterior Circulation Ischemic Stroke. Sci Rep. 2021;11(1):21370. doi:10.1038/s41598-021-00775-9 - Pubmed
Incoming Links
- Haemorrhagic transformation of ischaemic stroke
- Uraemic encephalopathy
- Hereditary angiopathy with nephropathy, aneurysms, and muscle cramps syndrome
- Dermal filler injections
- Fabry disease
- Artery of Percheron territory infarct
- Calcified cerebral embolus
- Large vessel occlusion
- Déjerine-Roussy syndrome
- Parinaud syndrome
- Three-territory sign (brain)
- Cytotoxic cerebral oedema
- Contrast-induced neurotoxicity
- Granulomatosis with polyangiitis (CNS manifestations)
- Luxury perfusion
- Bálint syndrome
- Ischaemic stroke (summary)
- Superior cerebellar artery infarct
- MR perfusion weighted imaging
- Intracardiac thrombus
- Occipital infarcts
- Lateral medullary (Wallenberg) syndrome
- Acute left PCA territory infarct
- Subacute cerebral infarct with gyriform enhancement
- Hyperdense MCA sign
- Acute left parietal infarct
- Chronic MCA infarct
- Cryptococcal meningoencephalitis with acute ischemic stroke
- Internal carotid artery dissection
- Cortical deafness due to infarct of both Heschl's gyri
- Acute infarction of bilateral anterior fornix
- Lateral medullary syndrome
- Lateral pontine syndrome
- Middle cerebral artery infarct
- Acute bilateral internal carotid artery occlusion
- Vertebrobasilar artery occlusion - diagnosis and treatment
- Bilateral ACA infarction due to azygos ACA embolism
- Bilateral ACA infarction
- ACA orbitofrontal infarct post DSA
- Question 2591
- Question 2415
- Question 2414
- Question 2413
- Question 2330
- Question 2324
- Question 2323
- Question 2318
- Question 2317
- Question 2311
- Question 1787
- Question 1748
- Question 1733
- Question 1732
- Question 1731
- Question 1730
- Question 1729
- Question 1728
- Question 1725
- Question 1724
Related articles: Stroke and intracranial haemorrhage
- code stroke CT (an approach)
- stroke in children and young adults
- infarct core
- ischemic penumbra
- luxury perfusion
- ghost infarct core
- multiphase CT angiography
- fogging phenomenon
- calcified cerebral embolus
- early DWI reversal
- ADC pseudonormalization
- T2 shine-through
- T2 blackout
- transient ischemic attack (TIA)
- intracranial atherosclerotic disease (ICAD)
- embolic shower
- CT angiography source image ASPECTS
- ASCOD classification
- Canadian Neurological Scale
- Heidelberg bleeding classification
- NIH Stroke Scale
- Mathew stroke scale
- modified Rankin scale
- Orgogozo Stroke Scale
- Scandinavian Stroke Scale
- modified treatment in cerebral infarction (mTICI) score
- expanded treatment in cerebral infarction (eTICI) score
- TOAST classification
- single-phase CTA collateral scores
- multiphase CTA collateral score
- carotid pseudo-occlusion
- clot meniscus sign
- disappearing basal ganglia sign
- hyperdense MCA sign
- MCA dot sign
- hypodense vessel sign
- prominent vessel sign
- salted pretzel sign
- susceptibility vessel sign
- tandem lesion
- three-territory sign
- frontal lobe infarct
- Gerstmann syndrome
- temporal lobe infarct
- alexia without agraphia syndrome : PCA
- cortical blindness syndrome (Anton syndrome): top of basilar or bilateral PCA
- Balint syndrome : bilateral PCA
- lacunar stroke syndromes
- lenticulostriate infarct
- artery of Percheron infarct
- Déjerine-Roussy syndrome (thalamic pain syndrome): thalamoperforators of PCA
- top of the basilar syndrome
- Benedikt syndrome : PCA
- Claude syndrome : PCA
- Nothnagel syndrome : PCA
- Weber syndrome : PCA
- Wernekink commissure syndrome
- Brissaud-Sicard syndrome
- facial colliculus syndrome
- Gasperini syndrome : basilar artery or AICA
- inferior medial pontine syndrome (Foville syndrome): basilar artery
- lateral pontine syndrome (Marie-Foix syndrome): basilar artery or AICA
- locked-in syndrome : basilar artery
- Millard-Gubler syndrome : basilar artery
- Raymond syndrome : basilar artery
- Babinski-Nageotte syndrome
- Cestan-Chenais syndrome
- hemimedullary syndrome (Reinhold syndrome)
- lateral medullary stroke syndrome (Wallenberg syndrome)
- medial medullary syndrome (Déjerine syndrome)
- Opalski syndrome
- anterior spinal artery syndrome
- sulcal artery syndrome
- posterior spinal artery syndrome
- anterior choroidal artery infarct
- subcallosal artery infarct
- basilar artery infarct
- superior cerebellar artery infarct
- anterior cerebellar artery infarct
- posterior inferior cerebellar artery infarct
- large vessel occlusion
- medium vessel occlusion
- endovascular clot retrieval (ECR)
- crossed cerebellar diaschisis
- cerebral intraparenchymal hyperattenuations post thrombectomy
- ABC/2 (volume estimation)
- black hole sign
- cashew nut sign
- CTA spot sign
- island sign
- satellite sign
- cerebral contusions
- cerebral microhemorrhage
- hemorrhagic venous infarct
- hypertensive intracranial hemorrhage
- jet hematoma
- basal ganglia hemorrhage
- remote cerebellar hemorrhage
- intraventricular hemorrhage (IVH)
- cerebral amyloid angiopathy
- Duret hemorrhage
- thalamic hemorrhage
- extradural versus subdural hemorrhage
- venous extradural hemorrhage
- intralaminar dural hemorrhage
- calcified chronic subdural hemorrhage
- berry aneurysm
- fusiform aneurysm
- mycotic aneurysm
- cortical superficial siderosis
- traumatic subarachnoid hemorrhage (tSAH)
- perimesencephalic subarachnoid hemorrhage (PMSAH)
- vasospasm following SAH
- Hunt and Hess grading system
- Fisher scale
- modified Fisher scale
- SDASH score
- WFNS grading system
- subpial hemorrhage
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

Ischemic Stroke Management: Posthospitalization and Transition of Care
Affiliation.
- 1 University of Iowa Carver College of Medicine, Iowa City, Iowa.
- PMID: 37440741
Ischemic stroke is a major cause of morbidity and mortality worldwide. Ischemic stroke and transient ischemic attack exist on a continuum of the same disease process. Ischemic stroke is common, and more than 85% of stroke risk is attributed to modifiable risk factors. The initial management of acute stroke is usually performed in the emergency department and hospital settings. Family physicians have a key role in follow-up, ensuring that a complete diagnostic evaluation has been performed, addressing modifiable risk factors, facilitating rehabilitation, and managing chronic sequelae. Secondary prevention of ischemic stroke includes optimization of chronic disease management (e.g., hypertension, type 2 diabetes mellitus, dyslipidemia), nonpharmacologic lifestyle interventions (e.g., diet changes, exercise, substance use counseling), and pharmacologic interventions. Dual antiplatelet therapy with aspirin and clopidogrel is generally indicated for minor noncardioembolic ischemic strokes and high-risk transient ischemic attacks and should be converted to single antiplatelet therapy after 21 to 90 days. Secondary prevention of cardioembolic stroke requires long-term anticoagulation. Direct oral anticoagulants are preferred over warfarin for patients with nonvalvular atrial fibrillation. Poststroke problems with mobility, balance, cognition, dysphagia, and depression are common. Rehabilitation involves a multidisciplinary, multimodal approach that includes physical therapy, speech therapy, and treatment of chronic pain and poststroke depression.
