Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center


LYMPHATIC FILARIASIS- A CASE REPORT

2019, JETIR
Filariasis is a disease group caused by filariae that affects humans and animals. Of the hundreds of described filarial parasites, only 8 species cause natural infections in humans. In this, repeated episodes of inflammation and lymphedema lead to lymphatic damage, chronic swelling and elephantiasis of the legs, arms, scrotum, vulva and breasts. Case presentation: A 72 year old male patient admitted in the hospital with chief complaints of fever, lower right limb swelling, and testicular pain with testicular swelling (mild). Upon laboratory investigations, it was found to be lymphatic filariasis. Discussion and Conclusion: More than 120 million people are infected. Imaging Ultrasound plays an important role in diagnosing filariasis. This case report deals with swelling of left lower limb and scrotum. The clinical manifestations of filariasis vary from person to person depending upon course of infection and worm load. Simple and cost-effective control strategies should be developed.
Related Papers
The American journal of tropical medicine and hygiene
Maged El-Setouhy
The purpose of this study was to explore the value of scrotal ultrasound as a means of evaluating Bancroftian filariasis. Color Doppler ultrasound examinations were performed to look for subclinical hydroceles and motile adult filarial worms (dancing worms) in dilated lymphatics. Sixty-one male subjects from a filariasis-endemic area in Egypt were studied including 19 clinically normal microfilaria (MF) carriers (seven with dancing worms and eight with subclinical hydroceles), 13 MF-negative subjects with positive filarial antigen test results (three with dancing worms and seven with subclinical hydroceles), 22 exposed subjects with no MF and negative antigen test results (no dancing worms, four subclinical hydroceles), and seven subjects with clinical filariasis (no dancing worms, seven hydroceles). Thus, all men tested with clinical filariasis and most clinically normal subjects with either microfilaremia or filarial antigenemia had abnormal ultrasound examination results. Ultraso...
Khairuddin Djawad
Filariasis is an infectious disease caused by a filarial worm infection transmitted by mosquito bites. The disease can result in reduced work productivity, disability and social stigma. This disease transmission process begins when a mosquito bite and suck the blood containing the microfilaria. Filarial infections have been grouped into three categories based on their location diseases of the disease: (1) lymphatics, (2) skin, and (3) body cavities. Morbidity is almost entirely due to the species that cause lymphatic diseases, and skin diseases to a lesser degree. A 28-year-old male came with a chief complaint of swollen right leg since four years ago which worsened in the last three months. Upon physical examination, edema, fibrosis, and hyper-pigmented plaques were present on the right lower extremity. The blood microfilariae examination was positive for Wuchereria bancrofti. The lymphedema did not resolve despite of antifilarial treatment and surgery was eventually performed to r...
Acta Cytologica
Arati Bhatia
International Journal of Contemporary Medical Research [IJCMR]
Rahul Solanki
Revista Do Instituto De Medicina Tropical De Sao Paulo
Guilherme Lima
Dermatologic Therapy
Natalia Mendoza
Ipyana Mwampagatwa , Bonaventura Mpondo
Lymphatic filariasis is frequently caused by Wuchereria banchrofti a widely distributed filarial worm throughout tropical regions of Asia and Africa. This worm is particularly prevalent in wet and humid areas. The common clinical manifestations of Banchroftian filariasis are acute adenolymphangitis, hydrocele, lymphedema and elephantiasis. Of these, elephantiasis appears to be most common in the legs. Pathology in the arms, scrotum and penis are also common. On the other hand elephantiasis of the vulva and the female breast is extremely rare and its occurrence deserves a mention in medical literature. Presented here is a 21 years old female who presented with progressive unilateral vulvar swelling over a period of two years. Microfilariae were found in peripheral blood film. The diagnosis of vulvar filarial elephantiasis was reached. The patient underwent reconstructive surgery and planned to be initiated on Diethylcarbamazine Citrate (DEC) which is a drug of choice in cases of filarial infestations. Keywords: Elephantiasis, Filariasis, Vulvar.
Siriraj Medical Journal
Sirichit Wongkamchai
Qatar Medical Journal
salem salah
Introduction: Filariasis is an endemic disease with worldwide distribution in tropical and subtropical regions. It is uncommon in Qatar. The conventional diagnostic procedure is the demonstration of microfilaria in blood smears. Even with its high incidence, it is unusual to detect microfilaria in fine needle aspiration cytology (FNAC) smears. Although the ‘filarial dance sign’ is rarely documented, it remains a classical ultrasonographic sign in lymphatic filariasis. Case presentation: We present a case of a 38-year-old male patient with fever, chills, shortness of breath and a tender warm swelling on his right thigh. Ultrasound of the thigh lesion showed the classical filarial dance sign. Subsequently FNAC from the lesion documented microfilaria in spite of absent peripheral blood eosinophilia and microfilaria. The patient underwent an incision and drainage of the thigh lesion and was treated with ivermectin and diethylcarbamazine. He was subsequently admitted to the medical ward ...
Acta medica Indonesiana
Winfrey Pangestu
A 51-year-old male came with the complaint of recurrent swelling in the scrotum and legs. Swelling of the scrotum first appeared 17 years ago in the left scrotum approximately the same size as an apple and underwent surgery. However, 2 years after surgery, the swelling reemerged and gradually increase in size in both scrotums. Left leg swelling began to emerge 5 years ago followed by right leg 3 years after. The patient lives in Sarmi regency Papua province (endemic).
RELATED PAPERS
SSRN Electronic Journal
Mustapha Balogun
Scientific Reports
Victor Multanen
Vojnosanitetski pregled
Vladimir Antic
Jurnal Hukum Bisnis Bonum Commune
Elsa Mellinda Saputri
Rivista Italiana di Costruttivismo Periodico Semestrale
Chemosensors
Frank Davis
IEEE Access
Jamal Hassan
EDUCAÇÃO E FILOSOFIA
Pedro Angelo Pagni
Journal of Economic Entomology
pio federico roversi
BMC medical informatics and decision making
Dina Alves Silva
Pediatric Pulmonology
Yvonne Belessis
Srpski arhiv za celokupno lekarstvo
Nikola Cerović
Tuncay Delibasi
Environment-Behaviour Proceedings Journal
Mohd Isahak
Journal of EMDR Practice and Research
Julio Gomez
Dmitry Cherkashin
Stephen Picken
Remote Sensing of Environment
Asmaa Abdelbaki
International Journal on Computational Science & Applications
Muthu kumar
Akmal Abdelfatah
Epidemiology, Biostatistics, and Public Health
Abdul Rehman Shah
Ranko Rajović , Andrea Debeljuh
American Journal of Veterinary Research
Ignacio Lopez
Environmental Toxicology and Pharmacology
Deepak Kumar Mittal
Janet Perkins
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Case #446 – June, 2017
Blood specimens were collected at night from members of a village in Myanmar endemic for lymphatic filariasis. Roughly one-third of the cases were symptomatic, with or without recurrent episodes of fever and various degrees of lymphedema. The objects in Figures A and B were observed on Giemsa-stained thick blood films from a few of the asymptomatic cases at 500x oil magnification; Figures C and D show close detail of the object in Figure B at 1000x oil magnification. The objects measured on average 200 micrometers in length. What is your diagnosis? Based on what criteria?
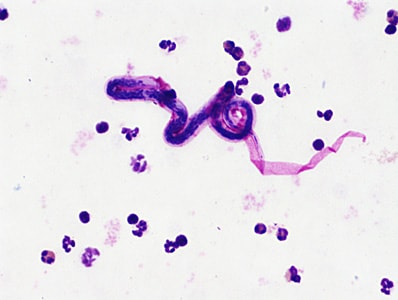
This was a case of lymphatic filariasis caused by Brugia malayi . Diagnostic morphologic features included:
- microfilaria whose sheaths stained pink with Giemsa and within the size range for B. malayi (175-230 micrometers)
- dense nuclear column in which individual nuclei are easily defined
- microfilariae with a relatively long head space and a tail with terminal and subterminal nuclei (Figure D )
More on lymphatic filariasis
Images presented in the dpdx case studies are from specimens submitted for diagnosis or archiving. On rare occasions, clinical histories given may be partly fictitious.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/ .
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform
Affiliations.
- 1 MRC Centre for Global Infectious Disease Analysis, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, London, United Kingdom.
- 2 London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, London, United Kingdom.
- 3 Infectious Diseases Data Observatory, University of Oxford, Oxford, United Kingdom.
- 4 ICMR-Vector Control Research Centre, Puducherry, India.
- 5 Department of Pathobiology and Population Sciences, Royal Veterinary College, Hatfield, United Kingdom.
- 6 Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
- 7 The Knowledge Centre, Bodleian Health Care Libraries, University of Oxford, Oxford, United Kingdom.
- 8 Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India.
- PMID: 38227595
- PMCID: PMC10817204
- DOI: 10.1371/journal.pntd.0011882
Background: Lymphatic filariasis (LF) is a neglected tropical disease (NTD) targeted by the World Health Organization for elimination as a public health problem (EPHP). Since 2000, more than 9 billion treatments of antifilarial medicines have been distributed through mass drug administration (MDA) programmes in 72 endemic countries and 17 countries have reached EPHP. Yet in 2021, nearly 900 million people still required MDA with combinations of albendazole, diethylcarbamazine and/or ivermectin. Despite the reliance on these drugs, there remain gaps in understanding of variation in responses to treatment. As demonstrated for other infectious diseases, some urgent questions could be addressed by conducting individual participant data (IPD) meta-analyses. Here, we present the results of a systematic literature review to estimate the abundance of IPD on pre- and post-intervention indicators of infection and/or morbidity and assess the feasibility of building a global data repository.
Methodology: We searched literature published between 1st January 2000 and 5th May 2023 in 15 databases to identify prospective studies assessing LF treatment and/or morbidity management and disease prevention (MMDP) approaches. We considered only studies where individual participants were diagnosed with LF infection or disease and were followed up on at least one occasion after receiving an intervention/treatment.
Principal findings: We identified 138 eligible studies from 23 countries, having followed up an estimated 29,842 participants after intervention. We estimate 14,800 (49.6%) IPD on pre- and post-intervention infection indicators including microfilaraemia, circulating filarial antigen and/or ultrasound indicators measured before and after intervention using 8 drugs administered in various combinations. We identified 33 studies on MMDP, estimating 6,102 (20.4%) IPD on pre- and post-intervention clinical morbidity indicators only. A further 8,940 IPD cover a mixture of infection and morbidity outcomes measured with other diagnostics, from participants followed for adverse event outcomes only or recruited after initial intervention.
Conclusions: The LF treatment study landscape is heterogeneous, but the abundance of studies and related IPD suggest that establishing a global data repository to facilitate IPD meta-analyses would be feasible and useful to address unresolved questions on variation in treatment outcomes across geographies, demographics and in underrepresented groups. New studies using more standardized approaches should be initiated to address the scarcity and inconsistency of data on morbidity management.
Copyright: © 2024 Freitas et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication types
- Systematic Review
- Albendazole / therapeutic use
- Diethylcarbamazine / therapeutic use
- Elephantiasis, Filarial* / drug therapy
- Elephantiasis, Filarial* / epidemiology
- Elephantiasis, Filarial* / prevention & control
- Filaricides* / therapeutic use
- Ivermectin / therapeutic use
- Prospective Studies
- Filaricides
- Diethylcarbamazine
- Albendazole
Grants and funding
- INV-004713/GATES/Bill & Melinda Gates Foundation/United States
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,973,697 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform.
Author information, affiliations.
- Freitas LT 1, 3, 6
- Halder JB 1, 3, 6
- Basáñez MG 1, 3, 6
- Balakrishnan V 2
- Srividya A 2
- Singh-Phulgenda S 3
- Guérin PJ 3
- Walker M 3, 6
- Harriss E 4
ORCIDs linked to this article
- Walker M | 0000-0001-8714-5365
- Jeyapal DR | 0000-0002-3359-3039
- Rahi M | 0000-0003-0932-0935
- Freitas LT | 0009-0003-3831-5788
Plos Neglected Tropical Diseases , 16 Jan 2024 , 18(1): e0011882 https://doi.org/10.1371/journal.pntd.0011882 PMID: 38227595 PMCID: PMC10817204
Abstract
Methodology, principal findings.
Conclusions
Free full text