- Anticoagulants / therapeutic use
- Atrial Fibrillation* / complications
- Atrial Fibrillation* / diagnosis
- Atrial Fibrillation* / therapy
- Diabetes Mellitus, Type 2* / drug therapy
- Ischemic Attack, Transient* / diagnosis
- Ischemic Attack, Transient* / therapy
- Ischemic Stroke* / drug therapy
- Patient Transfer
- Platelet Aggregation Inhibitors / therapeutic use
- Stroke* / etiology
- Stroke* / prevention & control
- Platelet Aggregation Inhibitors
- Anticoagulants
ORIGINAL RESEARCH article
Blood glucose to predict symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke with large infarct core: a prospective observational study.

- 1 Department of Neurology, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
- 2 Department of Neurology, Xinqiao Hospital and the Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China
- 3 Department of Pharmacy, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
Introduction: Symptomatic intracranial hemorrhage (sICH) is a serious complication of acute ischemic stroke (AIS) after endovascular treatment (EVT). Limited data exist regarding predictors and clinical implications of sICH after EVT, underscoring the significance of identifying risk factors to enhance prevention strategies. Therefore, the main objective of this study was to evaluate the incidence of sICH and identify its predictors after EVT in patients with large infarct core-AIS in the pre-circulation stage.
Methods: Using data from the EVT for the Pre-circulation Large Infarct Core-AIS Study, we enrolled patients who were treated with EVT from the Prospective Multicenter Cohort Study of Early Treatment in Acute Stroke (MAGIC) registry. Baseline demographics, medical history, vascular risk factors, blood pressure, stroke severity, radiographic features, and EVT details were collected. The patients were classified into three groups: without intracranial hemorrhage (ICH), with asymptomatic intracranial hemorrhage (aICH), and sICH, based upon the occurrence of sICH. The main outcomes were the occurrence of sICH according to the Heidelberg Bleeding Classification and functional condition at 90 days. Multivariate logistic regression analysis and receiver operating characteristic (ROC) curves were used to identify independent predictors of sICH after EVT.
Results: The study recruited a total of 490 patients, of whom 13.3% ( n = 65) developed sICH. Patients with sICH had less favorable outcomes than those without intracranial hemorrhage (ICH) and those with aICH (13.8% vs. 43.5% vs. 32.2%, respectively; p < 0.001). The overall mortality was 41.8% ( n = 205) at 90 days post-EVT. The univariate analysis revealed significant differences among the three groups in terms of blood glucose levels at admission, probability of favorable outcomes, incidence of brain herniation, and 90-day mortality. The multifactorial logistic regression analysis revealed that the blood glucose level at admission [odds ratio (OR) 1.169, p < 0.001, confidence interval (CI) 1.076–1.269] was an independent predictor of sICH. A blood glucose level of 6.95 mmol/L at admission was the best predictor of sICH, with an area under the ROC curve (AUC) of 0.685 (95% CI: 0.616–0.754).
Discussion: The study findings demonstrated that the probability of sICH after EVT was 13.3% in patients with pre-circulation large infarct core-AIS, and sICH increased the risk of an unfavorable prognosis. Higher blood glucose levels at admission were associated with sICH after EVT in patients with pre-circulation large infarct core AIS. These findings underscore the importance of early management strategies to mitigate this risk.
1 Introduction
As the second most lethal and disabling disease worldwide, acute ischemic stroke (AIS) has a serious impact on patient survival and quality of life ( 1 ). Approximately 20% of patients with AIS arrive at the hospital with a large infarct core, characterized by high mortality and poor prognosis, posing a global challenge in clinical management for this population ( 2 ). Endovascular therapy (EVT) has emerged as the preferred treatment option for AIS due to large vessel occlusion.
A previous study reported that, compared with patients treated with medication alone, those with large infarct-core AIS treated with EVT plus medication had a higher likelihood of reduced disability, independent ambulation, and favorable functional outcomes at 90 days ( 3 ). The Chinese Guidelines for Endovascular Treatment of Acute Ischemic Stroke 2023 recommend EVT for patients with large-vessel occlusion-induced AIS in the pre-circulation stage among those who meet the enrolment criteria of the ANGEL-ASPECT ( 4 ), RESCUE-Japan LIMIT ( 5 ), or SELECT 2 ( 6 ) studies ( 7 ).
Despite the success associated with EVT, intracranial hemorrhage (ICH) is a common complication, with a prevalence of 46.0–49.5% ( 8 ). In particular, symptomatic ICH (sICH) can reduce the benefit–risk ratio of treatment and increase the risk of mortality ( 9 ). However, limited data are available regarding the predictors and clinical relevance of sICH after EVT. Therefore, risk factors for sICH after EVT should be identified for the prevention of sICH and improvement in the efficacy of this new treatment strategy. Utilizing the database from the Prospective Multicenter Cohort Study of Early Treatment in Acute Stroke (MAGIC) study, the present study aimed to analyze potential predictors of sICH after EVT in patients with pre-circulation large infarct-core AIS.
2 Materials and methods
2.1 patients.
Patients were enrolled from the database of the MAGIC study, a nationwide prospective registry of consecutive patients who presented with acute, symptomatic, and radiologically confirmed pre-circulation large infarct core AIS. The MAGIC registry included patients with AIS with a large infarct core due to pre-circulation large-vessel occlusion who underwent EVT from November 2021 to February 2023. This prospective observational study was approved by the ethics committees of the participating centers.
The eligibility criteria for EVT were as follows: (1) age 18–80 years; (2) AIS due to anterior circulation large vessel occlusion, defined as occlusion of the internal carotid artery (ICA) or the M1 segment or M2 segment of the middle cerebral artery (MCA); (3) large ischemic core on non-contrast computed tomography (CT) findings [defined as Alberta stroke programme early CT score (ASPECTS) score of 0–5]; (4) pre-stroke score of 0 or 1 on the modified Rankin scale (mRS), assessed retrospectively (scores ranging from 0 to 6, with higher scores indicating greater disability and a score of 6 indicating death); and (5) symptom presentation within 24 h (the time metric of time last known well within 24 h was used if the presentation time was unavailable). The exclusion criteria were as follows: (1) no follow-up brain imaging data (CT or magnetic resonance imaging) at 24 h or when neurological deterioration occurred; (2) serious, advanced, or terminal illness; and (3) no mRS data at 90 days.
2.2 Clinical data collection
The collected data included baseline demographic data (age and sex), medical history, vascular risk factors (smoking, hypertension, hyperlipidemia, diabetes, and atrial fibrillation), blood pressure at admission (systolic and diastolic), stroke severity [National Institutes of Health Stroke Score (NIHSS)], radiographic features (ASPECTS, site of the occluded arteries), and EVT-related data (procedure process time, treatment methods, and recanalization). Additional data analyzed included blood glucose level at admission, triglyceride level, low-density cholesterol level, liver function, kidney function, and intravenous thrombolytic therapy (IVT).
The site of the occluded arteries was identified using CT angiography (CTA), magnetic resonance angiography (MRA), and/or cerebral digital subtraction angiography (DSA) reports and included the ICA and MCA. Anterior circulation lesions were defined using the ASPECTS score, which involved symptom onset to puncture (OTP) time and symptom onset to recanalization (OTR) time.
The patients in our study received EVT, which included intra-arterial thrombolysis, thrombectomy with stent retrievers, thromboaspiration, intracranial angioplasty, stent implantation, or a combination of these approaches at the discretion of the treatment surgeon.
2.3 Post-procedure evaluation
CT was usually performed 24 h after the procedure or whenever ICH was indicated by clinical symptoms. Successful recanalization was defined as a modified treatment in cerebral infarction (mTICI) with a score of 2b, 2c, or 3. The mRS score was evaluated at 90 days by a stroke neurologist during a scheduled post-stroke follow-up visit or via phone interview. Functional outcomes were evaluated according to the mRS as follows: complete recovery (mRS = 0–1), partial recovery, independence (mRS = 2), dependence (mRS = 3–5), and death (mRS = 6). Favorable and unfavorable outcomes were defined as an mRS score of 0–3 and > 3 ( 4 – 6 ), respectively.
2.4 Evaluation of ICH
According to the Heidelberg Bleeding Classification, ICH is diagnosed within 24 h after EVT. sICH and asymptomatic intracranial hemorrhage (aICH) were classified based on the presence or absence of neurological deficit exacerbations. The diagnosis of sICH was based on the association of ICH with any of the following conditions: (1) increase in NIHSS score by >4 points compared to the score prior to ICH; (2) increase in NIHSS score by >2 points in one category; and (3) deterioration leading to intubation, hemicraniectomy, external ventricular drain placement, or any other major intervention. Symptom deteriorations were required to be unexplainable by causes other than the observed ICH ( 10 ).
2.5 Statistical analysis
Measures with normal distribution are expressed as mean ± standard deviation of variance (ANOVA) with Bonferroni correction used for multiple comparisons. Continuous variables are presented as medians [interquartile range (IQR)] according to the type of non-normal distribution, and categorical variables are presented as frequencies (percentages). The Wilcoxon rank-sum test was used for comparison between two groups, and the Kruskal–Wallis test was used for multiple comparisons. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. A multivariate logistic regression analysis was performed to evaluate independent predictors for sICH, with the adjusted odds ratio (OR) and corresponding 95% confidence interval (CI) reported. Receiver operating characteristic (ROC) curve analysis was used to evaluate the optimal cutoff value for predicting sICH and to establish optimal cutoff points for the specificity and sensitivity values. Entered factors were those with at least marginal significance ( p < 0.1) in a univariate analysis. p -values of <0.05 were considered significant. Statistical analyses were performed using SPSS 23.0. (IBM, Armonk, NY, United States).
A total of 490 patients with pre-circulation large infarct-core AIS who were treated with EVT were enrolled in this study. They were divided into three groups: without ICH, with aICH, and with sICH. Overall, 115 patients (23.5%) had aICH after EVT within 24 h, whereas 65 patients (13.3%) had sICH. The proportions of reaching favorable functional outcomes in the three groups were 43.5, 32.2, and 13.8%, respectively ( p < 0.001) ( Figure 1 and Table 1 ). Significant differences were noted in the probability of developing brain herniation (21.6% vs. 30.4% vs. 58.5%, p < 0.001) and mortality at 90 days (35.2% vs. 44.3% vs. 69.2%, p < 0.001) ( Table 1 ).
Figure 1 . Distribution of 90-day mRS scores in patients without ICH, aICH, and sICH. Patients with sICH demonstrated the least favorable outcomes (mRS 0–3) 90 days post-index stroke, with a higher proportion experiencing mortality. The distribution of modified Rankin scale (mRS) scores underscores the impact of sICH on long-term prognosis. mRS, modified Rankin scale; ICH: intracranial hemorrhage; aICH, asymptomatic intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Table 1 . Baseline characteristics and outcomes of patients without ICH, with aICH, and sICH.
When comparing sICH based on the level of blood glucose at admission, a significant intergroup difference was noted (7.6 ± 2.8 vs. 8.0 ± 2.9 vs. 9.25 ± 3.5 mmol/L, respectively, p < 0.001) ( Table 1 ). The one-way ANOVA revealed that the levels of blood glucose at admission in the sICH group were significantly different from those in the without ICH and with aICH groups ( p < 0.001; p = 0.008, respectively). The count showed that the levels of blood glucose at admission in the sICH group were significantly different from those in the without ICH and with aICH groups (p < 0.001; p = 0.024, respectively, by the Bonferroni correction) ( Figure 2 ). In multivariate analysis, higher blood glucose levels at admission (OR 1.169, p < 0.001, CI 1.076–1.269) were associated with sICH after EVT in patients with pre-circulation large infarct-core AIS ( Table 2 ).
Figure 2 . Admission glucose levels in patients without ICH, aICH, and sICH. Significant differences in admission glucose levels were observed between groups without ICH, aICH, and sICH. The sICH group exhibited elevated blood glucose levels compared to the other two groups, emphasizing the association between admission hyperglycemia and symptomatic intracranial hemorrhage (after the Bonferroni correction).
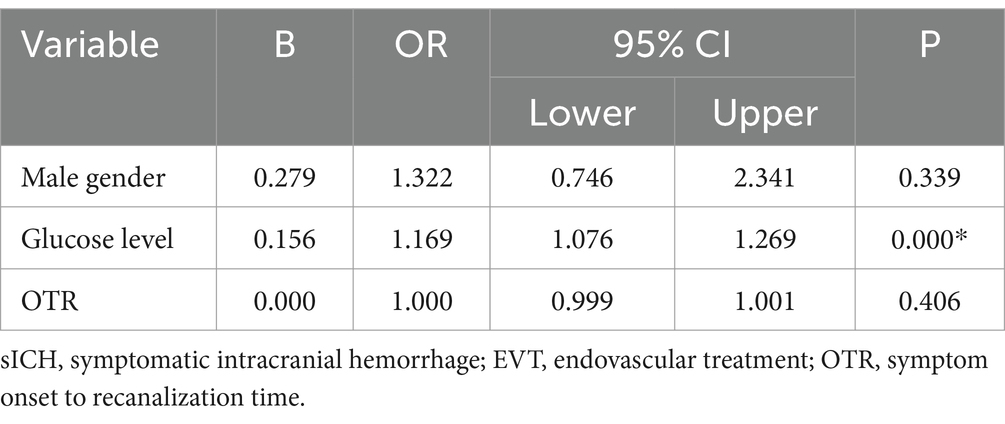
Table 2 . Multiple multifactorial logistic regression predicts sICH after EVT.
Figure 3 presents the results of the ROC analysis determining the prognostic value of blood glucose levels at admission to predict sICH. The area under the curve for the model was 0.685, with a 95% CI of 0.616–0.754, indicating that blood glucose levels at admission have a good discriminative ability. The optimal threshold was 6.95 mmol/L, corresponding to a sensitivity and specificity of 82.0 and 49.8%, respectively, with Youden’s index of 0.312. The positive predictive value was 19.2%, and the negative predictive value was 84.1%.