The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform
Luzia t. freitas.
1 MRC Centre for Global Infectious Disease Analysis, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, London, United Kingdom
2 London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, London, United Kingdom
3 Infectious Diseases Data Observatory, University of Oxford, Oxford, United Kingdom
Mashroor Ahmad Khan
4 ICMR-Vector Control Research Centre, Puducherry, India
Azhar Uddin
Julia b. halder.
5 Department of Pathobiology and Population Sciences, Royal Veterinary College, Hatfield, United Kingdom
Sauman Singh-Phulgenda
6 Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Jeyapal Dinesh Raja
Vijayakumar balakrishnan, eli harriss.
7 The Knowledge Centre, Bodleian Health Care Libraries, University of Oxford, Oxford, United Kingdom
Matthew Brack
Philippe j. guérin, maria-gloria basáñez, ashwani kumar.
8 Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
Martin Walker
Adinarayanan srividya.
- Associated Data
All relevant data are within the manuscript and its Supporting information files.
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) targeted by the World Health Organization for elimination as a public health problem (EPHP). Since 2000, more than 9 billion treatments of antifilarial medicines have been distributed through mass drug administration (MDA) programmes in 72 endemic countries and 17 countries have reached EPHP. Yet in 2021, nearly 900 million people still required MDA with combinations of albendazole, diethylcarbamazine and/or ivermectin. Despite the reliance on these drugs, there remain gaps in understanding of variation in responses to treatment. As demonstrated for other infectious diseases, some urgent questions could be addressed by conducting individual participant data (IPD) meta-analyses. Here, we present the results of a systematic literature review to estimate the abundance of IPD on pre- and post-intervention indicators of infection and/or morbidity and assess the feasibility of building a global data repository.
We searched literature published between 1 st January 2000 and 5 th May 2023 in 15 databases to identify prospective studies assessing LF treatment and/or morbidity management and disease prevention (MMDP) approaches. We considered only studies where individual participants were diagnosed with LF infection or disease and were followed up on at least one occasion after receiving an intervention/treatment.
We identified 138 eligible studies from 23 countries, having followed up an estimated 29,842 participants after intervention. We estimate 14,800 (49.6%) IPD on pre- and post-intervention infection indicators including microfilaraemia, circulating filarial antigen and/or ultrasound indicators measured before and after intervention using 8 drugs administered in various combinations. We identified 33 studies on MMDP, estimating 6,102 (20.4%) IPD on pre- and post-intervention clinical morbidity indicators only. A further 8,940 IPD cover a mixture of infection and morbidity outcomes measured with other diagnostics, from participants followed for adverse event outcomes only or recruited after initial intervention.
The LF treatment study landscape is heterogeneous, but the abundance of studies and related IPD suggest that establishing a global data repository to facilitate IPD meta-analyses would be feasible and useful to address unresolved questions on variation in treatment outcomes across geographies, demographics and in underrepresented groups. New studies using more standardized approaches should be initiated to address the scarcity and inconsistency of data on morbidity management.
- Author summary
Lymphatic filariasis (LF) is a debilitating parasitic disease that the World Health Organization (WHO) has earmarked for elimination by 2030 through a combination of mass distribution of anti-parasitic medicines and disease management approaches. Great strides have been made towards the elimination of LF as a public health problem, but nearly 900 million people still require treatment every year. In recent years, new combinations of medicines have been shown to improve the treatment of LF, yet there remain substantial gaps in understanding of apparent variability in treatment success and on the best treatment and management approaches to alleviate chronic morbidity. Some of these questions could be addressed through the development of an LF global data platform, which would enable pooled analyses of all available individual participant data. Here, we present the results of a systematic literature review of the LF treatment study landscape. We estimate the abundance of individual data on treatment of infection and morbidity and describe the characteristics of the studies and participants that have generated these data. We argue that collating and curating these data into a data LF platform could help to fill gaps in understanding of the best ways to treat infection and disease and enhance prospects of eliminating LF by 2030.
- Introduction
Lymphatic filariasis (LF) is a mosquito-borne neglected tropical disease (NTD) targeted by the World Health Organization (WHO) for elimination as a public health problem (EPHP), aiming at circa 80% of endemic countries validated for EPHP by 2030 [ 1 ]. Globally, approximately 50 million people are infected with the filarial nematodes ( Wuchereria bancrofti , Brugia malayi and B . timori) that cause LF, and 885 million people are estimated to be at risk of infection [ 2 ]. Moreover, approximately 36 million people are chronically debilitated by filarial lymphedema [ 3 ]. The global public health burden of LF in 2019 was estimated as 1.63 million disability-adjusted life years (DALYs) [ 4 ].
Since the inception of the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000, 17 countries have eliminated LF as a public health problem, primarily by repeated rounds of mass drug administration (MDA) with anti-filarial drugs to at-risk populations, a strategy known as preventive chemotherapy. The combination therapies of diethylcarbamazine plus albendazole (DA), or ivermectin plus albendazole (IA, used in Africa where co-endemicity with other filarial diseases, particularly onchocerciasis, complicates the use of diethylcarbamazine), were the cornerstones of MDA between 2000 and 2019, with more than 9 billion treatments distributed among endemic countries [ 2 ]. Since 2019, the use of a triple drug regimen combining ivermectin, diethlycarbamazine and albendazole (IDA), which has demonstrated superior efficacy compared to its dual therapy counterparts, (i.e., DA or IA) [ 5 – 8 ], has been increasingly adopted to accelerate progress towards LF elimination [ 9 , 10 ].
In addition to MDA, the LF elimination strategy also consists of morbidity management and disability prevention (MMDP) interventions for the various sequelae of chronic LF. The recommended minimum package of care includes treatment to kill remaining parasites (adult worms and microfilariae), management of lymphedema and elephantiasis to prevent episodes of acute dermatolymphangioadenitis (ADLA) and surgery for hydrocele [ 11 ]. However, the efficacy of each of these elements is understudied and a systematic review and meta-analysis of morbidity management outcomes related to ADLA [ 12 ] noted the need for standardisation, high degrees of study heterogeneity and insufficiency of information on the numbers of people requiring MMDP interventions.
Notwithstanding the successes of the GPELF in alleviating the global burden of LF, 45 countries require ongoing preventive chemotherapy and in India alone—where approximately 40% of global infections occur—this amounts to more than 400 million people considered at risk [ 2 ]. The urgency to meet the 2030 elimination goals has led to the rapid rollout of IDA as a strategy to accelerate LF elimination in many countries, including India [ 13 ]. Since Phase II and III clinical trials of IDA were only completed in 2018 (the latter demonstrating 96% clearance of microfilaraemia after 12 months, far superior to the 32% clearance among participants treated with DA) [ 5 , 14 ], this rollout has been done in conjunction with Phase IV trials that have generated safety and efficacy data from thousands of individuals in India, Southeast Asia and sub-Saharan Africa [ 6 – 8 ].
The rapid rollout of IDA was based, in part, on the findings of transmission dynamics modelling [ 15 ]. The models, which used assumptions on the microfilaricidal, macrofilaricidal and sterilizing effects of IDA and DA corresponding to clearance of microfilariae at 12 months after treatment of 100% for IDA and 23.1% for DA, indicated that IDA would have a profound impact on accelerating progress towards the elimination of LF. Yet, community trials have already revealed variable efficacy of the triple drug regimen in Côte d’Ivoire [ 7 ], Fiji [ 16 ], India [ 8 ] and Samoa [ 17 ]. Examining the findings of those publications does not allow to fully understand the risk factors associated with poorer treatment outcomes nor to conduct aggregated meta-analyses due to the heterogeneity of study endpoints. Collating, standardizing and analysing the IPD of recent studies would be a productive methodological approach to understand the factors determining variation in treatment responses [ 18 – 20 ].
The positive outcomes of sharing clinical data are well-recognised in the biomedical sciences [ 21 – 23 ], although adherence to data-sharing policies remains variable with the principles of Findability , Accessibility , Interoperability , and Reusability often not followed [ 24 ]. The need for managed repositories is of critical importance to avoid the unstructured and chaotic deposition of data over the internet, and the challenge of finding data [ 25 , 26 ]. The Infectious Diseases Data Observatory (IDDO) is an example of an established model based on a repository infrastructure that gathers dispersed and disparate IPD from scattered studies to create standardised datasets that allow researchers to address critical research gaps in a number of infectious diseases, beginning with the Worldwide Antimalarial Resistance Network (WWARN) in 2009 and extending to NTDs, including Chagas disease [ 27 ], visceral leishmaniasis [ 28 ], schistosomiasis and the soil-transmitted helminthiases [ 29 ], and emerging infections including Ebola and SARs-CoV-2 [ 30 ]. These platforms focus on the collation, curation and management of information-rich IPD, making them distinct from other population-level epidemiological data repositories such as the Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN) portal ( https://espen.afro.who.int ) and the WHO’s Global Health Observatory ( https://www.who.int/data/gho ).
The first step towards developing a data repository and reuse platform is a feasibility assessment. This entails a systematic literature review to estimate the quantity of IPD potentially available and, critically, the age of such data as a useful proxy of the likelihood of whether they will be available to be shared. Indeed, the fact that the likelihood of data being essentially lost increases with time is further testament to the imperative to archive and safeguard valuable IPD in actively managed repositories [ 31 ]. The systematic review also generates valuable study-level meta-data, which can be useful in identifying knowledge gaps in patient characteristics, diagnosis methods, treatment efficacy and safety outcomes [ 27 , 28 , 32 – 34 ].
Here, we present the results of a systematic literature review which characterises the published LF treatment studies and clinical trials landscape. We identify studies that have generated pre- and post-intervention IPD on infection and/or morbidity indicators and provide an overview of their essential study-level (meta-data) characteristics. We estimate the abundance of studies and associated IPD that could potentially be available and integrated into a LF data platform and discuss the feasibility and potential usability of such a platform to address knowledge gaps using IPD meta-analyses and identify where further primary research is required.
Systematic review search strategy
We searched the following databases on 5th May 2023: Ovid MEDLINE; Ovid Embase; Ovid Global Health; Scopus; Web of Science Core Collection; World Health Organization Global Index Medicus (AIM (AFRO), IMEMR (EMRO), IMSEAR (SEARO), LILACS (AMRO/OPAS)); Cochrane Database of Systematic Reviews; Cochrane Central Register of Controlled Trials; and Indmed, African Journals Online, ClinicalTrial.gov , WHO ICTRP and Ctri.nic.in. The search strategy was constructed using a comprehensive set of terms representing lymphatic filariasis, including parasite names, disease terms, and appropriate diagnostics. Keywords included (but were not limited to): bancroftian filariasis, brugian filariasis, elephantiasis, Wuchereria bancrofti , Brugia malayi , Brugia timori , lymphedema, circulating filarial antigen. Search strings were tailored for each database and included controlled terminology (e.g., MeSH terms) where relevant. The full search strategy for all databases is given in S1 Text . No limits were placed on language. The search was restricted to those published from 2000 to the date of search (5 th May 2023). The review is registered in the international Prospective Register of Systematic Reviews (PROSPERO) under the reference CRD42022319146.
All references were exported to an Endnote 20 library (Thomson Reuters, New York, NY) and deduplicated using a semi-automated method [ 35 , 36 ]. The de-duplicated references were loaded into a Covidence library ( www.covidence.org ), to facilitate collaborative screening and elimination of studies for the review. References were screened independently by two members of the review team (any two of LTF, MAK, AU and JBH). We first screened titles and abstracts for eligibility against the inclusion and exclusion criteria. Studies retained at this stage were then evaluated for eligibility by screening the full text.
Inclusion criteria for published studies on LF treatments
The search strategy was designed to retrieve all published registered trials and cohort studies on treatments for LF (infection and MMDP). For this review, we developed inclusion criteria for a subset of these, namely trials and observational studies published in full-text journal articles since 2000, due to the lower probability of IPD being retrievable and available from studies published prior to this [ 31 ].
The inclusion criteria were: (1) published after 2000; (2) availability of full text; (3) participants were diagnosed at baseline with LF by any (microscopy, serology, molecular, ultrasonographic, or clinical) method, and underwent an intervention; (4) a subset of study participants were followed post-intervention for assessment of intervention outcomes. Hence, the inclusion criteria were designed to cover all published studies on LF interventions (drug treatments and MMDP interventions) and which generated individual-level data (IPD) on intervention outcomes.
The exclusion criteria were: (1) non-human animal-only studies; (2) populations not tested for LF indicators; (3) non-primary research studies (books, letters, reviews); (4) case reports/series; (5) qualitative surveys; (6) retrospective studies other than for MMDP interventions; (7) studies published in conference abstracts only, and (8) studies with no follow-ups or follow-ups where individual participants could not have been followed through multiple (at least two) time points, e.g., repeated cross-sections.
Data extraction and management
A tailored variable data dictionary was prepared to facilitate and standardise the extraction process ( S1 Table ) through discussion with senior researchers (MGB, AK, MW, AS). A database was created using REDCap (Research Electronic Data Capture) [ 37 ] and validated through pilot extraction of variables. Data extraction was undertaken by three researchers (LTF, MAK and AU) and cross-verified among the three researchers and an additional researcher (JBH) was called if any disagreement was identified. The data extracted comprise details on: (1) study settings; (2) design/categories; (3) demographic characteristics; (4) details of drug treatment and MMDP interventions, and (5) an estimate of the number of potential IPD per study arm and at study level. We sourced support for translation of Portuguese publications, but other language publications (of particular note, Chinese) were not within the proficiencies of the review team and hence were not analysed in this review.
Data analysis
We analysed the study meta-data and characteristics using descriptive statistics, graphics and narrative synthesis. We estimated the amount of potential IPD generated by eligible studies based on reported study arm characteristics. For participants to be included in the estimate, a study arm must have tested or assessed participants for LF indicators (infection or disease), delivered drug treatment or another intervention (to all or a subset of participants) and subsequently followed up (all or a subset of) participants for the collection of post-intervention outcome data (this could include infection or disease indicators). We also made specific estimates on the amount of IPD which could be used to calculate responses to treatment of infection by: microfilaraemia (microfilariae in blood), circulating filarial antigen (CFA), ultrasound to detect worm nests, filarial dance sign (FDS) or adult worms by estimating the number of participants in those studies who were initially diagnosed by those methods as infection positive.
All analyses were done using Microsoft Excel and R software (version 4.2.2.).
Systematic review and screening
A total of 51,193 records were found in the database searches. Through initial deduplication, 33,937 articles were removed. The remaining 17,256 item titles and abstracts were screened. 16,589 irrelevant or further duplicate articles were excluded. From the remaining 667 full-text articles, a total of 147 full-text articles were included which reported on 138 distinct studies ( Fig 1 and S2 Text for reference list of all full-text studies). All the data extracted from the 147 full-text articles are included in S2 Table .

Geographical coverage and estimated abundance of individual participant data
Studies generating longitudinal (at least two time points, one pre- and one post-intervention) IPD on LF interventions (treatments of infection and/or morbidity management interventions) have been carried out in 23 countries ( Fig 2 ). Five of those countries (Brazil, Ghana, Haiti, India and Papua New Guinea) have had multiple studies conducted in each of the past three decades. Five of the 23 countries (Cook Islands, Egypt, Malawi, Sri Lanka and Thailand) have reached the EPHP of LF [ 2 ].

The 23 countries are shaded from red to yellow in accordance with the estimated abundance of IPD. The 39 countries shaded in blue are endemic for LF but had no studies identified. China is shaded green because of language barriers in assessing the abundance of IPD potentially available. The map was created using World ( naturalearthdata.com ) and the tmap package [ 38 ] for R (v. 4.2.2) [ 39 ].
Estimated individual participant data and timelines
We estimate that overall, 29,842 IPD on pre- and post-intervention outcomes have been generated worldwide from studies published between 2000 and 5 th May 2023, with Southeast Asia accounting for more than half of the data generated. Between 2000 and 2010, 83 studies were published, which generated an estimated 12,237 IPD and from 2011 to 2022, 55 studies generated 17,605 IPD ( Fig 3 ). Note, however, that a single study undertaken in India and published in 2021 [ 8 ] accounts for an estimated 8,803 IPD, i.e., 50% of all IPD generated between 2011 and 2022. We also estimated that, of the 138 eligible published studies, approximately 27% took place prior to 2000, with two studies having taken place as early as 1975. The months of longest follow-up ranged from less than one month to 26 years, with the most frequent longest follow-up time being approximately 12 months after intervention ( Fig 4 ). We identified 13 studies that followed participants for more than 5 years, 2 of those for more than 15 years [ 40 , 41 ].