Figure 3 . Receiver operating characteristic (ROC) curve for blood glucose levels predicting sICH. The ROC curve illustrates the predictive value of admission blood glucose levels for symptomatic intracranial hemorrhage (sICH). The curve highlights the discriminative ability of blood glucose levels, with an optimal threshold identified for predicting the occurrence of sICH after endovascular therapy (EVT).
4 Discussion
In our study, we explored potential predictors of sICH after EVT in patients with pre-circulation large infarct-core AIS and observed that the probability of sICH was 13.3% in patients who underwent EVT. Furthermore, sICH greatly reduced the probability of patients having a 90-day favorable prognosis and increased the risk of brain herniation and mortality. In addition, we demonstrated that the blood glucose level at admission was an independent predictor of sICH after EVT in AIS patients with large infarct cores, and the risk of sICH increased with higher blood glucose levels.
In our study, the occurrence of sICH was similar to that in the SELECT trial, which reported an increased probability of sICH in patients with large-vessel occlusion of large-core infarcts who were treated with EVT with an onset greater than 24 h ( 11 ). However, our results showed a higher value (13.3%) than that reported in a previous randomized controlled trial (4.4%) ( 12 ) and in the North American Solitaire Acute Stroke Study (9.9%) ( 13 ). This discrepancy may stem from the inclusion of patients in our study with large infarct cores in the anterior circulation and an ASPECTS score of ≤5, whereas previous EVT studies have largely excluded patients who had large infarct cores already present at the time of preoperative imaging. Several studies have demonstrated an association between larger cores on imaging and an increased risk of sICH after EVT ( 14 , 15 ). In most studies, an ASPECTS score of ≤5 was reported to be associated with an approximately 2-fold risk of ICH ( 16 , 17 ). Patients with large infarct cores have a high degree of blood–brain barrier disruption, and the development of ICH is closely related to blood–brain barrier damage. In previous models of cerebral ischemia in rats, loss of integrity of the matrix and basement membrane connecting the endothelial cells was observed by electron microscopy, and in some cases, glial cell protrusions were observed to be swollen or degenerative. These alterations affect the structure and function of the blood–brain barrier, which induces ICH by increasing its permeability ( 18 ).
Experimental results have shown that the ischemia–reperfusion-induced release and activation of metalloproteinases can cause rupture of the basement membrane, leading to ICH ( 19 , 20 ). The results of a previous meta-analysis demonstrated that, compared to pharmacological treatment, EVT did not increase the risk of sICH but improved the functional outcome in patients with large infarct cores ( 21 , 22 ). Based on the above observations, it is reasonable to attribute the higher incidence of sICH in our study to the larger infarct core rather than the EVT procedure itself.
Similar to the findings of Shen et al. ( 23 ), our results showed that patients with sICH had a higher risk of brain herniation and mortality and a greater likelihood of unfavorable outcomes than patients without ICH and with aICH. This emphasizes the critical nature of sICH as a potentially fatal complication that significantly influences patient functional outcomes, warranting emphasis on preventive measures.
In our study, blood glucose was identified as a predictor of sICH after EVT in AIS patients with large infarct cores in the anterior circulation. The blood glucose levels >6.95 mmol/L at admission were associated with an increased risk of sICH after EVT, comparable to a level of 6.6 [5.7–7.7] mmol/L reported in a previous study ( 24 ). Several previous studies have suggested that patients with hyperglycemia are at a high risk of ICH from different perspectives, such as a meta-analysis that retrospectively analyzed the clinical data of patients undergoing EVT and found that higher blood glucose levels at admission were associated with a higher incidence of sICH ( 25 , 26 ). Previous studies have shown that acute hyperglycemia increases blood–brain barrier disruption and ICH incidence in rat models ( 27 ). The results of Shen et al.’s ( 23 ) study suggest that high blood glucose levels are the strongest predictors of sICH after EVT. Another study showed that an elevated glycosylated hemoglobin level was significantly associated with an unfavorable prognosis and mortality in 90 days with AIS treated with EVT ( 28 ). The results of these studies are consistent with our findings; however, the mechanisms underlying how hyperglycemia enhances ICH remain unclear. The possible causes of ICH are as follows: (1) hyperglycemia exacerbates vascular wall dystrophy and hypoxia, resulting in more susceptible degeneration and necrosis of the vascular wall ( 29 , 30 ); (2) hyperglycemia leads to disruption of cellular metabolism, resulting in increased plasma osmolality and intracellular lactic acid buildup, ultimately leading to endothelial cell injury and acidosis ( 31 ); and (3) hyperglycemia can reportedly increase the activity of the matrix metalloproteinases (MMP-9 and MMP-3) in ischemic areas and aggravate blood–brain barrier dysfunction and post-reperfusion ICH ( 32 – 34 ). While it is hypothesized that maintaining stable blood glucose levels post-successful EVT may be crucial in preventing sICH ( 35 ), further studies are needed to confirm this.
This study has some limitations that should be considered when interpreting the results. First, as the study adopted a prospective observational design, we did not evaluate other potentially relevant variables, such as changes in perioperative blood pressure, use of heparin during EVT, and the effect of antiplatelet regimens on hemorrhagic conversion, which may influence the risk of ICH. Second, an incomplete evaluation index may not fully determine a causal connection, and the practical implications of these unresolved findings require future validation. Despite these limitations, the findings of this study provide insights into the predictive value of blood glucose levels at admission for sICH after EVT in pre-circulation large infarct core-AIS to facilitate early management.
5 Conclusion
In this multicenter study, the incidence of sICH after EVT was higher than that previously reported. Patients with pre-circulation large infarct core AIS who developed sICH after EVT had a higher incidence of unfavorable prognosis and mortality than those who did not develop sICH. Higher blood glucose levels at admission increased the risk of sICH after EVT in AIS patients with large infarct cores in the anterior circulation. It remains unclear whether controlling blood glucose levels prior to EVT can reduce the risk of sICH, and further investigation is required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Suzhou Dushu Lake Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY: Writing – original draft, Data curation, Formal analysis, Investigation. LY: Writing – original draft, Data curation, Methodology. XS: Writing – original draft, Formal analysis, Investigation, Software. XN: Formal analysis, Writing – original draft, Data curation, Funding acquisition, Methodology. SF: Data curation, Writing – original draft, Conceptualization, Investigation, Supervision. XX: Investigation, Supervision, Data curation, Writing – original draft, Formal analysis, Methodology. JM: Methodology, Data curation, Writing – original draft, Software, Validation. SY: Methodology, Writing – original draft, Conceptualization, Project administration, Resources, Supervision. ZW: Methodology, Writing – original draft, Formal analysis, Software, Validation. WZ: Validation, Writing – review & editing, Data curation, Funding acquisition, Project administration, Resources. DY: Funding acquisition, Project administration, Resources, Writing – review & editing, Data curation, Investigation, Formal analysis. YH: Investigation, Project administration, Writing – review & editing, Supervision, Visualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Science Fund for Distinguished Young Scholars (No. 81525008), the National Natural Science Foundation of China (NSFC) (82000237), and the Suzhou Science and Technology Plan Project (SZM2023017).
Acknowledgments
The authors would like to thank all the participants and their relatives in the study and the members of the survey teams.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hasan, TF, Todnem, N, Gopal, N, Miller, DA, Sandhu, SS, Huang, JF, et al. Endovascular thrombectomy for acute ischemic stroke. Curr Cardiol Rep . (2019) 21:112. doi: 10.1007/s11886-019-1217-6
Crossref Full Text | Google Scholar
2. Wu, C, and Ji, X. Angel-ASPECT: large infarct core acute Chinese protocol for endovascular treatment of ischaemic stroke. Chin J Stroke . (2023):247–9. doi: 10.3969/j.issn.1673-5765.2023.03.002
3. Palaiodimou, L, Sarraj, A, Safouris, A, Magoufis, G, Lemmens, R, Sandset, EC, et al. Endovascular treatment for large-core ischaemic stroke: a meta-analysis of randomised controlled clinical trials. J Neurol Neurosurg Psychiatry . (2023) 94:781–5. doi: 10.1136/jnnp-2023-331513
4. Huo, X, Ma, G, Tong, X, Zhang, X, Pan, Y, Nguyen, TN, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med . (2023) 388:1272–83. doi: 10.1056/NEJMoa2213379
5. Uchida, K, Shindo, S, Yoshimura, S, Toyoda, K, Sakai, N, Yamagami, H, et al. Association between Alberta stroke program early computed tomography score and efficacy and safety outcomes with endovascular therapy in patients with stroke from large-vessel occlusion: a secondary analysis of the recovery by endovascular salvage for cerebral ultra-acute embolism-Japan large ischemic core trial (RESCUE-Japan LIMIT). JAMA Neurol . (2022) 79:1260–6. doi: 10.1001/jamaneurol.2022.3285
6. Sarraj, A, Hassan, AE, Abraham, MG, Ortega-Gutierrez, S, Kasner, SE, Hussain, MS, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med . (2023) 388:1259–71. doi: 10.1056/NEJMoa2214403
7. Chinese Stroke Association, Neurological Intervention Branch of the Chinese Stroke Association, Interventional Group of the Stroke Prevention and Control Committee of the Chinese Preventive Medical Association . Chinese guidelines on endovascular treatment of acute Ischaemic stroke 2023. Chin J Stroke . (2023) 18:684–711. doi: 10.3969/j.issn.1673-5765.2023.06.010
8. Chinese Society of Neurology, Chinese Stroke Society . Consensus on diagnosis and treatment of hemorrhagic transformation after acute ischemic stroke in China 2019. Chin. J Neurol . (2019) 52:252–65. doi: 10.3760/cma.j.issn.1006-7876.2019.04.003
9. Darkhabani, Z, Nguyen, T, Lazzaro, MA, Zaidat, OO, Lynch, JR, Fitzsimmons, BF, et al. Complications of endovascular therapy for acute ischemic stroke and proposed management approach. Neurology . (2012) 79:S192–8. doi: 10.1212/WNL.0b013e31826958e3
10. von Kummer, R, Broderick, JP, Campbell, BC, Demchuk, A, Goyal, M, Hill, MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke . (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
11. Sarraj, A, Kleinig, TJ, Hassan, AE, Portela, PC, Ortega-Gutierrez, S, Abraham, MG, et al. Association of endovascular thrombectomy vs medical management with functional and safety outcomes in patients treated beyond 24 hours of last known well: the SELECT late study. JAMA Neurol . (2023) 80:172–82. doi: 10.1001/jamaneurol.2022.4714
12. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet . (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
13. Zaidat, OO, Castonguay, AC, Gupta, R, Sun, CJ, Martin, C, Holloway, WE, et al. North American solitaire stent retriever acute stroke registry: post-marketing revascularization and clinical outcome results. J NeuroIntervent Surg . (2014) 6:584–8. doi: 10.1136/neurintsurg-2013-010895
14. Phan, K, Saleh, S, Dmytriw, AA, Maingard, J, Barras, C, Hirsch, JA, et al. Influence of ASPECTS and endovascular thrombectomy in acute ischemic stroke: a meta-analysis. J Neurointerv Surg . (2019) 11:664–9. doi: 10.1136/neurintsurg-2018-014250
15. Seners, P, Wouters, A, Maïer, B, Boisseau, W, Gory, B, Heit, JJ, et al. Role of brain imaging in the prediction of intracerebral hemorrhage following endovascular therapy for acute stroke. Stroke . (2023) 54:2192–203. doi: 10.1161/STROKEAHA.123.040806
16. Winkelmeier, L, Heit, JJ, Adusumilli, G, Geest, V, Christensen, S, Kniep, H, et al. Hypoperfusion intensity ratio is correlated with the risk of parenchymal hematoma after endovascular stroke treatment. Stroke . (2023) 54:135–43. doi: 10.1161/STROKEAHA.122.040540
17. Janvier, P, Kerleroux, B, Turc, G, Pasi, M, Farhat, W, Bricout, N, et al. TAGE score for symptomatic intracranial hemorrhage prediction after successful endovascular treatment in acute ischemic stroke. Stroke . (2022) 202:2809–17. doi: 10.1161/STROKEAHA.121.038088
18. Haley, MJ, and Lawrence, CB. The blood-brain barrier after stroke: structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab . (2017) 37:456–70. doi: 10.1177/0271678X16629976
19. Fang, Z, He, QW, Li, Q, Chen, XL, Baral, S, Jin, HJ, et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J . (2016) 30:2097–107. doi: 10.1096/fj.201500126
20. Fujimura, M, Gasche, Y, Morita-Fujimura, Y, Massengale, J, Kawase, M, and Chan, PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res . (1999) 842:92–100. doi: 10.1016/s0006-8993(99)01843-0
21. Li, Q, Abdalkader, M, Siegler, JE, Yaghi, S, Sarraj, A, Campbell, BCV, et al. Mechanical thrombectomy for large ischemic stroke: a systematic review and meta-analysis. Neurology . (2023) 101:e922–32. doi: 10.1212/WNL.0000000000207536
22. Kobeissi, H, Adusumilli, G, Ghozy, S, Kadirvel, R, Brinjikji, W, Albers, GW, et al. Endovascular thrombectomy for ischemic stroke with large core volume: an updated, post-TESLA systematic review and meta-analysis of the randomized trials. Interv Neuroradiol . (2023) 28. doi: 10.1177/15910199231185738
23. Shen, H, Ma, Q, Jiao, L, Chen, F, Xue, S, Li, J, et al. Prognosis and predictors of symptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke. Front Neurol . (2022) 202:730940. doi: 10.3389/fneur.2021.730940
24. Chamorro, Á, Brown, S, Amaro, S, Hill, MD, Muir, KW, Dippel, DWJ, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke . (2019) 50:690–6. doi: 10.1161/STROKEAHA.118.023769
25. Neuberger, U, Kickingereder, P, Schönenberger, S, Schieber, S, Ringleb, PA, Bendszus, M, et al. Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation ischemic stroke. Neuroradiology . (2019) 61:461–9. doi: 10.1007/s00234-019-02180-6
26. Neuberger, U, Seker, F, Schönenberger, S, Nagel, S, Ringleb, PA, Bendszus, M, et al. Prediction of intracranial hemorrhages after mechanical thrombectomy of basilar artery occlusion. J Neurointerv Surg . (2019) 11:1181–6. doi: 10.1136/neurintsurg-2019-014939
27. Ennis, SR, and Keep, RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab . (2007) 27:1573–82. doi: 10.1038/sj.jcbfm.9600454
28. Wang, A, Cui, T, Wang, C, Zhu, Q, Zhang, X, Li, S, et al. Prognostic significance of admission glucose combined with hemoglobin A1c in acute ischemic stroke patients with reperfusion therapy. Brain Sci . (2022) 12:294. doi: 10.3390/brainsci12020294
29. Marik, PE, and Bellomo, R. Stress hyperglycemia: an essential survival response! Crit Care Med . (2013) 41:e93–4. doi: 10.1097/CCM.0b013e318283d124
30. Zhang, J, Li, X, Kwansa, H, Kim, YT, Yi, L, Hong, G, et al. Augmentation of poly(ADP ribose) polymerase-dependent neuronal cell death by acidosis. J Cereb Blood Flow Metab . (2017) 37:1982–93. doi: 10.1177/0271678X16658491