Infection and morbidity indicators
Most studies eligible for inclusion (138) have generated IPD on treatments of infection rather than treatments of morbidity (MMDP interventions). Of the estimated 29,842 IPD generated, 14,800 (~50%) comprise pre- and post-intervention (on at least one occasion) microfilaraemia, CFA and/or ultrasound (to detect adult worms) infection indicators, while 6,102 (~20%) comprise pre- and post-intervention morbidity indicators only, i.e., without infection indicators. An additional 300 IPD comprise both infection and morbidity indicators pre- and post-intervention. The remaining 8,940 IPD comprise data where either: (1) the diagnostic measurement was not of microfilariae, CFA, ultrasound or a clinical morbidity indicator; (2) participants were enrolled after the start of the study and thereafter had more than one follow-up (for example, during an observational study / MDA rollout), and/or (3) participants were followed up for adverse events (AEs) but not infection or morbidity indicators.
Diagnostics
We estimate that of the 14,800 IPD on pre- and post-intervention infection indicators, 4,424 comprise only CFA, 1,515 comprise only ultrasound indicators and 2,772 IPD comprise only microfilaraemia. The most common diagnostic used is CFA, with 10,212 IPD generated in combination with microfilaraemia and/or ultrasound and/or morbidity indicators. Microfilaraemia was measured in 8,861 IPD in combination with CFA and/or ultrasound indicators and/or morbidity indicators. Note that many IPD contain information from multiple diagnostics, so these estimates do not sum to the 14,800 IPD on pre- and post-intervention indicators. We could not estimate IPD for all specific diagnostic combinations given the information reported in individual studies ( Table 1 ).
a circulating filarial antigen
b given the information reported in individual studies it was not possible to estimate IPD for all specific combinations (e.g., microfilaraemia with CFA vs. microfilaraemia with ultrasound).
Study design
We identified 116 drug treatment studies. Fifty-six of these are randomized controlled trials, generating 7,918 IPD; 60 are non-randomized, either multi-arm studies or single-arm studies, including observational studies of cohorts followed up after participating in MDA, generating 7,458 potential IPD ( Table 2 ).
a mass drug administration
Demographic characteristics of study participants
The number of studies and estimated IPD from treatment of infection studies disaggregated by sex and minimum age eligibility are shown in Tables Tables3 3 and and4, 4 , respectively. These estimates include only the 47 studies where the number of participants by sex can be determined for those who are treated and followed at least once. Of the 91 studies for which these data were not available, some studies reported the demography of the sample population but did not report the age- and sex-structure of the recruited participants. It is noteworthy that 66 of the 109 studies that reported participant age included children <18 years (as well as adults).
a includes 1 community trial of diethylcarbamazine-fortified salt and 1 trial on participants co-infected with lymphatic filariasis and HIV
Drug regimens
The most frequently administered drug regimens from 100 of the 116 studies generating pre- and post-intervention infection data and the associated estimate of IPD are shown in Table 5 . These regimens account for 11,543 (78%) of the estimated 14,800 IPD from the 116 studies. The most common monotherapy regimens (studies and estimated IPD) are: diethylcarbamazine (47 with IPD 3,206); albendazole (12 with IPD 830); ivermectin (6 with IPD 233); and the anti- Wolbachia therapy doxycycline (5 with IPD 99). Corresponding values and estimates for combination regimens are: diethylcarbamazine plus albendazole (DA, 37 with IPD 4,423); ivermectin plus albendazole (IA, 15 with IPD 774); ivermectin plus diethylcarbamazine (4 with IPD 131); and ivermectin plus diethylcarbamazine plus albendazole (IDA, 10 with IPD 1,847).
a age-based fixed dosing 100 mg for 2–5 years and 200 mg for 6–14 and 300 mg for >14 years
Morbidity interventions
We identified 33 studies that collected morbidity indicators pre- and post-intervention. Some of these included interventions aimed specifically at treating and managing morbidities, while others examined the effect on morbidity outcomes of treatments primarily for infection. Due to the low anticipated numbers of eligible studies and low numbers of participants, we included retrospective as well as prospective studies for MMDP interventions only, to broaden the exploration of potentially useful IPD. Three of the included studies are retrospective. Eight studies had multiple interventions whose allocation was randomised.
Twenty-eight studies analysed the effect of interventions on limb lymphedema and related acute manifestations. Interventions for limb lymphedema usually include care packages comprising elements aimed at preventing further damage, and elements promoting lymph flow; some care packages only include one of these categories. There were two studies on the outcomes of surgery for lower limb lymphedema. Eight studies included hydrocele and/or penoscrotal lymphedema (three of which also included limb lymphedema). Outcomes for chronic and acute manifestations were reported heterogeneously.
We have estimated that, from studies published since 2000, approximately 29,842 IPD have been generated from 23 countries on LF infection and morbidity indicators measured before and after intervention. Approximately half of these data are on pre- and post-intervention infection indicators (i.e., treatment of infection studies) following mono-, dual- and triple drug therapies at various doses, covering the full demographic spectrum, from young children to old adults. These studies most frequently use microfilaraemia and/or CFA as outcome measures, with the most common longest follow-up time being 12 months after intervention, but some studies conduct follow-ups after more than 10 years. Approximately 20% of IPD comprises pre- and post-intervention morbidity indicators, revealing a relative paucity of information on the efficacy of morbidity management approaches, despite MMDP being one of the pillars of the LF elimination programme. These data could form the basis of an IPD data repository on the treatment of LF infection and disease.
Since 2000, great progress has been made towards the elimination of LF, with 17 countries having met the criteria for EPHP [ 2 ]. These criteria include sustaining infection levels below transmission assessment survey thresholds for at least four years after stopping MDA and providing essential care (MMDP) for known patients. These successes have largely been driven by the WHO’s strategy of distributing combinations of antiparasitic medicines to at-risk populations through MDA programmes. Yet, the 2030 target of validating EPHP in 58 (81%) countries [ 1 ] has renewed emphasis on the need for optimising treatments for LF to accelerate progress towards this goal. The development and rollout (excluding in areas co-endemic with other filariases in Africa) of triple drug IDA therapy [ 5 , 6 ] has enhanced the feasibility of EPHP within this timeframe, but as with all treatments, responses are variable, and understanding this variability and exploring how to optimise current strategies is needed for sustaining financial and political support to eliminate the disease.
Variation in responses to interventions can relate to geographical factors associated with both host and parasite populations, individual intensities of infection and severity of disease, age and sex differences in bioavailability, method of dosing (e.g., based on weight versus age) and adherence to treatment. Understanding variation requires moving focus from the population to the individual and detailed analyses of IPD, combining data from multiple studies to maximise the power of statistical inference. Traditional meta-analyses of study- or cohort-level data are limited by their inability to incorporate individual-level variables and are also more prone to biases arising from heterogeneity in study designs [ 42 ]. Individual participant data meta-analyses provide a solution to this, being able to adjust (statistically) for different study protocols and, therefore, not restricted to completely standardized designs [ 43 ]. But such analyses require access to detailed IPD with engagement and collaboration with study investigators to ensure a complete understanding of the original data so that variables and outcomes from multiple studies can be combined and standardized in a reliable and accurate manner. Hence, while the increasingly common sharing of IPD on journal and institutional servers is to be celebrated, it is not sufficient for the most effective data reuse [ 25 , 26 ].
Controlled data repositories provide a more effective means of data sharing, permitting IPD to be stored in one place using consistent standardization with dedicated resources for curation and collation. Some repositories, such as IDDO, also serve as collaborative hubs for reuse and reanalysis of data and promote fair and equitable data sharing practices that encourage the involvement of data contributors in further analyses [ 44 ]. This approach ensures the most robust and reliable scientific results and fosters trust across the scientific community that data will be shared and reused in an equitable and transparent manner. Meta-analyses of IPD hosted by IDDO have been used to inform treatment policies and clinical decision making for malaria [ 45 – 47 ], evaluate the safety of ivermectin in young children [ 48 ] and evaluate case definitions for SARS-CoV-2 [ 49 ].
The first step in evaluating the feasibility and potential utility of a data platform is performing a systematic review to estimate the abundance of IPD and characterise the landscape of studies that have generated them. Compared to similar exercises conducted for other NTDs—that identified studies conducted over similar timescales, although using somewhat different eligibility criteria—the estimated 29,842 IPD for LF is intermediate to the 20,517 IPD on schistosomiasis [ 32 ] and the 35,000 IPD for soil-transmitted helminthiases [ 33 ]. Unsurprisingly, most LF IPD have been generated from India, where approximately 40% of worldwide cases occur [ 2 ]. More notable is the relative scarcity of IPD from other countries where LF is endemic. For example, fewer IPD have been generated in Indonesia, with a population of approximately 275 million, compared to Papua New Guinea, with a population of around 10 million. There are also numerous countries in sub-Saharan Africa where no IPD have been identified from published studies over the past two decades. Although progress towards elimination in Africa is strong [ 50 ], this patchy geographic coverage of IPD could yet prove to be an impediment to further progress.
Geographic heterogeneity explains some of the variability in IPD from different drug regimens. For example, more than 4,000 IPD have been generated on various dose combinations of DA, and nearly 2,000 IPD on IDA, both used for MDA outside of Africa (and particularly in India). Yet for IA (150–200 μg/kg ivermectin + 400 mg albendazole), which remains the only regimen used for MDA within Africa, we could identify only 640 IPD from 14 studies conducted over the past two decades. This is a conspicuously scarce abundance of data for a regimen that is distributed annually to more than 100 million people [ 2 ]. This raises concerns about the depth of understanding on the efficacy of this regimen among the individuals comprising the vast and diverse African population. The fact that the 640 IPD come from 14 studies underscores that any future analysis including IA should aim to incorporate the IPD from all of these (small) studies to maximise inferential power.
A notable and distinguishing feature of the studies generating LF IPD is the frequent use of long follow-up times, typically at least one year after intervention. This largely reflects the more complex and protracted effects of current treatment options on filarial parasites, which tend to be more refractory than other nematodes (e.g., soil-transmitted helminths) or trematodes (e.g., schistosomes). For example, in onchocerciasis, while microfilariae are relatively susceptible to treatment, adult filariae macrofilariae often survive, albeit sterilized either temporarily or permanently or with a reduced lifespan [ 51 , 52 ]. The anti-macrofilarial activity of LF treatment options is less well studied, but the protracted decline in CFA (indicative of active macrofilarial infection) compared to the rapid and sustained clearance of microfilariae suggests similar sustained sterilization effects may operate [ 53 ]. In principle, the IPD identified here, where both CFA and microfilariae were measured could be combined and, where individuals have also been followed up for multiple years, help disentangle microfilaricidal and anti-macrofilarial activity.
For any data platform, the potential accessibility of IPD is strongly linked with the age of the data, falling by approximately 17% per year [ 31 ]. This serves as a reminder that without active engagement and participation with data repositories, valuable data can quickly become lost to reuse. This is both detrimental to scientific progress and may also be considered as not fulfilling ethical responsibilities to maximise the use of participants’ data [ 54 ]. Of the most recent (and therefore accessible) data generated since 2016, many are focused on IDA. An IPD meta-analysis of these data could identify individual factors associated with treatment while also accounting for geographical and other study-level heterogeneities (see for example [ 19 ] and [ 20 ] for similar analyses of responses to treatment of soil-transmitted helminthiasis and schistosomiasis respectively). This could help to explain some of the variation observed in the 12-month efficacies of IDA reported from Phase IV trials which have ranged from 63% in Fiji [ 16 ], 71% in Côte d’Ivoire [ 7 ] and 84% in India [ 8 ], to 94% in Haiti [ 55 ], 96% in Papua New Guinea [ 56 ] and 96.3% in Indonesia [ 57 ]. Indeed, in Fiji, unlike elsewhere, the efficacy of IDA was not superior to DA [ 16 ]. Moreover, integration of IPD from ongoing Phase III clinical trials of moxidectin [ 58 ] given with combinations of albendazole and diethylcarbamazine ( https://clinicaltrials.gov/study/NCT04410406 ) within such meta-analyses will also be important in generating comprehensive assessments of the relative efficacies of the different options available for treating LF infection.
While our quantification of IPD here has focused on pre- and post-intervention infection and morbidity indicators (i.e., longitudinal data measured before and at least once after intervention), these data will also frequently include information on safety and tolerability (although we did not quantify this explicitly because safety data typically do not require the longer follow-up of individuals that is required to assess infection or morbidity responses to intervention). Adverse events associated with the treatment of LF are common, albeit usually transient and seldom severe, and often relate to the killing of microfilariae [ 59 – 61 ]. This is common among filarial nematodes and indeed the severity of AEs induced by the rapid killing of Onchocerca volvulus and Loa loa microfilariae following treatment with diethylcarbamazine led to its withdrawal from use in Africa [ 62 ], although its re-introduction as part of IDA has been successfully trialled in areas non-endemic for onchocerciasis and loiasis [ 7 ], and strategies for its potential wider use in Africa were discussed [ 63 ]. It is routine for studies to report AEs at a cohort level, sometimes with more detailed individual analyses or clinical investigation of more serious events. But with abundant IPD, one could determine comprehensively whether the probability of AEs is associated with individual-level factors [ 59 ]. For example, a threshold level of L . loa microfilariae above which treatment with ivermectin (which also kills microfilariae) is contraindicated has been used during Test-and-(not) treat pilot field trials [ 64 ] because of an unacceptably high probability of severe AEs. Such a quantification could be extremely useful if DEC is to be reintroduced into Africa as part of IDA.
The efficacy of MMDP interventions has only been sparsely examined in published literature (e.g., [ 65 ]). The studies identified in this review where interventions for morbidity management were carried out were diverse with highly heterogeneous reporting of study-level meta-data and outcomes. Additionally, studies testing interventions for management of lymphedema or hydrocele may mix participants with both filarial and non-filarial causes (these studies were not included in this review). This explains why, hitherto, meta-analyses of morbidity management interventions for LF [ 12 ] have been restricted to only a small number of studies and on a subset of interventions and outcomes for particular complications. Further and more standardised studies are urgently needed in this domain and should include post-surgery follow-up of participants to quantify relapse rates and the occurrence of secondary infections. Additionally, as with data on infection, analysis of individual-level data would be especially valuable for understanding the drivers of variation in responses to treatment, and pooling (harmonised) data from individual studies would increase statistical power where subgroups of interest within individual studies are small.
The principal goal of this work was to identify studies and estimate the abundance of IPD on LF infection and morbidity indicators measured before and after intervention, which could potentially be integrated into a global data repository. Although we took a conservative approach to the estimation of IPD (e.g., counting only participants who were successfully followed at the last follow-up time), our estimates are based on information reported in publications and will be inexact. For example, it was often difficult to determine from the reported information whether the same cohort of individuals had participated before and after intervention, and in several studies additional participants were recruited after the initial intervention. It was also a challenge to avoid ‘double counting’ of IPD generated from single studies but reported in multiple publications and the often-limited reporting of study design made classification difficult. These challenges will naturally introduce uncertainty in our estimates of IPD. The difficulty in estimation using reported information is particularly apparent when trying to quantify IPD associated with individual-level variables. For example, most studies provided only age eligibility criteria of participants, so we were not able to estimate a detailed demographic breakdown of IPD. Similarly, for sex, only 47 of the 138 studies reported sufficient information to estimate IPD for males and females to reach conclusions on gender representativeness, although notably, we identified 19 studies that included male only participants and no female-only studies.
This review has highlighted a substantial number of recent studies on LF infection and morbidity responses to interventions, which if IPD were available, could be highly valuable in improving understanding of the factors that shape variability in responses to treatments. This is the first step in building a case for the utility and feasibility of an IPD data sharing and reuse platform that could maximise the power of these data. The next stage of this process will be to enhance engagement with the LF research community, seeking their commitment to effective data sharing and their expertise in defining and prioritising research questions that could be usefully answered using an operational platform.
- Supporting information
- Funding Statement
We acknowledge funding from the Bill & Melinda Gates Foundation (Grant number: INV-004713). LTF, JBH and MGB also acknowledge funding from the MRC Centre for Global Infectious Disease Analysis (MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
- Data Availability
- PLoS Negl Trop Dis. 2024 Jan; 18(1): e0011882.
Decision Letter 0
24 Oct 2023
Dear Dr Walker,
Thank you very much for submitting your manuscript "The lymphatic filariasis treatment study landscape: a systematic review of study characteristics and the case for an individual participant data platform" for consideration at PLOS Neglected Tropical Diseases. As with all papers reviewed by the journal, your manuscript was reviewed by members of the editorial board and by several independent reviewers. The reviewers appreciated the attention to an important topic. Based on the reviews, we are likely to accept this manuscript for publication, providing that you modify the manuscript according to the review recommendations.
Please prepare and submit your revised manuscript within 30 days. If you anticipate any delay, please let us know the expected resubmission date by replying to this email.
When you are ready to resubmit, please upload the following:
[1] A letter containing a detailed list of your responses to all review comments, and a description of the changes you have made in the manuscript.
Please note while forming your response, if your article is accepted, you may have the opportunity to make the peer review history publicly available. The record will include editor decision letters (with reviews) and your responses to reviewer comments. If eligible, we will contact you to opt in or out
[2] Two versions of the revised manuscript: one with either highlights or tracked changes denoting where the text has been changed; the other a clean version (uploaded as the manuscript file).
Important additional instructions are given below your reviewer comments.
Thank you again for your submission to our journal. We hope that our editorial process has been constructive so far, and we welcome your feedback at any time. Please don't hesitate to contact us if you have any questions or comments.
Sabine Specht
Academic Editor
PLOS Neglected Tropical Diseases
Francesca Tamarozzi
Section Editor
***********************
Reviewer's Responses to Questions
Key Review Criteria Required for Acceptance?
As you describe the new analyses required for acceptance, please consider the following:
-Are the objectives of the study clearly articulated with a clear testable hypothesis stated?
-Is the study design appropriate to address the stated objectives?
-Is the population clearly described and appropriate for the hypothesis being tested?
-Is the sample size sufficient to ensure adequate power to address the hypothesis being tested?
-Were correct statistical analysis used to support conclusions?
-Are there concerns about ethical or regulatory requirements being met?
Reviewer #1: The methods were clearly stated from the outset and were appropriate to achieve the stated aim of the review. The initial collection was clearly very extensive as shown by the very large attrition rate when removing duplicates from the large number of publication depositories. Overall no concerns
Reviewer #2: The authors have done a systematic review of literature to estimate the abundance of Individual participant data(IPD)on pre- and post-intervention indicators of LF infection and/or LF morbidity and assess the feasibility of building a global data repository. This is also aimed at developing a better strategy for elimination of LF. The study design is appropriate but the real data the authors could make available is small compared to the abundance of literature.Due to problems with the research studies itself the authors could select only 147 full text articles for analysis. I would like to congratulate the authors for the commendable and systematic efforts to identify he eligible articles.There are no ethical or regulatory concerns . The statistical methods used are appropriate
--------------------
-Does the analysis presented match the analysis plan?
-Are the results clearly and completely presented?
-Are the figures (Tables, Images) of sufficient quality for clarity?
Reviewer #1: The results are clearly and appropriately presented. The figures and tables are clear and provide additional information that assists in the understanding of the results.
Reviewer #2: The analysis is presented as per the plan itselfThe authors were looking for the IPD regarding management of infection (preventive chemotherapy) and that of management of disease, the morbidity management and disability prevention. The tables and figures explains well the results of analysis. The results are well presented
-Are the conclusions supported by the data presented?
-Are the limitations of analysis clearly described?
-Do the authors discuss how these data can be helpful to advance our understanding of the topic under study?
-Is public health relevance addressed?
Reviewer #1: The conclusions are clear and the limitations are adequately discussed. Since the main aim of this work was to identify material currently available to advance public health interventions in LF, these are covered in detail. Overall, this paper provides the benchmark for future work collection and curating individual patient data in LF and to plan prospective data collection. Overall, the aims of the study and publication of the material are achieved.
Reviewer #2: The authors had limitations to obtain the IPD on morbidity management and disability prevention. The Global program to eliminate LF and its preventive chemotherapy is a well designed public health program and lot of data have been already generated on that. But the other strategy of MMDP has not been taken up by all the countries and the data that could be made available in this study is minimal only. This is the limitation of tis study also
Editorial and Data Presentation Modifications?
Use this section for editorial suggestions as well as relatively minor modifications of existing data that would enhance clarity. If the only modifications needed are minor and/or editorial, you may wish to recommend “Minor Revision” or “Accept”.
Reviewer #1: The paper is well written and easily understandable. it should be accepted without the need for additional modification.
Reviewer #2: Here the goal of the authors is to develop a global data repository for LF infection and LF morbidity. For this the authors have resorted to doing the systematic eview of the available studies. The massive data available on LF elimination data- the preventive chemotherapy is the Global health observatory for lymphatic filariasis. Here the data of the number of ppeople to whom preventive chemotherapy was given, percentage consumption in the country and lal details are available. But authors have not mentioned about this at all. As a reviewer I would like to have an opinion from the authors who have done lot of work, on how this repository will help them or not help them to achieve their goals of this study. Data on MMDP also will be available but may not be full proof.
The authors may be encouraged to get details of global health observatory LF and may be added here in this manuscript how that would help to develop a repository important for LF elimination
I am not in a position to give recommendation but once this is done this can be considered for acceptance
Summary and General Comments
Use this section to provide overall comments, discuss strengths/weaknesses of the study, novelty, significance, general execution and scholarship. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. If requesting major revision, please articulate the new experiments that are needed.
Reviewer #1: The output of this work has considerable importance and has the potential to answer many of the questions that have dogged the LF community. The identification of areas where data is lacking, for example in MMDP studies and in treatment data from Africa, is an important step, although those in the field would be able to identify these from their own experience. The key step is to identify what data is often missing from data sets and ensure that these are collected in the future. The need to develop guidelines for collection and presentation is essential and probably reaches across all studies and diseases. It came as a surprise that even simple things like age and sex are an issue in these data sets.
It is impressive that the data cut off for analysis was as late as May this year, meaning that an enormous amount of work in writing has been achieved in a very short space of time.
Reviewer #2: All the authors have done lot of committed work from the designing, collection of data, analysis and writing the manuscript. Similarly lot of efforts have gone into writing this manuscript also. So this is to be considered as a good write up in this context which could become an inspiration to other researchersSo with revisions this may be accepted. The authors have also explained the limitations of the study
PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #1: Yes: John Horton
Reviewer #2: No
Figure Files:
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email us at gro.solp@serugif .
Data Requirements:
Please note that, as a condition of publication, PLOS' data policy requires that you make available all data used to draw the conclusions outlined in your manuscript. Data must be deposited in an appropriate repository, included within the body of the manuscript, or uploaded as supporting information. This includes all numerical values that were used to generate graphs, histograms etc.. For an example see here: http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.1001908#s5 .
Reproducibility:
To enhance the reproducibility of your results, we recommend that you deposit your laboratory protocols in protocols.io, where a protocol can be assigned its own identifier (DOI) such that it can be cited independently in the future. Additionally, PLOS ONE offers an option to publish peer-reviewed clinical study protocols. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article's retracted status in the References list and also include a citation and full reference for the retraction notice.
Author response to Decision Letter 0
19 Dec 2023
Submitted filename: Responses to Reviewers.docx
Decision Letter 1
22 Dec 2023
We are pleased to inform you that your manuscript 'The lymphatic filariasis treatment study landscape: a systematic review of study characteristics and the case for an individual participant data platform' has been provisionally accepted for publication in PLOS Neglected Tropical Diseases.
Before your manuscript can be formally accepted you will need to complete some formatting changes, which you will receive in a follow up email. A member of our team will be in touch with a set of requests.
Please note that your manuscript will not be scheduled for publication until you have made the required changes, so a swift response is appreciated.
IMPORTANT: The editorial review process is now complete. PLOS will only permit corrections to spelling, formatting or significant scientific errors from this point onwards. Requests for major changes, or any which affect the scientific understanding of your work, will cause delays to the publication date of your manuscript.
Should you, your institution's press office or the journal office choose to press release your paper, you will automatically be opted out of early publication. We ask that you notify us now if you or your institution is planning to press release the article. All press must be co-ordinated with PLOS.
Thank you again for supporting Open Access publishing; we are looking forward to publishing your work in PLOS Neglected Tropical Diseases.
Best regards,
***********************************************************
Acceptance letter
10 Jan 2024
We are delighted to inform you that your manuscript, "The lymphatic filariasis treatment study landscape: a systematic review of study characteristics and the case for an individual participant data platform," has been formally accepted for publication in PLOS Neglected Tropical Diseases.
We have now passed your article onto the PLOS Production Department who will complete the rest of the publication process. All authors will receive a confirmation email upon publication.
The corresponding author will soon be receiving a typeset proof for review, to ensure errors have not been introduced during production. Please review the PDF proof of your manuscript carefully, as this is the last chance to correct any scientific or type-setting errors. Please note that major changes, or those which affect the scientific understanding of the work, will likely cause delays to the publication date of your manuscript. Note: Proofs for Front Matter articles (Editorial, Viewpoint, Symposium, Review, etc...) are generated on a different schedule and may not be made available as quickly.
Soon after your final files are uploaded, the early version of your manuscript will be published online unless you opted out of this process. The date of the early version will be your article's publication date. The final article will be published to the same URL, and all versions of the paper will be accessible to readers.
Thank you again for supporting open-access publishing; we are looking forward to publishing your work in PLOS Neglected Tropical Diseases.
Shaden Kamhawi
co-Editor-in-Chief
Paul Brindley
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pntd.0011882
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics.

Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04410406
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis.
Macfarlane CL , Budhathoki SS , Johnson S , Richardson M , Garner P
Cochrane Database Syst Rev , 1:CD003753, 08 Jan 2019
Cited by: 13 articles | PMID: 30620051 | PMCID: PMC6354574
Albendazole for lymphatic filariasis.
Addiss D , Critchley J , Ejere H , Garner P , Gelband H , Gamble C , International Filariasis Review Group
Cochrane Database Syst Rev , (1):CD003753, 01 Jan 2004
Cited by: 6 articles | PMID: 14974034
An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis.
Horton J , Witt C , Ottesen EA , Lazdins JK , Addiss DG , Awadzi K , Beach MJ , Belizario VY , Dunyo SK , Espinel M , Gyapong JO , Hossain M , Ismail MM , Jayakody RL , Lammie PJ , Makunde W , Richard-Lenoble D , Selve B , Shenoy RK , [...] Weerasooriya MV
Parasitology , 121 Suppl:S147-60, 01 Jan 2000
Cited by: 58 articles | PMID: 11386686
How Thailand eliminated lymphatic filariasis as a public health problem.
Rojanapanus S , Toothong T , Boondej P , Thammapalo S , Khuanyoung N , Santabutr W , Prempree P , Gopinath D , Ramaiah KD
Infect Dis Poverty , 8(1):38, 27 May 2019
Cited by: 14 articles | PMID: 31130143 | PMCID: PMC6535972
Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: seeking synergies and preventing severe adverse events during mass drug administration campaigns.
Cano J , Basáñez MG , O'Hanlon SJ , Tekle AH , Wanji S , Zouré HG , Rebollo MP , Pullan RL
Parasit Vectors , 11(1):70, 31 Jan 2018
Cited by: 15 articles | PMID: 29382363 | PMCID: PMC5791223
Funding
Funders who supported this work.
Bill & Melinda Gates Foundation (1)
Grant ID: INV-004713
7 publication s
Bill and Melinda Gates Foundation (1)
5 publication s
Medical Research Council (1)
Mrc centre for global infectious disease analysis.
Professor Neil Ferguson, Imperial College London
Grant ID: MR/R015600/1
1467 publication s
Europe PMC is part of the ELIXIR infrastructure
Advertisement
Lymphatic filariasis in Asia: a systematic review and meta-analysis
- Helminthology - Review
- Published: 08 January 2021
- Volume 120 , pages 411–422, ( 2021 )
Cite this article

- Negar Bizhani ORCID: orcid.org/0000-0002-3510-8770 1 ,
- Saeideh Hashemi Hafshejani ORCID: orcid.org/0000-0002-7705-6729 1 ,
- Neda Mohammadi ORCID: orcid.org/0000-0002-2542-4518 2 ,
- Mehdi Rezaei ORCID: orcid.org/0000-0002-0056-1073 3 &
- Mohammad Bagher Rokni ORCID: orcid.org/0000-0002-1048-2512 1
7478 Accesses
15 Citations
Explore all metrics
Lymphatic filariasis (LF) is an important neglected parasitic disease according to the World Health Organization. In this study, we aimed to determine the prevalence of human LF in Asia using a systematic review and meta-analysis approach. Records from 1990 to 2018 in reputable databases including PubMed, Science Direct, Embase, and Cochrane Library were searched using a panel of related keywords. All 48 countries of Asia were searched one by one in combination with the keywords. In all, 41,742 cases identified in this study were included in the analysis. According to our findings, the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). There was no major trend in the cumulative prevalence of LF over time. Some countries in Asia including China, Japan, Vietnam, and South Korea succeeded in eliminating LF as a public health problem, but others still need to monitor the disease. Based on the initiative of the WHO starting in 2000, some countries in Asia succeeded in eliminating LF as a public health problem. Other countries have taken steps to eliminate the disease with variable degrees of success. These efforts might be affected by issues such as climate change.
Similar content being viewed by others

Re-drawing the Maps for Endemic Mycoses

A Review of Leishmaniasis: Current Knowledge and Future Directions

Severe malaria
Avoid common mistakes on your manuscript.
Introduction
The term neglected tropical diseases (NTDs) arose from the recommendation by the Working Group on Monitoring and Evaluation of the Strategic and Technical Advisory Group for NTDs (WHO 2020 ). Among NTDs, parasitic diseases are prominent, and many studies have been conducted on different aspects of their risks and complications (Torgerson et al. 2014 ). According to the WHO (WHO 2020 ), the following diseases are considered NTDs: Chagas disease, dracunculiasis (guinea-worm disease), echinococcosis, foodborne trematodiases, human African trypanosomiasis (sleeping sickness), leishmaniasis, lymphatic filariasis, onchocerciasis (river blindness), schistosomiasis, soil-transmitted helminthiasis, and taeniasis/cysticercosis.
Filariasis is an important parasitic disease caused by roundworms of the Filarioidea superfamily, which are parasites residing in the blood and tissues of humans. In humans, filariasis is caused by Wuchereria bancrofti , Brugia malayi , Loa loa , Onchocerca volvulus , and Dirofilaria spp. Lymphatic filariasis (LF), in which the adult worms are found in the lymphatic system, is considered the most important form of filariasis, and is also known as elephantiasis. It is transmitted by mosquitoes of the genera Culex , Mansonia , and Anopheles (Solgi et al. 2017 ; WHO 2013 ).
Nearly 63% of 1.34 billion people worldwide are at risk of LF, and about 50% of the 120 million infected people live in the South-East Asia Region. This region bears approximately 57% of the total global burden of an estimated 5.1 million disability-adjusted life years (DALYs) lost due to LF. Nine countries in this region are endemic for LF. India, Nigeria (in Africa), Bangladesh and Indonesia together account for 70% of LF in the world, although 80 countries are considered endemic for the disease (WHO 2013 ). In the year 2016, a total of 1189 (587.7 to 2114.9) DALYs (thousands) were reported for all ages (Collaborators 2017 ).
Countries and areas considered at high risk include central Africa and the Nile delta, Madagascar, Turkey, the Middle East, India, Pakistan, Sri Lanka, Myanmar, Thailand, Malaysia, Vietnam, South Korea, Indonesia, the Philippines, Timor, southern China, Haiti, Dominican Republic, Guyana, French Guinea, and costal Brazil (Hotez and Ehrenberg 2010 ; Utzinger et al. 2010 ).
The disease has variable symptoms caused mostly because adult worms occupy and block the lymphatic vessels. Depending on the kind of filariasis, the patient shows a spectrum of signs and symptoms including elephantiasis, lymphedema, hydrocele, chyluria, chylous diarrhea, and chylorrhagia (Addiss 2010 ; Kabatereine et al. 2010 ). Some asymptomatic cases, which may become chronic, have also been reported.
Clinical manifestations including episodic attacks of lymphadenitis and lymphangitis (fever, pain in the affected area, tender red streaks) along with fever and malaise are attributed to acute LF, while some cases of acute attacks have been reported for chronic manifestations. The patients show lumps in the subcutaneous tissue, breasts or testicles due to reactions to adult worms or related granulomas (Al-Shaham and Sood 2010 ).
After the World Health Assembly (WHA) on 1997 and based on Resolution 50.29 to eliminate LF, a Global Programme to Eliminate LF (GPELF) was initiated in 2000 to eliminate LF in 2020. The main strategies were mass drug administration (MDA) using a two-drug combination of diethylcarbamazine (DEC) and albendazole, and a transmission assessment survey (TAS) (WHO 2013 ).
In this review, we aimed to collect and analyze data concerning the situation of human filariasis in Asian countries by searching publications between 1990 and 2018 recorded in reliable databases.
Search strategy
The PubMed, Science Direct, Embase, and Cochrane Library databases were searched using a panel of keywords that included, but was not limited to, human filariasis, lymphatic filariasis, Wuchereria bancrofti , Brugia malayi , Brugia timori , prevalence, epidemiology, and all Asia countries in turn ( https://www.worldatlas.com/articles/how-many-countries-are-in-asia.html ). The countries included in the searches were Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, East Timor, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar, Nepal, North Korea, Oman, Pakistan, Philippines, Qatar, Saudi Arabia, Singapore, South Korea, Sri Lanka, State of Palestine, Syria, Tajikistan, Thailand, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, Vietnam and Yemen.
We included relevant studies conducted from 1990 to 2018 and published in English. We initially screened studies based on their title and abstract, followed by availability of the full text. If only the abstract was available, information from the study was considered only in the Discussion section. Items such as books and Letters to the Editors were excluded from the study.
The inclusion criteria were (1) conducted between 1990 and 2018, (2) English language, (3) availability of the full text, (4) original or review article as publication type, and (5) involving human patients.
The exclusion criteria were (1) studies in animals only, and (2) case report or letter to the editor as publication type.
As shown in Fig. 1 , a total of 3305 records were initially found in the database searches. After initial screening, 2440 duplicate articles were deleted. The remaining 790 items were then screened to locate relevant content. The remaining 75 articles were then checked to verify that they met the inclusion criteria, and as a result, a total of 19 studies were included in the analysis.
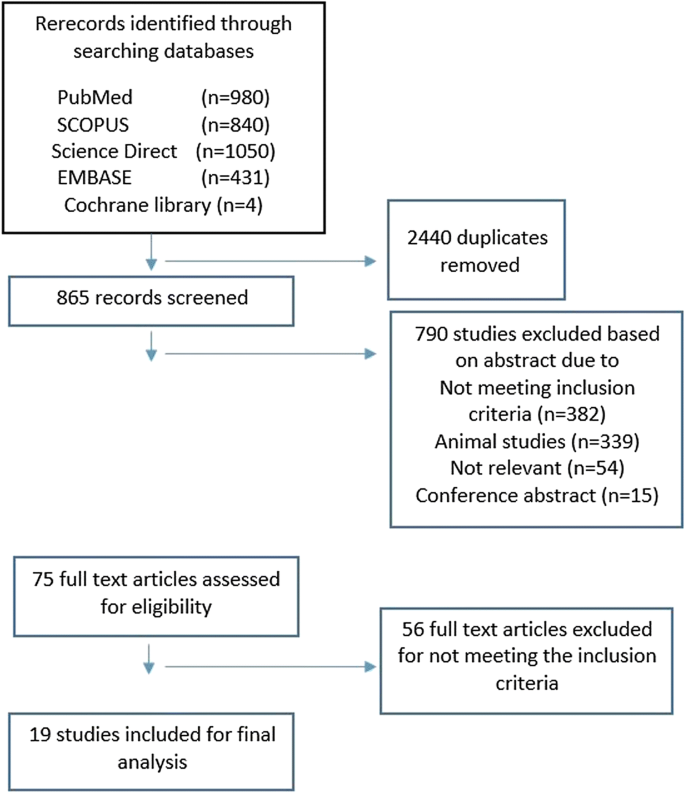
Flow diagram of search and selection of relevant articles

Statistical analysis
The data were analyzed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria.). The pooled prevalence was estimated with its 95% confidence interval (CI). Chi-squared, tau-squared, and I-squared statistics were calculated to assess heterogeneity of the studies. Due to significant heterogeneity, a random effects model was used to estimate the pooled prevalence of filariasis. Publication bias was evaluated using a funnel plot. Results with p values less than 0.05 were considered statistically significant.
Results and discussion
Figure 2 shows the distribution of LF in Asia. The differences in prevalence among countries are discussed in detail below.
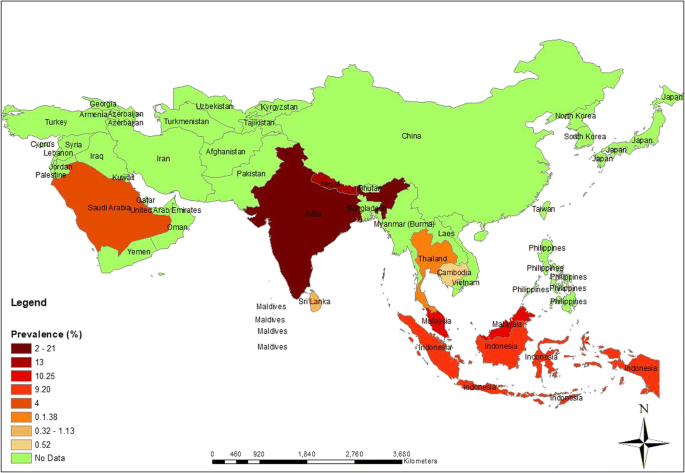
Distribution of lymphatic filariasis in Asia (Original figure)
A total of 41,742 cases synthesized in the present study were included in the analysis. According to the results of the present meta-analysis, reported in Fig. 3 , the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). The results of chi-squared, tau-squared and I -squared tests revealed significant heterogeneity among the studies, so a random effects model was used to pool the prevalences from all included studies (tau 2 = .53, chi 2 = 1618.02, p value < 0.001, I 2 = 99%).
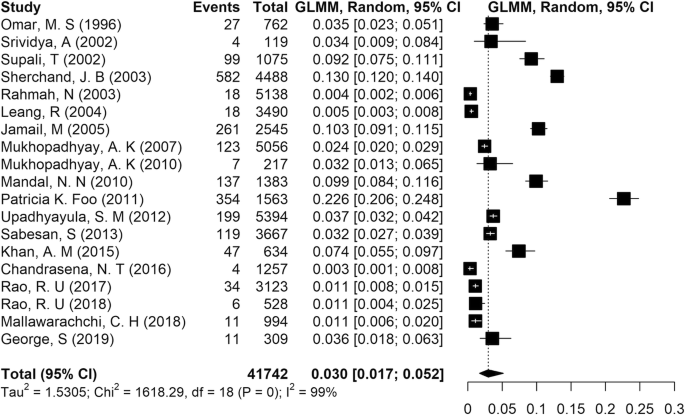
Forest plot showing the pooled prevalence of lymphatic filariasis in Asia
Cumulative meta-analysis was performed to estimate the trend in LF prevalence with time in Asia. The results showed that there was no major trend in cumulative prevalence (Fig. 4 ). A funnel plot drawn to check for the existence of publication bias in the studies (Fig. 5 ) showed no bias.
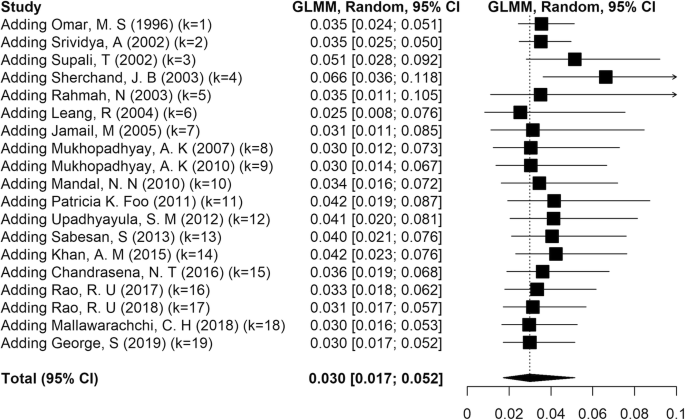
Cumulative forest plot showing the trends in lymphatic filariasis prevalence in Asia
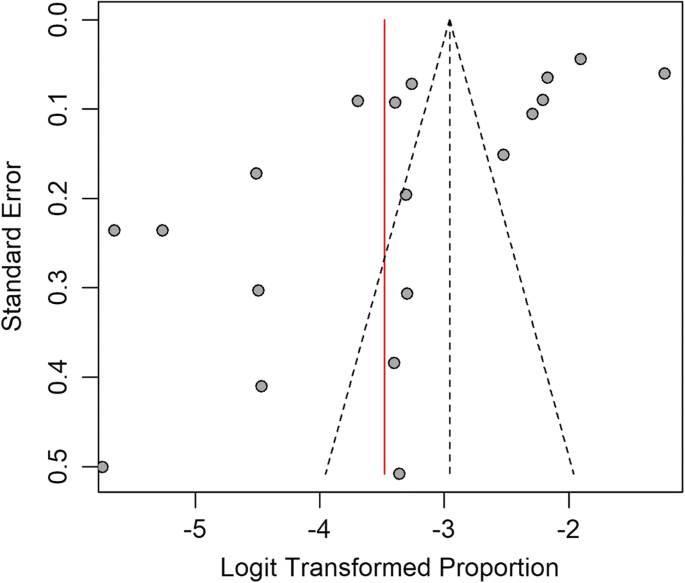
Funnel plot to assess the presence of publication bias
The highest prevalence of infection was reported in India as 21% (Foo et al. 2011 ), and the lowest rate was from Sri Lanka as 0.32% (Chandrasena et al. 2016 ). We noted that most studies done in India reported a prevalence of LF between 2.4 and 21% (Foo et al. 2011 ; George et al. 2019 ; Khan et al. 2015 ; Mandal et al. 2010 ; Mukhopadhyay 2010 ; Mukhopadhyay et al. 2007 ; Sabesan et al. 2013 ; Srividya et al. 2002 ; Upadhyayula et al. 2012 ).
Lymphatic filariasis due to Brugia spp. was reported from Indonesia (Supali et al. 2002 ), Malaysia (Jamail et al. 2005 ), Sri Lanka (Iddawela et al. 2015 ; Mallawarachchi et al. 2018 ), and Thailand (Rahmah et al. 2003 ). Dirofilaria spp. was reported in humans in Iran (Ashrafi et al. 2010 ; Ghasemi et al. 2020 ; Jamshidi et al. 2008 ; Tavakolizadeh and Mobedi 2009 ), Israeli (Gutierrez et al. 1995 ), and Taiwan (Li et al. 2013 ), although these cases are not considered NTDs. Because the aim of the study was to document the situation of LF only in humans, the many studies that reported animal filariasis in Asia were excluded from the present analysis.
The situation of LF in individual Asian countries is reported below.
Lymphatic filariasis has a long history in India dating back to the 6th century B.C. In 1995, the National Filaria Control Programme (NFCP) was started (Agrawal and Sashindran 2006 ). The disease has been reported in India since 1945 (Ahmad 1945 ). Unfortunately, there are no detailed data in the literature from that time. A survey conducted in 1981 showed that of 24,946 persons examined for LF, 8–41% had microfilaremia (Rajagopalan et al. 1989 ).
Many more recent studies from India have been published regarding the prevalence of LF (Foo et al. 2011 ; George et al. 2019 ; Khan et al. 2015 ; Mandal et al. 2010 ; Mukhopadhyay 2010 ; Mukhopadhyay et al. 2007 ; Sabesan et al. 2013 ; Srividya et al. 2002 ; Upadhyayula et al. 2012 ). Bancroftian filariasis was reported in all of these studies. India harbors nearly 40% of 120 million cases of infection with LF in the world (George et al. 2019 ). The DALYs lost in India due to FL has been reported as 2.06 million, resulting in an annual wage loss of US $811 million (Ottesen 2000 ). The six states regarded as having high endemicity in India are Uttar Pradesh, Bihar, Jharkhand, Orissa, Kerala, and Gujarat (Raju et al. 2010 ). The global LF program is currently being conducted with the main targets of eliminating LF and interrupting transmission using MDA (Ottesen 2000 ). The drugs DEC and albendazole are used as part of the program to support its aims. Accordingly, they may reduce the microfilaremia more than 95% after 2 annul rounds (Ottesen 2000 ).
In Malaysia, LF has been reported with two agents: W. bancrofti and B. malayi (Al-Abd et al. 2014 ). The first report of LF we noted in the literature dates to 1968, but no details were available (Yap et al. 1968 ). The earliest report we could find described microfilaria as sub-periodic B. malayi in 82 persons among 1613 people examined in seven villages in Serian District (Rubis et al. 1981 ). The disease is not widespread in the country, and occurs only in some states of Peninsular Malaysia including Terengganu, Kelantan, Pahang, Selangor, and Johor as well as the very small Sabah and Sarawak regions (Al-Abd et al. 2014 ). In Malaysia, an MDA program is being conducted with the two drugs noted earlier for India. The program covered all endemic areas from 2004 to 2008. It appears that the program has not been completely successful and requires additional efforts to reach the WHO goals (Al-Abd et al. 2014 ; Noordin et al. 2017 ). In a study by Rahmah et al. (Rahmah et al. 2003 ) with B. malayi recombinant antigen for ELISA testing, among 5138 children in Malaysia, 0.35% showed seropositivity. Positive cases included 13 boys and 5 girls between 7 and 12 years old.
Lymphatic filariasis has been reported from Sri Lanka for hundreds of years, with high endemicity (Rao et al. 2018 ). The earliest documented report found microfilaremia in 19.1% of houses examined for LF in Ceylon (Abdulcader et al. 1966 ). The country implemented an MDA program with DEC and albendazole from 2002 to 2006 in all endemic parts of the country. A TAS was conducted in endemic areas in 2013 (WHO 2014 ) to determine the prevalence of filarial antigenemia in young schoolchildren, and found a prevalence of less than 2% with 95% certainty (Chu et al. 2013 ). Rao et al. believed this method was not sensitive to detect ongoing transmission of W. bancrofti in many areas in Sri Lanka (Rao et al. 2018 ). They reported high rates of circulating filarial antigenemia (3%, confidence interval [CI]: 1.8–4.9) and microfilaremia (1%, CI: 0.5–2.5%), and noted that “circulating filarial antigenemia rates were 2.8-fold higher in males than females”. Anti-filarial antibodies were detected in young children at a prevalence of 5.7% (Rao et al. 2018 ). According to the WHO, although Sri Lanka could eliminate LF in 2016, surveillance efforts and interventions should be continued to monitor the problem in endemic areas (WHO 2016 ). It is believed that according to antigen prevalence data, adult males account for most persistent filarial infections in Sri Lanka (Irvine et al. 2018 ; Rao et al. 2017 , 2018 ).
The reported cases of LF in this country were 9.2% and 1.98% (Ginandjar et al. 2018 ; Supali et al. 2002 ). In 1980, the prevalence of microfilaremia was 19.5%, but it had been reduced to 4.7% by 2014 (Lee and Ryu 2019 ). The Indonesian Ministry of Health planned an MDA program in 2015 to monitor LF in 106 endemic districts. Based on further verifications, there was little success in monitoring the disease, such that 29 provinces continued to have problem in Eastern Indonesia in 2016 (Lee and Ryu 2019 ; Wibawa and Satoto 2016 ). A known endemic district is Pekalongan, where 62 cases with chronic LF were reported. There is a risk that the disease will spread if it is not controlled (Ginandjar et al. 2018 ). In a study of elementary schoolchildren in Indonesia in 2015, the prevalence was 1.98% and the agent was W. bancrofti . Although most infected students were older ones and males, no significant differences were reported (Ginandjar et al. 2018 ). In another study (Supali et al. 2002 ), both bancroftian filariasis and B. timori were reported in separate districts but no mixed infections were detected. Among 586 cases studied for B. timori , 27% showed microfilaremia. Males showed more infection than females. Regarding clinical manifestations, 13% of cases showed lymphedema in the legs, but no hydrocele or genital lymphedema were reported.
Another country in Asia, which is listed by the WHO as an endemic country for LF, is Nepal (Sherchand et al. 2003 ). The only agent of LF in Nepal is W. bancrofti , transmitted by Culex quinquefasciatus . Our survey of the literature disclosed no data regarding the history of LF in Nepal in the past, and identified only some reports which testify to the endemic nature of LF (Pradhan et al. 1998 ; Rana Krishna 2003 ). According to the Department of Health Services (DoHS) ( 2020 ) and the Epidemiology and Disease Control Division Teku ( 2018 ) and based on a survey from 2001 to 2012, the prevalence of LF was reported to range between 1 and 39% with average of 13%. It was also reported that 25 million people are at risk of LF in 61 out of 75 districts in Nepal. All these 61 endemic districts have received six rounds of MDA. Since 2018, 14 districts have been considered nonendemic. The MDA program was stopped in 36 districts in light of a successful TAS, but 25 districts were scheduled for MDA in 2019. Morbidity data recorded during the MDA from 61 districts showed a total of 28,529 cases of LF, among which most (19,907) were hydrocele, 5704 were elephantiasis, and cases 2918 involved hand and breast swelling and other LF manifestations (Epidemiology and Disease Control Division Teku 2018 ). At present, many health workers have learned to manage the disease and teach people how to prevent, manage and cure the LF. A referral system is available, along with treatment free of charge for infected people. The government of Nepal has implemented six rounds of MDA, instead of the five rounds implemented in other countries, to ensure the elimination of LF as a public health problem (Epidemiology and Disease Control Division Teku 2018 ). These rounds used a single dose of albendazole plus DEC as the baseline for MDA. A study of 4488 people in Nepal showed 13% seropositivity for LF. A higher rate of infection was reported among males (57.4%), but the difference between males and females was not significant. In addition, seropositivity was highest in the group of people 46 to 50 years old, with the lowest rate in the 36-to-40-year-old age group, but here again the difference was not significant (Sherchand et al. 2003 ).
A noteworthy feature of Thailand is this country’s extensive border with Myanmar. It has been documented that there is no significant rate of LF in Thai people, and the disease has been reported only in one southern province. Regarding the history of LF in Thailand, an old study reported that among 4112 persons examined in many villages, 863 were positive for microfilariae, of whom 215 showed filarial disease (Iyengar 1953 ). Currently, most cases of LF are reported in immigrants (Nithikathkul et al. 2006 ; Rojanapanus et al. 2019 ). Many immigrants reside in Thailand, so many studies have focused on this population group. In a long-term study from 2002 to 2017, LF was investigated in 23,477 immigrants. The results showed that 0.7% (range 0.1 to 2.7%) of them were seropositive (Rojanapanus et al. 2019 ). In contrast, during the same period, no positive cases were detected among Thai people in nearby areas. In another study of 2462 people of Thai origin, 1.38% were positive for B. malayi microfilariae The highest prevalence (4.69%) was reported in the 45-to-60-year-old group , and the lowest rate (0.37%) in the less than 15-year-old age group. The rate of infection was threefold as high in males as in females (Triteeraprapab et al. 2001 ). Toothong et al. evaluated the efficacy of MDA in 2015, and found that 75% of immigrants received DEC, which was below the standard. Barriers to receiving DEC were lack of official documents, unemployed status, daily employment, short-term immigrant status, and living in a fishery area for immigrants (Toothong et al. 2015 ). Currently, an LF surveillance program is conducted by the government every 2 years, targeted especially to immigrants. The perspectives seem promising in terms of eliminating LF as a public health problem (Rojanapanus et al. 2019 ).
Saudi Arabia
In Saudi Arabia, there are no significant concern at present regarding LF. Most cases have been reported among foreigner workers or as case reports (El-Moamly et al. 2012 ; Haleem et al. 2002 ; Omar 1996 ). In a study conducted by El-Moamly et al., among 647 foreigner workers from countries endemic for LF, 32 (5%) were positive for W. bancrofti microfilaremia according to membrane filtration and microscopy, 142 (22%) according to ELISA, and 128 (20%) according to an immunochromatographic test (ICT) (El-Moamly et al. 2012 ). Thus, FL in this country mostly a potential problem because of the huge number of foreigner workers, but not in people of Saudi origin. Another study of foreigner workers ( n = 762) to determine the rate of LF showed that 3.5% of the participants were positive. A total of 259 Indian males had an mf density of 6.0/20 mm 3 of blood. To date, only W. bancrofti has been detected as the only causal agent of LF, and in 1996, it was first suggested that Culex pipiens mosquitoes might be a potential vector for introduced LF in this country (Omar 1996 ). These results show that people of Saudi origin are at risk of the disease and should be aware of the risk. In one of the few studies of LF in people of Saudi origin from 1981 to 2001 at a military hospital, three cases were reported. They included a 68-year-old woman, a 78-year-old woman, and a 32-year-old man, all of Saudi origin. The authors warned that indigenous filariasis is present in Saudi Arabia, and that this disease should be considered in patients who show compatible signs or symptoms on physical examination (Haleem et al. 2002 ).
The earliest report of existing LF in this country dates back to 1956, when microfilaria was discovered in mosquitoes (Urbani 1997 ). The first case of LF was reported in 1995, followed by other positive cases in a single village (Leang et al. 2004 ). Cambodia contains both B. malayi and W. bancrofti microfilariae (Khieu et al. 2018 ; Leang et al. 2004 ). During a survey in 2004 by Leang et al., LF was investigated in 3490 people with a compendium of methods. The results showed that 0.52% were infected with filariasis according to the WB ICT card test, and that 0.23% were infected according to night blood examination (Leang et al. 2004 ). Both B. malayi and W. bancrofti were detected as the agents. The Cambodian Ministry of Health inaugurated an initiative in 2003 to control and eliminate NTDs including LF, by 2015. In overall terms this target was achieved, thanks in part to positive collaboration among the responsible organizations. In addition, MDA covered more than 70% of the country in five consecutive rounds from 2005 to 2009. This achievement was possible thanks to a compendium of training, allocation of two reference hospitals, MDA, screening tests, and other measures (Khieu et al. 2018 ). It was also announced that antigenemia in schoolchildren decreased from 1 to 0% during the years 2010–2013 and 2015. Together, these achievements led the WHO to announce that Cambodia was free of LF in June 2016 (Khieu et al. 2018 ).
China is among the countries which has not only certified the elimination of LF as a public health problem, but has also has entered the post-elimination survivable phase (Fang and Zhang 2019 ). Previously, both W. bancrofti and B. malayi infections were prevalent in this country. In the 1980s, 31 million cases of LF were estimated including 22 million as bancroftian filariasis and 9 million as malayan filariasis (Anonymous 1991 ). Sixteen provinces were involved then, including 864 counties and cities. The nature of filariasis in these areas was bancroftian ( n = 463), malayan ( n = 217), and mixed infections ( n = 184) (Sun and Chen 1992 ). In 1995, elimination was first announced in Guanxi, and the province to eliminate the disease was Anhui in 2006. Thereafter, the WHO announced that China was the first country in the world to officially succeed in eliminating LF as a public health problem (De-Jian et al. 2013 ). Some strong points helped China to combat the disease, e.g., an emphasis on control of infectious sources, three rounds of DEC, and establishing a threshold for LF transmission interruption (Fang and Zhang 2019 ). Although the present situation is ideal, the government plans to appraise the TSA in some previously endemic areas during the next 2 years.
Other countries
Vietnam is among the countries that, according to the WHO, has successfully eliminated LF (Cane 2020 ; Serrano et al. 2020 ). Reports of the disease in this country date from the 1900s (Meyrowitsch et al. 1998 ). A study in 1998 surveyed 135,000 people from 24 provinces, and found a prevalence of microfilaremia, attributed primarily to B. malayi , ranging from 0.9 to 5.5% (Meyrowitsch et al. 1998 ).
The Philippines reported LF caused by both W. bancrofti and B. malayi from 1951 onwards. With the establishment of the National Filariasis Control Programme in 1963, the government tried to identify endemic areas (Leonardo et al. 2020 ). Forty-six provinces (of a total of 81) had cases of LF. It is reported that the disease was more prevalent in adults than children, and in males compared to females (Kron et al. 2000 ; Leonardo et al. 2020 ). Like other countries at risk for LF, the Philippines started to combat the disease using a compendium of MDA, morbidity management and prophylaxis from 2000. Before that, 40 million people were at risk of infection, and in 1998 the national prevalence rate of LF was 9.7 cases per 1000 population (Galvez Tan 2003 ; Kron et al. 2000 ; Rubite 2018 ). Five rounds of MDA were implemented in 38 endemic provinces. Following on from this achievement, the government hopes to eliminate LF in the country by 2020.
The Republic of Korea is documented to be free of LF (Cheun et al. 2009 ; Cheun et al. 2017 ). Official reports of LF in this country date back to 1927. Although W. bancrofti was initially identified as the culprit species, later B. malayi was determined to be the correct culprit (Senoo 1943 ). In the 1950s, it was reported that 12.1% of the population was positive for microfilaria caused by B. malayi (Senoo and Lincicome 1951 ). Later studies showed that the infection was remained present in Korea (Cheun et al. 2017 ); however, at present, the country is documented to be free of LF. This achievement was made possible by government initiatives including the installation of mosquito nets and sanitation in houses (Cheun et al. 2009 ). During a follow-up study conducted by Cheun et al., 83 patients with an earlier diagnosis of LF were surveyed, and no cases were detected in many of them, although 31 patients could not be traced for different reasons (Cheun et al. 2017 ).
Bangladesh still has not eliminated LF but has made significant progress towards this goal (Karim et al. 2019 ; Shamsuzzaman et al. 2017 ). An MDA program was successfully implemented in the country, when it was assumed that 70 million people were at risk of LF. The species involved was identified as W. bancrofti transmitted by Culex sp. In 2001, 34 out of 64 districts were endemic for microfilaremia, at a rate of 1 to 19% (Hafiz et al. 2015 ; Karim et al. 2019 ; Shamsuzzaman et al. 2017 ). Overall, 19 endemic areas successfully completed the MDA program while 15 others were excluded from MDA monitoring because of their low endemicity (WHO 2011 ). One recent study (Karim et al. 2019 ) detected 43,678 clinical cases in 19 highly endemic districts, including cases of leg and/or arm lymphedema, hydrocele, female breast lymphedema or genital swelling. It shows that the government should implement additional measures to eliminate the infection. In another study to detect clinical cases of LF in 30 villages of Nilphamari District in Bangladesh, among 1242 participants, 4.4% were found to have LF-related clinical conditions. The most frequent clinical manifestations were hydrocele in males and leg lymphedema in females (Hafiz et al. 2015 ).
In Myanmar, the prevalence of LF remains high. The species involved is W. bancrofti , transmitted by Culex quinquefasciatus (Aye et al. 2018 ; de Meillon et al. 1967 ). In accordance with the WHO, the government of Myanmar decided in 2020 to launch a program to eliminate LF (Research et al. 1998 ). As a result, it was found that about 41 million people (about 80% of the total population) were infected with LF in 45 of 65 districts. An MDA program was implemented in endemic areas. To appraise the degree of success, two studies were conducted in 2008 and 2014 (Aye et al. 2018 ; WHO 2004 ). The results did not show complete elimination, but varying degress of progress were made and in some areas, the outcome was significant. The rate of filarial antigen positivity ranged from 0 to > 25% (Aye et al. 2018 ). A recent study showed the overall prevalence of infection to be 2.63% based on antigenemia and 1.03% based on microfilaremia (Dickson et al. 2018 ). No cases of lymphedema were found among participants, but 2.78% of males showed hydrocele. The present situation demonstrates promising features. Satisfactory measurements have been implemented with positive perspectives for eliminating the disease in the near future.
In Turkey, single case of filariasis was reported in an 11-year-old girl in a southern region, with swelling in both her legs (Cengiz et al. 2006 ). The species of the parasite has not been reported. Her family were reported to be free from the disease.
Japan is another country in Asia that has successfully eliminated LF (Ichimori et al. 2007 ). The initiative to combat the infection was started in the 1970s and ended in 1999. The high level of cooperation among the population was an important factor in eliminating not only LF but also many other parasitic diseases.
In Oman, the LF situation presents no serious concerns. Only sporadic cases have been reported, most of which were imported. A study of 250 children aged 17 to 18 years in 2004 detected no positive cases (Al Awaidy et al. 2010 ), and the authors concluded that LF is nonendemic in Oman. In another study from 1999 to 2013, 5 cases of filariasis were reported, of which 4 cases were travel-associated infections. The type of filariasis was not reported in this study (Al-Abri et al. 2015 ).
An important factor which has a decisive influence on the prognosis of LF is climate change. As a vector-borne infectious disease, LF is considered among the parasitic diseases affected by climate change. Accordingly, it is expected that LF could readily spread to new areas and worsen the situation (Short et al. 2017 ). Soil and plant canopy moisture levels are factors which directly influence the distribution of LF because they affect mosquito larvae breeding sites (Thompson et al. 1996 ). Changing temperature and precipitation patterns will thus affect soil moisture levels and mosquito populations. In Africa, it is reported that based on the level of climate change, the population at risk of LF may increase from 543 to 804 million to as much as 1.65–1.86 billion by 2050 (Slater and Michael 2012 ).
A limitation of our study was that some articles not accessible. Although we used some abstracts to obtain a clear-cut picture of the LF situation in Asia, the lack of access to information in some full texts may somewhat compromise the integrity of the output. To offset this drawback as far as possible, we tried to review all 48 countries individually to ensure reliability.
We found no cases of LF in other countries in Asia, although other kinds of filariasis were reported (Negahban et al. 2007 ; Parsa et al. 2020 ; Reddy 2013 ; Rokni 2008 ; Simón et al. 2012 ).
After the WHO announced a major initiative to eliminate LF in 2000, considerable progress has been made. Many countries have succeeded in eliminating the disease in accordance with the goals set by the WHO, while other countries should still take additional steps. Our study shows that in general, the outcomes have been satisfactory, and measures recommended by the WHO were observed to an adequate extent. One disappointing aspect is that unfortunately the region encompasses many political and social issues in some countries, including immigration, disruption, and poverty. These problems hinder efforts to attain all the aims proposed by the WHO to eliminate the disease in all countries.
According to evaluations conducted by the WHO and some governments in the region, LF is on track to be controlled and eliminated, yet some important factors including climate changes and especially the deadly new disease caused by SARS-CoV-2 will undoubtedly affect future efforts. Increasing declines in financial and economic resources may foreseeably prevent further efforts to control and eradicate filariasis. In order to continue the fight against the disease and prevent its spread, it is necessary for health authorities to consider the following points:
Regular training for health workers
Systematic surveillance management
Direct Network Report system
Establishing new and effective diagnostic methods
TAS and morbidity datasets should be developed for post-elimination surveillance strategies
Long-term reporting of new cases
Patient access to care (lymphedema management and hydrocele surgery)
Patient outreach and identification activities
Integration of LF clinical care into the primary health care system
Establishing a system of travel health service, e.g., increasing physicians’ awareness of travel-associated infections and passenger inspection programs for countries with high LF endemicity due to high numbers of immigrants
Eventually, five public health strategies recommended by the WHO to monitor neglected tropical diseases should be implemented by all involved countries: expansion of preventive chemotherapy, intensified case detection and case management, improved vector control, appropriate veterinary public health measures, and provision of safe water, sanitation, and hygiene.
Abdulcader MHM, Rajakone P, Rajendran K, Aponso L (1966) Age, sex, and house distribution of Wuchereria bancrofti Microfilaremia in Ceylon. Am J Trop Med Hyg 15:519–522. https://doi.org/10.4269/ajtmh.1966.15.519
Article CAS PubMed Google Scholar
Addiss DG (2010) Global elimination of lymphatic filariasis: addressing the public health problem. PLoS Negl Trop Dis 4:e741. https://doi.org/10.1371/journal.pntd.0000741
Article PubMed PubMed Central Google Scholar
Agrawal VK, Sashindran VK (2006) Lymphatic filariasis in India: problems, challenges and new initiatives. Med J Armed Forces India 62:359–362. https://doi.org/10.1016/s0377-1237(06)80109-7
Article CAS PubMed PubMed Central Google Scholar
Ahmad N (1945) Problems of filariasis with reference to post-war planning in India. J Indian Med Assoc 14:306–309
CAS PubMed Google Scholar
Al Awaidy ST, Bawikar S, Patel PK, Kurup P, Sonal GS, Al Mahrooqi S, Ramzy R (2010) Absence of lymphatic filariasis infection among secondary-school children in Oman. East Mediterr Health J 16:1059–1063
Article Google Scholar
Al-Abd NM, Nor ZM, Ahmed A, Al-Adhroey AH, Mansor M, Kassim M (2014) Lymphatic filariasis in Peninsular Malaysia: a cross-sectional survey of the knowledge, attitudes, and practices of residents. Parasit Vectors 7:545. https://doi.org/10.1186/s13071-014-0545-z
Al-Abri SS, Abdel-Hady DM, Al Mahrooqi SS, Al-Kindi HS, Al-Jardani AK, Al-Abaidani IS (2015) Epidemiology of travel-associated infections in Oman 1999-2013: a retrospective analysis. Travel Med Infect Dis 13:388–393. https://doi.org/10.1016/j.tmaid.2015.08.006
Al-Shaham AA, Sood S (2010) Recurrent furunculosis as a cause of isolated penile lymphedema: a case report. J Med Case Rep 4:196. https://doi.org/10.1186/1752-1947-4-196
Anonymous (1991) Major achievements and experience in filariasis control in the People’s Republic of China. National Technical Steering Group for Filariasis Control and Research. Chin Med J (Engl) 104:446–453
Google Scholar
Ashrafi K, Golchai J, Geranmayeh S (2010) Human subcutaneous dirofilariasis due to Dirofilaria ( Nochtiella ) repens : clinically suspected as cutaneous fascioliasis. Iran J Public Health 39:105–109