31. Desilles, JP, Syvannarath, V, Ollivier, V, Journé, C, Delbosc, S, Ducroux, C, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke . (2017) 48:1932–40. doi: 10.1161/STROKEAHA.117.017080
32. Hafez, S, Abdelsaid, M, El-Shafey, S, Johnson, MH, Fagan, SC, and Ergul, A. Matrix metalloprotease 3 exacerbates hemorrhagic transformation and worsens functional outcomes in hyperglycemic stroke. Stroke . (2016) 47:843–51. doi: 10.1161/STROKEAHA.115.011258
33. Kamada, H, Yu, F, Nito, C, and Chan, PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke Soci Theatre Res . (2007) 38:1044–9. doi: 10.1161/01.STR.0000258041.75739.cb
34. Ironside, N, Chen, CJ, Chalhoub, RM, Wludyka, P, Kellogg, RT, Al Kasab, S, et al. Risk factors and predictors of intracranial hemorrhage after mechanical thrombectomy in acute ischemic stroke: insights from the stroke Thrombectomy and aneurysm registry (STAR). J Neurointerv Surg . (2023) 15:e312–22. doi: 10.1136/jnis-2022-019513
35. Kim, TJ, Lee, JS, Park, SH, and Ko, SB. Short-term glycemic variability and hemorrhagic transformation after successful endovascular thrombectomy. Transl Stroke Res . (2021) 12:968–75. doi: 10.1007/s12975-021-00895-4
Keywords: large infarct core, symptomatic intracranial hemorrhage, endovascular treatment, acute ischemic stroke, glucose
Citation: Yang Y, Yang L, Shi X, Ni X, Fan S, Xu X, Ma J, Yang S, Wang Z, Zi W, Yang D and Hao Y (2024) Blood glucose to predict symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke with large infarct core: a prospective observational study. Front. Neurol . 15:1367177. doi: 10.3389/fneur.2024.1367177
Received: 08 January 2024; Accepted: 11 April 2024; Published: 01 May 2024.
Reviewed by:
Copyright © 2024 Yang, Yang, Shi, Ni, Fan, Xu, Ma, Yang, Wang, Zi, Yang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Hao, [email protected] ; Dahong Yang, [email protected]
† These authors have contributed equally to this work and share first authorship
‡ These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Submit a Manuscript
- Advanced search

American Journal of Neuroradiology
Advanced Search
Concurrent Moyamoya-like Intracranial Steno-Occlusive Disease and Dural Arteriovenous Fistulas
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Vaishnavi L. Rao
- ORCID record for James P. Klaas
- ORCID record for Giuseppe Lanzino
- Figures & Data
- Supplemental
- Info & Metrics
The simultaneous presentation of intracranial steno-occlusive disease, Moyamoya disease, or Moyamoya-like vasculopathy and dural arteriovenous fistulas (DAVFs) has been documented in very few case reports worldwide. We aimed to better characterize this association by reviewing the clinical and radiologic findings of 4 patients with concurrent intracranial steno-occlusive disease or Moyamoya-like vasculopathy and DAVFs evaluated in our institution. All 4 patients were of Asian descent. One patient presented with ischemic stroke secondary to intracranial stenosis, 2 presented with symptoms related to the DAVF, and the diagnosis was incidental in the fourth patient. Three patients underwent embolization of the DAVF, which was followed by surgical ligation in 2. One patient underwent extracranial-intracranial bypass for Moyamoya-like intracranial steno-occlusive disease. One patient is being managed conservatively with close follow-up. Our case series details findings in 4 patients with associated intracranial steno-occlusive disease and DAVFs. Further studies and reporting of similar cases are necessary to establish whether this is pure coincidence or if there is indeed a relationship between these 2 conditions, especially in certain ethnic groups.
- ABBREVIATIONS:
Moyamoya disease (MMD), Moyamoya-like vasculopathy, and intracranial steno-occlusive disease are cerebrovascular conditions with a higher incidence in East Asian countries than in the Western Hemisphere. 1 Dural arteriovenous fistulas (DAVFs) are relatively uncommon cerebrovascular entities characterized by abnormal shunting between the meningeal arteries and the dural venous sinuses or subarachnoid veins, 2 accounting for 10%–15% of intracranial vascular malformations, 3 with an incidence as high as 0.29 per 100,000 person-years in Japan. 4 There have been very few case reports worldwide documenting the co-occurrence of these 2 disease entities before surgical intervention or head trauma. Here, we describe an institutional series of 4 patients presenting with concurrent Moyamoya-like vascular changes or intracranial steno-occlusive disease and DAVFs.
- CASE SERIES
We identified all patients with intracranial steno-occlusive disease, Moyamoya, Moyamoya-like vasculopathy, and DAVFs from a prospectively maintained database of cases evaluated and/or treated by the senior author (G.L.) at our institution between January 2008 and 2023. The diagnosis of idiopathic Moyamoya or Moyamoya-like vasculopathy was made on the basis of the presence of both stenosis or occlusion of the terminal ICA or the proximal middle and/or anterior cerebral artery and an abnormal network of blood vessels on cerebral angiography, according to the 2021 diagnostic criteria for MMD published by the Ministry of Health, Labor and Welfare in Japan. 5 More recently, patients underwent high-resolution MR imaging with vessel wall enhancement to distinguish eccentric (more consistent with intracranial atherosclerosis) from concentric (more consistent with idiopathic vasculopathy) enhancing patterns. 6 , 7 The Cognard system was used to classify the type of DAVF, because it is useful for predicting the associated risk and guiding management decisions. 8
This retrospective study was reviewed and approved by our institutional review board (19–001663), and all protocols were followed in accordance with institutional review board guidelines. A common denominator of these patients was their Asian ethnicity. Demographics, clinical presentation, and treatment are summarized in the Online Supplemental Data. Angiographic findings are presented in Figs 1–4 . MR vessel wall imaging was available for cases 2 and 4, which presented more recently after vessel wall imaging was incorporated into practice at our institution. For these patients, vessel wall imaging demonstrated circumferential vessel wall enhancement involving the distal left ICA in case 2 and lack of any vessel wall enhancement whatsoever in case 4, suggesting a steno-occlusive picture outside intracranial atherosclerosis. For cases 1 and 3, we elected to treat the DAVFs because they were symptomatic, while in case 2, we attempted treatment of the DAVF because of the retrograde cortical venous drainage/venous hypertension and its potential role in aggravating perfusion issues related to the steno-occlusive disease. For case 4, we elected for observation of the DAVF because of its asymptomatic status/incidental finding and potential risks associated with embolization (ie, compromise of retinal blood flow supply) or surgical therapy (brain manipulation in the setting of steno-occlusive disease and marginal perfusion). The steno-occlusive disease in each patient was medically managed with aspirin monotherapy, while patient 2 underwent surgical revascularization for recurrent left-hemispheric infarcts.