CAS PubMed PubMed Central Google Scholar
Aye NN, Lin Z, Lon KN, Linn NYY, Nwe TW, Mon KM, Ramaiah K, Betts H, Kelly-Hope LA (2018) Mapping and modelling the impact of mass drug adminstration on filariasis prevalence in Myanmar. Infect Dis Poverty 7:56. https://doi.org/10.1186/s40249-018-0420-9
Cane L (2020) A bright future: eliminating lymphatic filariasis in Vietnam. https://medium.com/@RTI_INTL_DEV/a-bright-future-eliminating-lymphatic-filariasis-in-vietnam-776bae245c8c . Accessed 11 June 2020
Cengiz N, Savaş L, Uslu Y, Anarat A (2006) Filariasis in a child from southern Turkey: a case report. Turk J Pediatr 48:152–154
PubMed Google Scholar
Chandrasena NT, Premaratna R, Samarasekera DS, de Silva NR (2016) Surveillance for transmission of lymphatic filariasis in Colombo and Gampaha districts of Sri Lanka following mass drug administration. Trans R Soc Trop Med Hyg 110:620–622. https://doi.org/10.1093/trstmh/trw067
Article PubMed Google Scholar
Cheun HI, Kong Y, Cho SH, Lee JS, Chai JY, Lee JS, Lee JK, Kim TS (2009) Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol 47:323–335. https://doi.org/10.3347/kjp.2009.47.4.323
Cheun HI et al (2017) Follow-up study of patients previously diagnosed with lymphatic filariasis in Korea. Osong Public Health Res Perspect 8:421–424. https://doi.org/10.24171/j.phrp.2017.8.6.10
Chu BK, Deming M, Biritwum NK, Bougma WR, Dorkenoo AM, el-Setouhy M, Fischer PU, Gass K, Gonzalez de Peña M, Mercado-Hernandez L, Kyelem D, Lammie PJ, Flueckiger RM, Mwingira UJ, Noordin R, Offei Owusu I, Ottesen EA, Pavluck A, Pilotte N, Rao RU, Samarasekera D, Schmaedick MA, Settinayake S, Simonsen PE, Supali T, Taleo F, Torres M, Weil GJ, Won KY (2013) Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis 7:e2584. https://doi.org/10.1371/journal.pntd.0002584
Collaborators GDaH (2017) Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1260–1344. https://doi.org/10.1016/s0140-6736(17)32130-x
de Meillon B, Grab B, Sebastian A (1967) Evaluation of Wuchereria bancrofti infection in Culex pipiens fatigans in Rangoon, Burma. Bull World Health Organ 36:91–100
PubMed PubMed Central Google Scholar
De-Jian S, Xu-Li D, Ji-Hui D (2013) The history of the elimination of lymphatic filariasis in China. Infect Dis Poverty 2:30. https://doi.org/10.1186/2049-9957-2-30
Department of Health Services (DoHS) T (2020) Kathmandu Lymphatic filariasis elimination. https://www.mohp.gov.np/eng/program/communicable-disease/lymphatic-fliariasis#:~:text=Lymphatic%20Filariasis%20(LF)%20is%20a,%3C1%25%20to%2039%25 . Accessed 7 June 2020
Dickson BFR, Graves PM, Aye NN, Nwe TW, Wai T, Win SS, Shwe M, Douglass J, Bradbury RS, McBride WJ (2018) The prevalence of lymphatic filariasis infection and disease following six rounds of mass drug administration in Mandalay Region, Myanmar. PLoS Negl Trop Dis 12:e0006944. https://doi.org/10.1371/journal.pntd.0006944
El-Moamly AA, El-Sweify MA, Hafez MA (2012) Using the AD12-ICT rapid-format test to detect Wuchereria bancrofti circulating antigens in comparison to Og4C3-ELISA and nucleopore membrane filtration and microscopy techniques. Parasitol Res 111:1379–1383. https://doi.org/10.1007/s00436-012-2870-5
Epidemiology and Disease Control Division Teku K, Nepal (2018) Lymphatic filariasis elimination program Annual report (2017/18). http://www.edcd.gov.np/resources/download/lymphatic-filariasisannual-report-201718 . Accessed 4 June 2020
Fang Y, Zhang Y (2019) Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China. Infect Dis Poverty 8:66. https://doi.org/10.1186/s40249-019-0578-9
Foo PK, Tarozzi A, Mahajan A, Yoong J, Krishnan L, Kopf D, Blackburn BG (2011) High prevalence of Wuchereria bancrofti infection as detected by immunochromatographic card testing in five districts of Orissa, India, previously considered to be non-endemic. Trans R Soc Trop Med Hyg 105:109–114. https://doi.org/10.1016/j.trstmh.2010.10.006
Galvez Tan JZ (2003) The elimination of lymphatic filariasis: a strategy for poverty alleviation and sustainable development - perspectives from the Philippines. Filaria J 2:12. https://doi.org/10.1186/1475-2883-2-12
George S, Joy TM, Kumar A, Panicker KN, George LS, Raj M, Leelamoni K, Nair P (2019) Prevalence of neglected tropical diseases (leishmaniasis and lymphatic filariasis) and malaria among a migrant labour settlement in Kerala, India. J Immigr Minor Health 21:563–569. https://doi.org/10.1007/s10903-018-0767-9
Ghasemi E, Shamsinia S, Taghipour A, Anvari D, Bahadory S, Shariatzadeh SA, Kordi B, Majidiani H, Borji H, Chaechi Nosrati M, Yousefi A, Shams M (2020) Filarial worms: a systematic review and meta-analysis of diversity in animals from Iran with emphasis on human cases. Parasitology 147(9):909–921. https://doi.org/10.1017/S003118202000058X
Ginandjar P, Saraswati LD, Suparyanto D, Sakundarno M, Supali T (2018) The prevalence of lymphatic filariasis in elementary school children living in endemic areas: a baseline survey prior to mass drug administration in Pekalongan District-Indonesia. Iran J Public Health 47:1484–1492
Gutierrez Y, Misselevich I, Fradis M, Podoshin L, Boss JH (1995) Dirofilaria repens infection in northern Israel. Am J Surg Pathol 19:1088–1091. https://doi.org/10.1097/00000478-199509000-00014
Hafiz I, Graves P, Haq R, Flora MS, Kelly-Hope LA (2015) Clinical case estimates of lymphatic filariasis in an endemic district of Bangladesh after a decade of mass drug administration. Trans R Soc Trop Med Hyg 109:700–709. https://doi.org/10.1093/trstmh/trv084
Haleem A, Al Juboury M, Al Husseini H (2002) Filariasis: a report of three cases. Ann Saudi Med 22:77–79. https://doi.org/10.5144/0256-4947.2002.77
Hotez PJ, Ehrenberg JP (2010) Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv Parasitol 72:31–53. https://doi.org/10.1016/s0065-308x(10)72002-9
Ichimori K, Graves PM, Crump A (2007) Lymphatic filariasis elimination in the Pacific: PacELF replicating Japanese success. Trends Parasitol 23:36–40. https://doi.org/10.1016/j.pt.2006.11.005
Iddawela D, Ehambaram K, Wickramasinghe S (2015) Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka. Asian Pac J Trop Med 8:1022–1026. https://doi.org/10.1016/j.apjtm.2015.11.010
Irvine MA, Kazura JW, Hollingsworth TD (2018) Understanding heterogeneities in mosquito-bite exposure and infection distributions for the elimination of lymphatic filariasis. Proc Biol Sci 285(1871):20172253. https://doi.org/10.1098/rspb.2017.2253
Iyengar MO (1953) Filariasis in Thailand. Bull World Health Organ 9:731–766
Jamail M, Andrew K, Junaidi D, Krishnan AK, Faizal M, Rahmah N (2005) Field validation of sensitivity and specificity of rapid test for detection of Brugia malayi infection. Trop Med Int Health 10:99–104. https://doi.org/10.1111/j.1365-3156.2004.01334.x
Jamshidi A, Jamshidi M, Mobedi I, Khosroara M (2008) Periocular dirofilariasis in a young woman: a case report. Korean J Parasitol 46:265–267. https://doi.org/10.3347/kjp.2008.46.4.265
Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH (2010) How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis 4:e755. https://doi.org/10.1371/journal.pntd.0000755
Karim MJ, Haq R, Mableson HE, Sultan Mahmood ASM, Rahman M, Chowdhury SM, Rahman A, Hafiz I, Betts H, Mackenzie C, Taylor MJ, Kelly-Hope LA (2019) Developing the first national database and map of lymphatic filariasis clinical cases in Bangladesh: another step closer to the elimination goals. PLoS Negl Trop Dis 13(7):e0007542. https://doi.org/10.1371/journal.pntd.000754213:e0007542
Khan AM, Dutta P, Sarmah CK, Baruah NK, Das S, Pathak AK, Sarmah P, Hussain ME, Mahanta J (2015) Prevalence of lymphatic filariasis in a tea garden worker population of Dibrugarh (Assam), India after six rounds of mass drug administration. J Vector Borne Dis 52:314–320
Khieu V, Or V, Tep C, Odermatt P, Tsuyuoka R, Char MC, Brady MA, Sidwell J, Yajima A, Huy R, Ramaiah KD, Muth S (2018) How elimination of lymphatic filariasis as a public health problem in the Kingdom of Cambodia was achieved. Infect Dis Poverty 7:15. https://doi.org/10.1186/s40249-018-0394-7
Kron M, Walker E, Hernandez L, Torres E, Libranda-Ramirez B (2000) Lymphatic filariasis in the Philippines. Parasitol Today 16:329–333. https://doi.org/10.1016/S0169-4758(00)01705-1
Leang R, Socheat D, Bin B, Bunkea T, Odermatt P (2004) Assessment of disease and infection of lymphatic filariasis in Northeastern Cambodia. Trop Med Int Health 9:1115–1120. https://doi.org/10.1111/j.1365-3156.2004.01311.x
Lee J, Ryu JS (2019) Current status of parasite infections in Indonesia: a literature review. Korean J Parasitol 57:329–339. https://doi.org/10.3347/kjp.2019.57.4.329
Leonardo L, Hernandez L, Magturo TC, Palasi W, Rubite JM, de Cadiz A, Moendeg K, Fornillos RJ, Tabios IK, Mistica M, Fontanilla IK (2020) Current status of neglected tropical diseases (NTDs) in the Philippines. Acta Trop 203:105284. https://doi.org/10.1016/j.actatropica.2019.105284
Li CY, Chang YL, Lee YC (2013) Human pulmonary dirofilariasis coexisting with intercostal neurilemmoma: a case report and literature review. J Formos Med Assoc 112:644–647. https://doi.org/10.1016/j.jfma.2012.07.016
Mallawarachchi CH, Nilmini Chandrasena TGA, Premaratna R, Mallawarachchi S, de Silva NR (2018) Human infection with sub-periodic Brugia spp. in Gampaha District, Sri Lanka: a threat to filariasis elimination status. Parasit Vectors 11:68. https://doi.org/10.1186/s13071-018-2649-3
Mandal NN, Bal MS, Das MK, Achary KG, Kar SK (2010) Lymphatic filariasis in children: age dependent prevalence in an area of India endemic for Wuchereria bancrofti infection. Trop Biomed 27:41–46
Meyrowitsch DW, Toan ND, Hao HT, Dan NT, Michael E (1998) A review of the present status of lymphatic filariasis in Vietnam. Acta Trop 70:335–347. https://doi.org/10.1016/S0001-706X(98)00037-0
Mukhopadhyay AK (2010) Lymphatic filariasis in Andhra Pradesh Paper Mill Colony, Rajahmundry, India after nine rounds of MDA programme. J Vector Borne Dis 47:55–57
Mukhopadhyay AK, Patnaik SK, Babu PS (2007) Status of lymphatic filariasis in parts of east Godavari district of Andhra Pradesh, India. J Vector Borne Dis 44:72–74
Negahban S, Daneshbod Y, Atefi S, Daneshbod K, Sadjjadi SM, Hosseini SV, Bedayat GR, Abidi H (2007) Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates: a case report. Acta Cytol 51:567–570. https://doi.org/10.1159/000325796
Nithikathkul C, Wannapinyosheep S, Saichua P, Nithikathkul M (2006) Filariasis: the disease will come to be the problem of Thailand. JSHMR 24:6
Noordin R, Mohd Zain SN, Yunus MH, Sahimin N (2017) Seroprevalence of lymphatic filariasis among migrant workers in Peninsular Malaysia. Trans R Soc Trop Med Hyg 111:370–372. https://doi.org/10.1093/trstmh/trx062
Omar MS (1996) A survey of bancroftian filariasis among South-East Asian expatriate workers in Saudi Arabia. Trop Med Int Health 1:155–160. https://doi.org/10.1111/j.1365-3156.1996.tb00021.x
Ottesen EA (2000) The global programme to eliminate lymphatic filariasis. Trop Med Int Health 5:591–594. https://doi.org/10.1046/j.1365-3156.2000.00620.x
Parsa R, Sedighi A, Sharifi I, Bamorovat M, Nasibi S (2020) Molecular characterization of ocular dirofilariasis: a case report of Dirofilaria immitis in south-eastern Iran. BMC Infect Dis 20:520. https://doi.org/10.1186/s12879-020-05182-5
Pradhan SP, Shrestha I, Palikhey N, Uprety RP (1998) Epidemiological study of lymphatic filariasis in Gokarna village development committee of Kathmandu valley during August and Septem. J Nepal Hlth Res Council 2:13–17
Rahmah N, Lim BH, Azian H, Ramelah TS, Rohana AR (2003) Short communication: use of a recombinant antigen-based ELISA to determine prevalence of brugian filariasis among Malaysian schoolchildren near Pasir Mas, Kelantan-Thailand border. Trop Med Int Health 8:158–163. https://doi.org/10.1046/j.1365-3156.2003.01004.x
Rajagopalan PK, Das PK, Subramanian S, Vanamail P, Ramaiah KD (1989) Bancroftian filariasis in Pondicherry, south India: 1. Pre-control epidemiological observations. Epidemiol Infect 103:685–692. https://doi.org/10.1017/s0950268800031083
Raju K, Jambulingam P, Sabesan S, Vanamail P (2010) Lymphatic filariasis in India: epidemiology and control measures. J Postgrad Med 56:232–238. https://doi.org/10.4103/0022-3859.68650
Rana Krishna J (2003) A brief study on the epidemiology of filariasis in Nepal. J Nepal Med Assoc 11:155–168. https://doi.org/10.31729/jnma.1561
Rao RU, Samarasekera SD, Nagodavithana KC, Dassanayaka TDM, Punchihewa MW, Ranasinghe USB, Weil GJ (2017) Reassessment of areas with persistent Lymphatic Filariasis nine years after cessation of mass drug administration in Sri Lanka. PLoS Negl Trop Dis 11:e0006066. https://doi.org/10.1371/journal.pntd.0006066
Rao RU, Samarasekera SD, Nagodavithana KC, Goss CW, Punchihewa MW, Dassanayaka TDM, Ranasinghe USB, Mendis D, Weil GJ (2018) Comprehensive assessment of a hotspot with persistent Bancroftian filariasis in coastal Sri Lanka. Am J Trop Med Hyg 99:735–742. https://doi.org/10.4269/ajtmh.18-0169
Reddy MV (2013) Human dirofilariasis: an emerging zoonosis. Trop Parasitol 3:2–3
Research UNWBWSPf, Training in Tropical D, Mapping WUJPfH, World Health Organization. Division of Tropical D (1998) Research on rapid geographical assessment of Bancroftian filariasis. World Health Organization, Geneva
Rojanapanus S, Toothong T, Boondej P, Thammapalo S, Khuanyoung N, Santabutr W, Prempree P, Gopinath D, Ramaiah KD (2019) How Thailand eliminated lymphatic filariasis as a public health problem. Infect Dis Poverty 8:38. https://doi.org/10.1186/s40249-019-0549-1
Rokni MB (2008) The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol 102:283–295. https://doi.org/10.1179/136485908x300805
Rubis P, Chang MS, Nagum AJ, Jau JL (1981) Parasitological and entomological studies on filariasis in seven villages, Serian District, Sarawak, East Malaysia. Southeast Asian J Trop Med Public Health 12:30–35
Rubite JM (2018) Initiatives on NTDs in the Philippines. In: the First ASEAN LF Forum, Manila, Philippines, 11-12 July, 2018
Sabesan S, Raju KH, Subramanian S, Srivastava PK, Jambulingam P (2013) Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis 13:657–665. https://doi.org/10.1089/vbz.2012.1238
Senoo T (1943) Detection of microfilaria malayi brug in Korea. Nippon Kiseichu Gakkai Kiji 15:36
Senoo T, Lincicome RD (1951) Malayan filariasis; incidence and distribution in Southern Korea. U S Armed Forces Med J 2:1483–1489
Ruel E. Serrano, Tmong Udui, Vu Lam Binh, Morel E (2020) Three more countries eliminate lymphatic filariasis. https://www.who.int/westernpacific/news/detail/08-10-2018-three-more-countries-eliminate-lymphatic-filariasis . Accessed 11 June 2020
Shamsuzzaman AK et al (2017) The significant scale up and success of Transmission Assessment Surveys ‘TAS’ for endgame surveillance of lymphatic filariasis in Bangladesh: one step closer to the elimination goal of 2020. PLoS Negl Trop Dis 11:e0005340. https://doi.org/10.1371/journal.pntd.0005340
Sherchand JB, Obsomer V, Thakur GD, Hommel M (2003) Mapping of lymphatic filariasis in Nepal. Filaria J 2:7. https://doi.org/10.1186/1475-2883-2-7
Short EE, Caminade C, Thomas BN (2017) Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis (Auckl) 10:1178633617732296. https://doi.org/10.1177/1178633617732296
Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E, Montoya-Alonso JA (2012) Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev 25:507–544. https://doi.org/10.1128/cmr.00012-12
Slater H, Michael E (2012) Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS One 7:e32202. https://doi.org/10.1371/journal.pone.0032202
Solgi R, Sadjjadi SM, Mohebali M, Djadid ND, Raz A, Zakeri S, Zarei Z (2017) Susceptibility of Anopheles stephensi (Diptera: Culicidae) to Dirofilaria immitis (Spirurida: Onchocercidae). Russ J Nematol 25(2):121–127. https://doi.org/10.24411/0869-6918-2017-00005
Srividya A, Michael E, Palaniyandi M, Pani SP, Das PK (2002) A geostatistical analysis of the geographic distribution of lymphatic filariasis prevalence in southern India. Am J Trop Med Hyg 67:480–489. https://doi.org/10.4269/ajtmh.2002.67.480
Sun DJ, Chen PL (1992) Filariasis surveillance at the post-control stage in China. Southeast Asian J Trop Med Public Health 23:369–376
Supali T et al (2002) High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. Am J Trop Med Hyg 66:560–565. https://doi.org/10.4269/ajtmh.2002.66.560
Tavakolizadeh S, Mobedi I (2009) Orbital dirofilariasis in Iran: a case report. Korean J Parasitol 47:397–399. https://doi.org/10.3347/kjp.2009.47.4.397
Thompson DF, Malone JB, Harb M, Faris R, Huh OK, Buck AA, Cline BL (1996) Bancroftian filariasis distribution and diurnal temperature differences in the southern Nile delta. Emerg Infect Dis 2:234–235. https://doi.org/10.3201/eid0203.960313
Toothong T, Tipayamongkholgul M, Suwannapong N, Suvannadabba S (2015) Evaluation of mass drug administration in the program to control imported lymphatic filariasis in Thailand. BMC Public Health 15:975. https://doi.org/10.1186/s12889-015-2325-x
Torgerson PR, de Silva NR, Fèvre EM, Kasuga F, Rokni MB, Zhou XN, Sripa B, Gargouri N, Willingham AL, Stein C (2014) The global burden of foodborne parasitic diseases: an update. Trends Parasitol 30:20–26. https://doi.org/10.1016/j.pt.2013.11.002
Triteeraprapab S, Karnjanopas K, Pruksakorn C, Sai-Ngam A, Yentakam S, Loymak S (2001) Lymphatic filariasis caused by Brugia malayi in an endemic area of Narathiwat Province, southern of Thailand. J Med Assoc Thailand = Chotmaihet Thangphaet 84(Suppl 1):S182–S188
Upadhyayula SM, Mutheneni SR, Kadiri MR, Kumaraswamy S, Nagalla B (2012) A cohort study of lymphatic filariasis on socio economic conditions in Andhra Pradesh, India. PLoS One 7:e33779. https://doi.org/10.1371/journal.pone.0033779
Urbani C (1997) Control of schistosomiasis and other helminthiasis in Cambodia. Me´decins Sans Frontie`res Switzerland, internal report n/d
Utzinger J, Bergquist R, Olveda R, Zhou XN (2010) Important helminth infections in Southeast Asia diversity, potential for control and prospects for elimination. Adv Parasitol 72:1–30. https://doi.org/10.1016/s0065-308x(10)72001-7
WHO (2004) Report on the mid-term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec 79:457–468. http://www.who.int/neglected_diseases/resources/who_wer7940/en/ . Accessed 17 June 2020
WHO (2011) Global programme to eliminate lymphatic filariasis: a manual for National Elimination Programmes (Monitoring and Epidemiological Assessment of Mass Drug Administration). http://www.who.int/lymphatic_filariasis/resources/9789241501484/ . Accessed 14 Jun2 2020
WHO (2013) Towards eliminating lymphatic filariasis: progress in the South-East Asia Region (2001–2011).
WHO (2014) Global programme to eliminate lymphatic filariasis: progress report, 2013. Wkly Epidemiol Rec 89:409–418
WHO (2016) Maldives and Sri Lanka eliminate lymphatic filariasis. http://www.searo.who.int/mediacentre/releases/2016/1626/en/ . Accessed 3 June 2020
WHO (2020) Neglected tropical diseases. https://www.who.int/neglected_diseases/diseases/en/ . Accessed 3 June 2020
Wibawa T, Satoto TBT (2016) Magnitude of neglected tropical diseases in Indonesia at postmillennium development goals era. J Trop Med 2016:5716785–5716789. https://doi.org/10.1155/2016/5716785
Yap LF, Ramachandran CP, Balasingam E (1968) A parasitological study of Pulau Pinang and Pulau Perhentian Kechil, off Trengganu, West Malaysia. I. Malaria and filariasis. Med J Malaya 23:118–122
Download references
Acknowledgments
The sincere cooperation of Dr A Alizadeh, is highly appreciated. We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for editing the English in the manuscript.
Availability of data and material
All data, analyses, and Excel spreadsheets are available on request.
The study was funded by a grant awarded by the National Institute for Medical Research Development, Ministry of Health and Medical Education, Iran (No. 977187).
Author information
Authors and affiliations.
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Negar Bizhani, Saeideh Hashemi Hafshejani & Mohammad Bagher Rokni
Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Neda Mohammadi
Department of Forestry and Landscape Architecture, Konkuk University, Seoul, Republic of Korea
Mehdi Rezaei
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Negar BIZHANI, Saeideh HASHEMI HAFSHEJANI1, and Mohammad Bager ROKNI. Analysis and graph preparation were performed by Neda MOHAMMADI and Mehdi Rezaei. The first draft of the manuscript was written by Mohammad Bager ROKNI and Negar BIZHANI. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad Bagher Rokni .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflicts of interest.
Code availability
Not applicable
Additional information
Section Editor: Ramaswamy Kalyanasundaram
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Bizhani, N., Hashemi Hafshejani, S., Mohammadi, N. et al. Lymphatic filariasis in Asia: a systematic review and meta-analysis. Parasitol Res 120 , 411–422 (2021). https://doi.org/10.1007/s00436-020-06991-y
Download citation
Received : 06 July 2020
Accepted : 25 November 2020
Published : 08 January 2021
Issue Date : February 2021
DOI : https://doi.org/10.1007/s00436-020-06991-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lymphatic filariasis
- Neglected tropical diseases
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Lymphatic filariasis in Asia: a systematic review and meta-analysis
Negar bizhani.
1 Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Saeideh Hashemi Hafshejani
Neda mohammadi.
2 Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Mehdi Rezaei
3 Department of Forestry and Landscape Architecture, Konkuk University, Seoul, Republic of Korea
Mohammad Bagher Rokni
Associated data.
All data, analyses, and Excel spreadsheets are available on request.
Lymphatic filariasis (LF) is an important neglected parasitic disease according to the World Health Organization. In this study, we aimed to determine the prevalence of human LF in Asia using a systematic review and meta-analysis approach. Records from 1990 to 2018 in reputable databases including PubMed, Science Direct, Embase, and Cochrane Library were searched using a panel of related keywords. All 48 countries of Asia were searched one by one in combination with the keywords. In all, 41,742 cases identified in this study were included in the analysis. According to our findings, the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). There was no major trend in the cumulative prevalence of LF over time. Some countries in Asia including China, Japan, Vietnam, and South Korea succeeded in eliminating LF as a public health problem, but others still need to monitor the disease. Based on the initiative of the WHO starting in 2000, some countries in Asia succeeded in eliminating LF as a public health problem. Other countries have taken steps to eliminate the disease with variable degrees of success. These efforts might be affected by issues such as climate change.
Introduction
The term neglected tropical diseases (NTDs) arose from the recommendation by the Working Group on Monitoring and Evaluation of the Strategic and Technical Advisory Group for NTDs (WHO 2020 ). Among NTDs, parasitic diseases are prominent, and many studies have been conducted on different aspects of their risks and complications (Torgerson et al. 2014 ). According to the WHO (WHO 2020 ), the following diseases are considered NTDs: Chagas disease, dracunculiasis (guinea-worm disease), echinococcosis, foodborne trematodiases, human African trypanosomiasis (sleeping sickness), leishmaniasis, lymphatic filariasis, onchocerciasis (river blindness), schistosomiasis, soil-transmitted helminthiasis, and taeniasis/cysticercosis.
Filariasis is an important parasitic disease caused by roundworms of the Filarioidea superfamily, which are parasites residing in the blood and tissues of humans. In humans, filariasis is caused by Wuchereria bancrofti , Brugia malayi , Loa loa , Onchocerca volvulus , and Dirofilaria spp. Lymphatic filariasis (LF), in which the adult worms are found in the lymphatic system, is considered the most important form of filariasis, and is also known as elephantiasis. It is transmitted by mosquitoes of the genera Culex , Mansonia , and Anopheles (Solgi et al. 2017 ; WHO 2013 ).
Nearly 63% of 1.34 billion people worldwide are at risk of LF, and about 50% of the 120 million infected people live in the South-East Asia Region. This region bears approximately 57% of the total global burden of an estimated 5.1 million disability-adjusted life years (DALYs) lost due to LF. Nine countries in this region are endemic for LF. India, Nigeria (in Africa), Bangladesh and Indonesia together account for 70% of LF in the world, although 80 countries are considered endemic for the disease (WHO 2013 ). In the year 2016, a total of 1189 (587.7 to 2114.9) DALYs (thousands) were reported for all ages (Collaborators 2017 ).
Countries and areas considered at high risk include central Africa and the Nile delta, Madagascar, Turkey, the Middle East, India, Pakistan, Sri Lanka, Myanmar, Thailand, Malaysia, Vietnam, South Korea, Indonesia, the Philippines, Timor, southern China, Haiti, Dominican Republic, Guyana, French Guinea, and costal Brazil (Hotez and Ehrenberg 2010 ; Utzinger et al. 2010 ).
The disease has variable symptoms caused mostly because adult worms occupy and block the lymphatic vessels. Depending on the kind of filariasis, the patient shows a spectrum of signs and symptoms including elephantiasis, lymphedema, hydrocele, chyluria, chylous diarrhea, and chylorrhagia (Addiss 2010 ; Kabatereine et al. 2010 ). Some asymptomatic cases, which may become chronic, have also been reported.
Clinical manifestations including episodic attacks of lymphadenitis and lymphangitis (fever, pain in the affected area, tender red streaks) along with fever and malaise are attributed to acute LF, while some cases of acute attacks have been reported for chronic manifestations. The patients show lumps in the subcutaneous tissue, breasts or testicles due to reactions to adult worms or related granulomas (Al-Shaham and Sood 2010 ).
After the World Health Assembly (WHA) on 1997 and based on Resolution 50.29 to eliminate LF, a Global Programme to Eliminate LF (GPELF) was initiated in 2000 to eliminate LF in 2020. The main strategies were mass drug administration (MDA) using a two-drug combination of diethylcarbamazine (DEC) and albendazole, and a transmission assessment survey (TAS) (WHO 2013 ).
In this review, we aimed to collect and analyze data concerning the situation of human filariasis in Asian countries by searching publications between 1990 and 2018 recorded in reliable databases.
Search strategy
The PubMed, Science Direct, Embase, and Cochrane Library databases were searched using a panel of keywords that included, but was not limited to, human filariasis, lymphatic filariasis, Wuchereria bancrofti , Brugia malayi , Brugia timori , prevalence, epidemiology, and all Asia countries in turn ( https://www.worldatlas.com/articles/how-many-countries-are-in-asia.html ). The countries included in the searches were Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, East Timor, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar, Nepal, North Korea, Oman, Pakistan, Philippines, Qatar, Saudi Arabia, Singapore, South Korea, Sri Lanka, State of Palestine, Syria, Tajikistan, Thailand, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, Vietnam and Yemen.
We included relevant studies conducted from 1990 to 2018 and published in English. We initially screened studies based on their title and abstract, followed by availability of the full text. If only the abstract was available, information from the study was considered only in the Discussion section. Items such as books and Letters to the Editors were excluded from the study.
The inclusion criteria were (1) conducted between 1990 and 2018, (2) English language, (3) availability of the full text, (4) original or review article as publication type, and (5) involving human patients.
The exclusion criteria were (1) studies in animals only, and (2) case report or letter to the editor as publication type.
As shown in Fig. Fig.1, 1 , a total of 3305 records were initially found in the database searches. After initial screening, 2440 duplicate articles were deleted. The remaining 790 items were then screened to locate relevant content. The remaining 75 articles were then checked to verify that they met the inclusion criteria, and as a result, a total of 19 studies were included in the analysis.

Flow diagram of search and selection of relevant articles
Statistical analysis
The data were analyzed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria.). The pooled prevalence was estimated with its 95% confidence interval (CI). Chi-squared, tau-squared, and I-squared statistics were calculated to assess heterogeneity of the studies. Due to significant heterogeneity, a random effects model was used to estimate the pooled prevalence of filariasis. Publication bias was evaluated using a funnel plot. Results with p values less than 0.05 were considered statistically significant.
Results and discussion
Figure Figure2 2 shows the distribution of LF in Asia. The differences in prevalence among countries are discussed in detail below.

Distribution of lymphatic filariasis in Asia (Original figure)
A total of 41,742 cases synthesized in the present study were included in the analysis. According to the results of the present meta-analysis, reported in Fig. Fig.3, 3 , the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). The results of chi-squared, tau-squared and I -squared tests revealed significant heterogeneity among the studies, so a random effects model was used to pool the prevalences from all included studies (tau 2 = .53, chi 2 = 1618.02, p value < 0.001, I 2 = 99%).

Forest plot showing the pooled prevalence of lymphatic filariasis in Asia
Cumulative meta-analysis was performed to estimate the trend in LF prevalence with time in Asia. The results showed that there was no major trend in cumulative prevalence (Fig. (Fig.4). 4 ). A funnel plot drawn to check for the existence of publication bias in the studies (Fig. (Fig.5) 5 ) showed no bias.