- Download figure
- Open in new tab
- Download powerpoint
Case 1. A 57-year-old Asian man who presented with several weeks of progressive myelopathy. A , Conventional angiography shows a craniocervical junction AVF ( white circle ) with a large arterialized draining vein ( arrowhead ) arising from the dura adjacent to the point where the vertebral artery pierces the dura, supplied by branches of the left vertebral artery ( arrow ). B , The same injection demonstrates the venous drainage of the fistula down the anterior and posterior spinal veins, which are filled early ( arrowhead) . C , A right ICA injection, anterior-posterior view shows incidental occlusion of the M1 segment with Moyamoya-like collaterals ( white circle ) reconstituting the distal MCA territory in addition to leptomeningeal collaterals ( arrowhead ) from the distal anterior cerebral artery. He underwent surgical ligation of the fistula after failed embolization. The asymptomatic MCA occlusion was treated medically.
Case 2. A 63-year-old Asian man who presented with multiple left-hemisphere infarcts during 1 year. A , A left common carotid artery injection shows a left sphenoparietal DAVF ( circle ) supplied by the left middle meningeal artery with exclusive retrograde cortical venous drainage (arrowheads ). B , There is delayed filling of the left ICA ( arrow ), which is occluded distal to the origin of the ophthalmic artery ( arrowheads ). C , Selective right ICA injection, lateral view, shows severe stenosis of the right supraclinoid ICA ( arrows ). The patient underwent partial embolization of the DAVF followed by surgical ligation and left superficial temporal artery–to-MCA bypass.
Case 3, A 74-year-old Asian woman who presented with episodic vision loss, papilledema, and cognitive decline. A , Conventional angiography, selective left ICA injection, shows an incidental left supraclinoid ICA occlusion as well as a left transverse-sigmoid junction DAVF with arterial feeders from the left meningohypophyseal artery ( arrows ). B , External carotid artery injection, lateral view, shows a high-flow transverse-sigmoid sinus DAVF. The DAVF was occluded via a transvenous approach with resolution of symptoms at follow up. Low-dose aspirin was prescribed for the incidental carotid occlusion.
Case 4. A 42-year-old Asian man with right-sided headache. A , Selective right ICA injection, anterior-posterior view, early arterial phase, shows occlusion of the MCA with a Moyamoya network ( white circle ). B , Selective right ICA injection, lateral view, early arterial phase, shows a lack of opacification in the MCA territory and early filling of the superior sagittal sinus ( arrowheads ). C , Right ICA selective injection, magnified oblique view, shows enlarged and tortuous distal ophthalmic artery branches ( arrowheads ) feeding a dural/pial AVF ( white circle ), with early opacification of a cortical vein draining into the superior sagittal sinus.
We describe 4 patients of Asian ethnicity with concomitant idiopathic Moyamoya disease, Moyamoya-like vasculopathy, or steno-occlusive disease and intracranial DAVFs. The rare concurrent appearance of intracranial steno-occlusive disease and DAVFs as seen in our 4 cases has been previously documented in only a few single case reports and usually after surgical therapy or trauma. De novo formation of DAVFs in patients with Moyamoya-like vasculopathy has been observed in a delayed fashion following direct bypass, 9 , 10 indirect bypass, 11 and head trauma; 12 however, there have been a very few cases of simultaneous presentation in the absence of prior surgery or trauma. These include a patient with unilateral MMD who presented with intraventricular hemorrhage and was found to have an ipsilateral transverse-sigmoid DAVF, which was ultimately treated with endovascular embolization, 13 similar to case 3 in our series, as well as ipsilateral cavernous and tentorial DAVFs observed in patients with MMD in China 14 and Spain, 15 respectively.
A proposed mechanism for the formation of DAVFs in these patients is that aberrant flow dynamics in intracranial steno-occlusive disease leads to an increased demand and decreased perfusion, with the subsequent ischemia triggering an angiogenic response in the dural vasculature, resulting in arteriovenous shunting. 16 Indeed, increased expression of proangiogenic factors has been reported in the dura of patients with MMD, including vascular endothelial growth factor 17 and fibroblast growth factor. 18 These factors are also involved in the formation of DAVFs. 19
Of note, all 4 patients in our series are of East Asian ethnicity, suggesting that a genetic association may potentially exist in cases of concurrent intracranial steno-occlusive disease and DAVF. None of the previous case reports we came across specified the patients’ ethnicity, so we could not confirm such an association on the basis of prior literature. Nevertheless, variants of the HLA genes and the RNF213 susceptibility gene have been found to be associated with East Asian patients with MMD. 20 , 21 Unfortunately, the patients included in our study did not undergo genetic testing to evaluate these pathogenic variants; however, this association would be an interesting area of future study. Overall, intracranial stenosis is more prevalent in Asians, 22 often with a younger mean age of onset and independent of vascular risk factors compared with Caucasians. 23
In our small institutional case series, we describe the simultaneous presentation of the two entities of Moyamoya pattern/steno-occlusive intracranial disease and DAVF in 4 patients of East Asian descent. While our series does not establish causality, future reporting and study of similar cases may further determine whether a potential relationship exists between ethnicity and the concurrence of these cerebrovascular phenomena. As more patients like these are identified, the pathophysiology of these two conditions can be more clearly elucidated and the presence of an underlying genetic association can be investigated.
- Acknowledgments
We acknowledge Peggy Chihak and Arielle Davis for their help in formatting the figures and manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org .
- Reynolds MR ,
- Lanzino G ,
- Newton TH ,
- Cronqvist S
- Fujimura M ,
- Takahashi J , et al
- Mossa-Basha M ,
- de Havenon A ,
- Becker KJ , et al
- Gao X , et al
- Cognard C ,
- Pierot L , et al
- Feroze AH ,
- Kushkuley J ,
- Choudhri O , et al
- Hanaoka M ,
- Matsubara S ,
- Satoh K , et al
- Wilkinson DA ,
- Griauzde JM , et al
- Zaletel M ,
- Surlan-Popović K ,
- Pretnar-Oblak J , et al
- Killory BD ,
- Gonzalez LF ,
- Wait SD , et al
- Lv X , et al
- Cajal Campo B ,
- Delgado Acosta F
- Lawton MT ,
- Jacobowitz R ,
- Spetzler RF
- Sakamoto S ,
- Yamasaki F , et al
- Hoshimaru M ,
- Takahashi JA ,
- Kikuchi H , et al
- Uranishi R ,
- Mertens R ,
- Graupera M ,
- Gerhardt H , et al
- Hiraide T ,
- Momoi M , et al
- Johnston SC
- Hurford R ,
- Feng X , et al
- Received November 15, 2023.
- Accepted after revision January 15, 2024.
- © 2024 by American Journal of Neuroradiology
Thank you for your interest in spreading the word on American Journal of Neuroradiology.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles.
- Google Scholar
Cited By...
- No citing articles found.
This article has not yet been cited by articles in journals that are participating in Crossref Cited-by Linking.
More in this TOC Section
- Association between CT Perfusion Parameters and Hemorrhagic Transformation after Endovascular Treatment in Acute Ischemic Stroke: Results from the ESCAPE-NA1 Trial
- Contrast Staining in Noninfarcted Tissue after Endovascular Treatment of Acute Ischemic Stroke
Similar Articles

IMAGES
VIDEO
COMMENTS
Ischemic Stroke Syndromes. ... This correlates to the classical presentation of contralateral hemiparesis, facial paralysis, and sensory loss in the face and upper extremity. The lower extremity may be involved, but upper extremity symptoms usually predominate. Gaze preferences towards the side of the lesion may be seen.
Stroke should be considered in any patient presenting with an acute neurologic deficit (focal or global) or altered level of consciousness. No historical feature distinguishes ischemic from hemorrhagic stroke, although nausea, vomiting, headache, and a sudden change in the patient's level of consciousness are more common in hemorrhagic ...
An ischemic stroke is caused by a blocked artery in the brain. A hemorrhagic stroke is caused by leaking or bursting of a blood vessel in the brain. Some people may have only a temporary disruption of blood flow to the brain, known as a transient ischemic attack (TIA). A TIA doesn't cause lasting symptoms. Ischemic stroke
Ischemic stroke can also result from lacunar infarcts. These small (≤ 1.5 cm) infarcts result from nonatherothrombotic obstruction of small, perforating arteries that supply deep cortical structures; the usual cause is lipohyalinosis (degeneration of the media of small arteries and replacement by lipids and collagen).Emboli may cause lacunar infarcts.
An ischemic stroke happens when an artery in the brain is blocked. There are two types of ischemic stroke: Embolic Stroke: In an embolic stroke, a blood clot or plaque fragment forms, usually in the heart or the large arteries leading to the brain, and then moves through the arteries to the brain. In the brain, the clot blocks a blood vessel ...
An ischemic stroke is the most common type of stroke. It occurs when a blood clot or fatty plaque lodges in a blood vessel within the brain, blocking blood flow. Because brain cells begin to die within minutes of the interruption of blood flow, it's crucial for an ischemic stroke to be diagnosed and treated as quickly as possible. ...
Each year in the United States, approximately 700,000 people have an acute ischemic stroke. 1 Before modern treatments, early mortality was 10%. 2 Among survivors, one half had moderate-to-severe ...
Ischemic stroke usually results when an artery to the brain is blocked, often by a blood clot and/or a fatty deposit due to atherosclerosis. Symptoms occur suddenly and may include muscle weakness, paralysis, lost or abnormal sensation on one side of the body, difficulty speaking, confusion, problems with vision, dizziness, and loss of balance ...
The subacute and long-term assessment and management of patients who have suffered a stroke includes physical therapy and testing to determine the precise etiology of the event so as to prevent recurrence. The acute management differs. Immediate goals include minimizing brain injury, treating medical complications, and moving toward uncovering ...
An ischemic stroke is a life-threatening medical condition that happens when there's a lack of blood flow to a part of your brain. These usually happen because of blood clots, but they can also happen for other reasons. Ischemia (pronounced "iss-key-me-uh") is when cells in your body don't have enough blood flow, which causes them to die.
Ischemic strokes occur when blood supply is cut off to part of the brain. This type of stroke accounts for the majority of all strokes. The blocked blood flow in an ischemic stroke may be caused by a blood clot or by atherosclerosis, a disease which causes narrowing of the arteries over time. Ischemic strokes can be caused by a blockage anywhere along the arteries feeding the brain.
Types of Stroke: Ischemic Stroke. Ischemic stroke is the most common type of stroke, accounting for about 87% of strokes. An ischemic stroke occurs when a clot blocks a vessel supplying blood to the brain. The artery becomes narrowed or clogged, cutting off blood flow to brain cells. Slide 7: Types of Stroke: Hemorrhagic Stroke
A stroke is a debilitating emergency event that can greatly affect speech and movement, and is potentially fatal. One type is an ischemic stroke. Strokes have several signs that can alert someone ...
It's the most common type of stroke, and around 85% of strokes in the UK are ischaemic strokes. The other 15% of strokes are due to bleeding in or around the brain, known as haemorrhagic stroke. A transient ischaemic attack (TIA or mini-stroke) is the same as a stroke but the symptoms only last for a short amount of time.
Ischemic stroke is an episode of neurological dysfunction due to focal infarction in the central nervous system attributed to arterial thrombosis, embolization, ... Clinical presentation. An ischemic stroke typically presents with rapid onset neurological deficit, which is determined by the area of the brain that is involved. ...
Risk factors of ischemic stroke. You're more likely to have an ischemic stroke if you: Are over age 60; Have high blood pressure, heart disease, high cholesterol, or diabetes; Have an irregular ...
Ischemic stroke (see the image below) is characterized by the sudden loss of blood circulation to an area of the brain, resulting in a corresponding loss of neurologic function. Acute ischemic stroke is caused by thrombotic or embolic occlusion of a cerebral artery and is more common than hemorrhagic stroke. Maximum intensity projection (MIP ...
Summary. Ischemic and hemorrhagic strokes have different causes. When a blood clot or other debris blocks a blood vessel leading to part of the brain, it causes an ischemic stroke. Hemorrhagic ...
Ischemic stroke is a major cause of morbidity and mortality worldwide. Ischemic stroke and transient ischemic attack exist on a continuum of the same disease process. Ischemic stroke is common, and more than 85% of stroke risk is attributed to modifiable risk factors. The initial management of acute …
83 time of ischemic or hemorrhagic stroke presentation, we further stratified by their primary 84 presentation, whether patients presented with COVID -19 first and developed a stroke during 85 their hospitalization (N=45) (stroke -first) or whether stroke was the initial presenting symptom
1 Introduction. As the second most lethal and disabling disease worldwide, acute ischemic stroke (AIS) has a serious impact on patient survival and quality of life ().Approximately 20% of patients with AIS arrive at the hospital with a large infarct core, characterized by high mortality and poor prognosis, posing a global challenge in clinical management for this population ().
The simultaneous presentation of intracranial steno-occlusive disease, Moyamoya disease, or Moyamoya-like vasculopathy and dural arteriovenous fistulas (DAVFs) has been documented in very few case reports worldwide. ... One patient presented with ischemic stroke secondary to intracranial stenosis, 2 presented with symptoms related to the DAVF ...
setting of acute ischemic infarction MRI DWI MRI ADC. Moyamoya Moyamoya disease is an idiopathic, progressive, non-inflammatory, non-atherosclerotic, vaso-occlusive disease characterized by ... • Most frequent cause of stroke in Asian children Presentation
No association was observed between day-to-day variation in any pollutant and risk of total stroke, ischemic stroke, or specific etiologies of ischemic stroke. We observed a positive association between risk of hemorrhagic stroke and NO2 and NOx in the 3 days prior to stroke with OR of 1.24 (95% CI: 1.01, 1.52) and 1.18 (95% CI: 1.03, 1.34) per ...
The webcast will remain available for playback on our website, under investor relations - events and presentations, following the earnings call and for 12 months thereafter. ... company committed to improving the lives of people suffering from serious diseases with a focus on acute ischemic stroke. DiaMedica's lead candidate DM199 ...