Cumulative forest plot showing the trends in lymphatic filariasis prevalence in Asia

Funnel plot to assess the presence of publication bias
The highest prevalence of infection was reported in India as 21% (Foo et al. 2011 ), and the lowest rate was from Sri Lanka as 0.32% (Chandrasena et al. 2016 ). We noted that most studies done in India reported a prevalence of LF between 2.4 and 21% (Foo et al. 2011 ; George et al. 2019 ; Khan et al. 2015 ; Mandal et al. 2010 ; Mukhopadhyay 2010 ; Mukhopadhyay et al. 2007 ; Sabesan et al. 2013 ; Srividya et al. 2002 ; Upadhyayula et al. 2012 ).
Lymphatic filariasis due to Brugia spp. was reported from Indonesia (Supali et al. 2002 ), Malaysia (Jamail et al. 2005 ), Sri Lanka (Iddawela et al. 2015 ; Mallawarachchi et al. 2018 ), and Thailand (Rahmah et al. 2003 ). Dirofilaria spp. was reported in humans in Iran (Ashrafi et al. 2010 ; Ghasemi et al. 2020 ; Jamshidi et al. 2008 ; Tavakolizadeh and Mobedi 2009 ), Israeli (Gutierrez et al. 1995 ), and Taiwan (Li et al. 2013 ), although these cases are not considered NTDs. Because the aim of the study was to document the situation of LF only in humans, the many studies that reported animal filariasis in Asia were excluded from the present analysis.
The situation of LF in individual Asian countries is reported below.
Lymphatic filariasis has a long history in India dating back to the 6th century B.C. In 1995, the National Filaria Control Programme (NFCP) was started (Agrawal and Sashindran 2006 ). The disease has been reported in India since 1945 (Ahmad 1945 ). Unfortunately, there are no detailed data in the literature from that time. A survey conducted in 1981 showed that of 24,946 persons examined for LF, 8–41% had microfilaremia (Rajagopalan et al. 1989 ).
Many more recent studies from India have been published regarding the prevalence of LF (Foo et al. 2011 ; George et al. 2019 ; Khan et al. 2015 ; Mandal et al. 2010 ; Mukhopadhyay 2010 ; Mukhopadhyay et al. 2007 ; Sabesan et al. 2013 ; Srividya et al. 2002 ; Upadhyayula et al. 2012 ). Bancroftian filariasis was reported in all of these studies. India harbors nearly 40% of 120 million cases of infection with LF in the world (George et al. 2019 ). The DALYs lost in India due to FL has been reported as 2.06 million, resulting in an annual wage loss of US $811 million (Ottesen 2000 ). The six states regarded as having high endemicity in India are Uttar Pradesh, Bihar, Jharkhand, Orissa, Kerala, and Gujarat (Raju et al. 2010 ). The global LF program is currently being conducted with the main targets of eliminating LF and interrupting transmission using MDA (Ottesen 2000 ). The drugs DEC and albendazole are used as part of the program to support its aims. Accordingly, they may reduce the microfilaremia more than 95% after 2 annul rounds (Ottesen 2000 ).
In Malaysia, LF has been reported with two agents: W. bancrofti and B. malayi (Al-Abd et al. 2014 ). The first report of LF we noted in the literature dates to 1968, but no details were available (Yap et al. 1968 ). The earliest report we could find described microfilaria as sub-periodic B. malayi in 82 persons among 1613 people examined in seven villages in Serian District (Rubis et al. 1981 ). The disease is not widespread in the country, and occurs only in some states of Peninsular Malaysia including Terengganu, Kelantan, Pahang, Selangor, and Johor as well as the very small Sabah and Sarawak regions (Al-Abd et al. 2014 ). In Malaysia, an MDA program is being conducted with the two drugs noted earlier for India. The program covered all endemic areas from 2004 to 2008. It appears that the program has not been completely successful and requires additional efforts to reach the WHO goals (Al-Abd et al. 2014 ; Noordin et al. 2017 ). In a study by Rahmah et al. (Rahmah et al. 2003 ) with B. malayi recombinant antigen for ELISA testing, among 5138 children in Malaysia, 0.35% showed seropositivity. Positive cases included 13 boys and 5 girls between 7 and 12 years old.
Lymphatic filariasis has been reported from Sri Lanka for hundreds of years, with high endemicity (Rao et al. 2018 ). The earliest documented report found microfilaremia in 19.1% of houses examined for LF in Ceylon (Abdulcader et al. 1966 ). The country implemented an MDA program with DEC and albendazole from 2002 to 2006 in all endemic parts of the country. A TAS was conducted in endemic areas in 2013 (WHO 2014 ) to determine the prevalence of filarial antigenemia in young schoolchildren, and found a prevalence of less than 2% with 95% certainty (Chu et al. 2013 ). Rao et al. believed this method was not sensitive to detect ongoing transmission of W. bancrofti in many areas in Sri Lanka (Rao et al. 2018 ). They reported high rates of circulating filarial antigenemia (3%, confidence interval [CI]: 1.8–4.9) and microfilaremia (1%, CI: 0.5–2.5%), and noted that “circulating filarial antigenemia rates were 2.8-fold higher in males than females”. Anti-filarial antibodies were detected in young children at a prevalence of 5.7% (Rao et al. 2018 ). According to the WHO, although Sri Lanka could eliminate LF in 2016, surveillance efforts and interventions should be continued to monitor the problem in endemic areas (WHO 2016 ). It is believed that according to antigen prevalence data, adult males account for most persistent filarial infections in Sri Lanka (Irvine et al. 2018 ; Rao et al. 2017 , 2018 ).
The reported cases of LF in this country were 9.2% and 1.98% (Ginandjar et al. 2018 ; Supali et al. 2002 ). In 1980, the prevalence of microfilaremia was 19.5%, but it had been reduced to 4.7% by 2014 (Lee and Ryu 2019 ). The Indonesian Ministry of Health planned an MDA program in 2015 to monitor LF in 106 endemic districts. Based on further verifications, there was little success in monitoring the disease, such that 29 provinces continued to have problem in Eastern Indonesia in 2016 (Lee and Ryu 2019 ; Wibawa and Satoto 2016 ). A known endemic district is Pekalongan, where 62 cases with chronic LF were reported. There is a risk that the disease will spread if it is not controlled (Ginandjar et al. 2018 ). In a study of elementary schoolchildren in Indonesia in 2015, the prevalence was 1.98% and the agent was W. bancrofti . Although most infected students were older ones and males, no significant differences were reported (Ginandjar et al. 2018 ). In another study (Supali et al. 2002 ), both bancroftian filariasis and B. timori were reported in separate districts but no mixed infections were detected. Among 586 cases studied for B. timori , 27% showed microfilaremia. Males showed more infection than females. Regarding clinical manifestations, 13% of cases showed lymphedema in the legs, but no hydrocele or genital lymphedema were reported.
Another country in Asia, which is listed by the WHO as an endemic country for LF, is Nepal (Sherchand et al. 2003 ). The only agent of LF in Nepal is W. bancrofti , transmitted by Culex quinquefasciatus . Our survey of the literature disclosed no data regarding the history of LF in Nepal in the past, and identified only some reports which testify to the endemic nature of LF (Pradhan et al. 1998 ; Rana Krishna 2003 ). According to the Department of Health Services (DoHS) ( 2020 ) and the Epidemiology and Disease Control Division Teku ( 2018 ) and based on a survey from 2001 to 2012, the prevalence of LF was reported to range between 1 and 39% with average of 13%. It was also reported that 25 million people are at risk of LF in 61 out of 75 districts in Nepal. All these 61 endemic districts have received six rounds of MDA. Since 2018, 14 districts have been considered nonendemic. The MDA program was stopped in 36 districts in light of a successful TAS, but 25 districts were scheduled for MDA in 2019. Morbidity data recorded during the MDA from 61 districts showed a total of 28,529 cases of LF, among which most (19,907) were hydrocele, 5704 were elephantiasis, and cases 2918 involved hand and breast swelling and other LF manifestations (Epidemiology and Disease Control Division Teku 2018 ). At present, many health workers have learned to manage the disease and teach people how to prevent, manage and cure the LF. A referral system is available, along with treatment free of charge for infected people. The government of Nepal has implemented six rounds of MDA, instead of the five rounds implemented in other countries, to ensure the elimination of LF as a public health problem (Epidemiology and Disease Control Division Teku 2018 ). These rounds used a single dose of albendazole plus DEC as the baseline for MDA. A study of 4488 people in Nepal showed 13% seropositivity for LF. A higher rate of infection was reported among males (57.4%), but the difference between males and females was not significant. In addition, seropositivity was highest in the group of people 46 to 50 years old, with the lowest rate in the 36-to-40-year-old age group, but here again the difference was not significant (Sherchand et al. 2003 ).
A noteworthy feature of Thailand is this country’s extensive border with Myanmar. It has been documented that there is no significant rate of LF in Thai people, and the disease has been reported only in one southern province. Regarding the history of LF in Thailand, an old study reported that among 4112 persons examined in many villages, 863 were positive for microfilariae, of whom 215 showed filarial disease (Iyengar 1953 ). Currently, most cases of LF are reported in immigrants (Nithikathkul et al. 2006 ; Rojanapanus et al. 2019 ). Many immigrants reside in Thailand, so many studies have focused on this population group. In a long-term study from 2002 to 2017, LF was investigated in 23,477 immigrants. The results showed that 0.7% (range 0.1 to 2.7%) of them were seropositive (Rojanapanus et al. 2019 ). In contrast, during the same period, no positive cases were detected among Thai people in nearby areas. In another study of 2462 people of Thai origin, 1.38% were positive for B. malayi microfilariae The highest prevalence (4.69%) was reported in the 45-to-60-year-old group , and the lowest rate (0.37%) in the less than 15-year-old age group. The rate of infection was threefold as high in males as in females (Triteeraprapab et al. 2001 ). Toothong et al. evaluated the efficacy of MDA in 2015, and found that 75% of immigrants received DEC, which was below the standard. Barriers to receiving DEC were lack of official documents, unemployed status, daily employment, short-term immigrant status, and living in a fishery area for immigrants (Toothong et al. 2015 ). Currently, an LF surveillance program is conducted by the government every 2 years, targeted especially to immigrants. The perspectives seem promising in terms of eliminating LF as a public health problem (Rojanapanus et al. 2019 ).
Saudi Arabia
In Saudi Arabia, there are no significant concern at present regarding LF. Most cases have been reported among foreigner workers or as case reports (El-Moamly et al. 2012 ; Haleem et al. 2002 ; Omar 1996 ). In a study conducted by El-Moamly et al., among 647 foreigner workers from countries endemic for LF, 32 (5%) were positive for W. bancrofti microfilaremia according to membrane filtration and microscopy, 142 (22%) according to ELISA, and 128 (20%) according to an immunochromatographic test (ICT) (El-Moamly et al. 2012 ). Thus, FL in this country mostly a potential problem because of the huge number of foreigner workers, but not in people of Saudi origin. Another study of foreigner workers ( n = 762) to determine the rate of LF showed that 3.5% of the participants were positive. A total of 259 Indian males had an mf density of 6.0/20 mm 3 of blood. To date, only W. bancrofti has been detected as the only causal agent of LF, and in 1996, it was first suggested that Culex pipiens mosquitoes might be a potential vector for introduced LF in this country (Omar 1996 ). These results show that people of Saudi origin are at risk of the disease and should be aware of the risk. In one of the few studies of LF in people of Saudi origin from 1981 to 2001 at a military hospital, three cases were reported. They included a 68-year-old woman, a 78-year-old woman, and a 32-year-old man, all of Saudi origin. The authors warned that indigenous filariasis is present in Saudi Arabia, and that this disease should be considered in patients who show compatible signs or symptoms on physical examination (Haleem et al. 2002 ).
The earliest report of existing LF in this country dates back to 1956, when microfilaria was discovered in mosquitoes (Urbani 1997 ). The first case of LF was reported in 1995, followed by other positive cases in a single village (Leang et al. 2004 ). Cambodia contains both B. malayi and W. bancrofti microfilariae (Khieu et al. 2018 ; Leang et al. 2004 ). During a survey in 2004 by Leang et al., LF was investigated in 3490 people with a compendium of methods. The results showed that 0.52% were infected with filariasis according to the WB ICT card test, and that 0.23% were infected according to night blood examination (Leang et al. 2004 ). Both B. malayi and W. bancrofti were detected as the agents. The Cambodian Ministry of Health inaugurated an initiative in 2003 to control and eliminate NTDs including LF, by 2015. In overall terms this target was achieved, thanks in part to positive collaboration among the responsible organizations. In addition, MDA covered more than 70% of the country in five consecutive rounds from 2005 to 2009. This achievement was possible thanks to a compendium of training, allocation of two reference hospitals, MDA, screening tests, and other measures (Khieu et al. 2018 ). It was also announced that antigenemia in schoolchildren decreased from 1 to 0% during the years 2010–2013 and 2015. Together, these achievements led the WHO to announce that Cambodia was free of LF in June 2016 (Khieu et al. 2018 ).
China is among the countries which has not only certified the elimination of LF as a public health problem, but has also has entered the post-elimination survivable phase (Fang and Zhang 2019 ). Previously, both W. bancrofti and B. malayi infections were prevalent in this country. In the 1980s, 31 million cases of LF were estimated including 22 million as bancroftian filariasis and 9 million as malayan filariasis (Anonymous 1991 ). Sixteen provinces were involved then, including 864 counties and cities. The nature of filariasis in these areas was bancroftian ( n = 463), malayan ( n = 217), and mixed infections ( n = 184) (Sun and Chen 1992 ). In 1995, elimination was first announced in Guanxi, and the province to eliminate the disease was Anhui in 2006. Thereafter, the WHO announced that China was the first country in the world to officially succeed in eliminating LF as a public health problem (De-Jian et al. 2013 ). Some strong points helped China to combat the disease, e.g., an emphasis on control of infectious sources, three rounds of DEC, and establishing a threshold for LF transmission interruption (Fang and Zhang 2019 ). Although the present situation is ideal, the government plans to appraise the TSA in some previously endemic areas during the next 2 years.
Other countries
Vietnam is among the countries that, according to the WHO, has successfully eliminated LF (Cane 2020 ; Serrano et al. 2020 ). Reports of the disease in this country date from the 1900s (Meyrowitsch et al. 1998 ). A study in 1998 surveyed 135,000 people from 24 provinces, and found a prevalence of microfilaremia, attributed primarily to B. malayi , ranging from 0.9 to 5.5% (Meyrowitsch et al. 1998 ).
The Philippines reported LF caused by both W. bancrofti and B. malayi from 1951 onwards. With the establishment of the National Filariasis Control Programme in 1963, the government tried to identify endemic areas (Leonardo et al. 2020 ). Forty-six provinces (of a total of 81) had cases of LF. It is reported that the disease was more prevalent in adults than children, and in males compared to females (Kron et al. 2000 ; Leonardo et al. 2020 ). Like other countries at risk for LF, the Philippines started to combat the disease using a compendium of MDA, morbidity management and prophylaxis from 2000. Before that, 40 million people were at risk of infection, and in 1998 the national prevalence rate of LF was 9.7 cases per 1000 population (Galvez Tan 2003 ; Kron et al. 2000 ; Rubite 2018 ). Five rounds of MDA were implemented in 38 endemic provinces. Following on from this achievement, the government hopes to eliminate LF in the country by 2020.
The Republic of Korea is documented to be free of LF (Cheun et al. 2009 ; Cheun et al. 2017 ). Official reports of LF in this country date back to 1927. Although W. bancrofti was initially identified as the culprit species, later B. malayi was determined to be the correct culprit (Senoo 1943 ). In the 1950s, it was reported that 12.1% of the population was positive for microfilaria caused by B. malayi (Senoo and Lincicome 1951 ). Later studies showed that the infection was remained present in Korea (Cheun et al. 2017 ); however, at present, the country is documented to be free of LF. This achievement was made possible by government initiatives including the installation of mosquito nets and sanitation in houses (Cheun et al. 2009 ). During a follow-up study conducted by Cheun et al., 83 patients with an earlier diagnosis of LF were surveyed, and no cases were detected in many of them, although 31 patients could not be traced for different reasons (Cheun et al. 2017 ).
Bangladesh still has not eliminated LF but has made significant progress towards this goal (Karim et al. 2019 ; Shamsuzzaman et al. 2017 ). An MDA program was successfully implemented in the country, when it was assumed that 70 million people were at risk of LF. The species involved was identified as W. bancrofti transmitted by Culex sp. In 2001, 34 out of 64 districts were endemic for microfilaremia, at a rate of 1 to 19% (Hafiz et al. 2015 ; Karim et al. 2019 ; Shamsuzzaman et al. 2017 ). Overall, 19 endemic areas successfully completed the MDA program while 15 others were excluded from MDA monitoring because of their low endemicity (WHO 2011 ). One recent study (Karim et al. 2019 ) detected 43,678 clinical cases in 19 highly endemic districts, including cases of leg and/or arm lymphedema, hydrocele, female breast lymphedema or genital swelling. It shows that the government should implement additional measures to eliminate the infection. In another study to detect clinical cases of LF in 30 villages of Nilphamari District in Bangladesh, among 1242 participants, 4.4% were found to have LF-related clinical conditions. The most frequent clinical manifestations were hydrocele in males and leg lymphedema in females (Hafiz et al. 2015 ).
In Myanmar, the prevalence of LF remains high. The species involved is W. bancrofti , transmitted by Culex quinquefasciatus (Aye et al. 2018 ; de Meillon et al. 1967 ). In accordance with the WHO, the government of Myanmar decided in 2020 to launch a program to eliminate LF (Research et al. 1998 ). As a result, it was found that about 41 million people (about 80% of the total population) were infected with LF in 45 of 65 districts. An MDA program was implemented in endemic areas. To appraise the degree of success, two studies were conducted in 2008 and 2014 (Aye et al. 2018 ; WHO 2004 ). The results did not show complete elimination, but varying degress of progress were made and in some areas, the outcome was significant. The rate of filarial antigen positivity ranged from 0 to > 25% (Aye et al. 2018 ). A recent study showed the overall prevalence of infection to be 2.63% based on antigenemia and 1.03% based on microfilaremia (Dickson et al. 2018 ). No cases of lymphedema were found among participants, but 2.78% of males showed hydrocele. The present situation demonstrates promising features. Satisfactory measurements have been implemented with positive perspectives for eliminating the disease in the near future.
In Turkey, single case of filariasis was reported in an 11-year-old girl in a southern region, with swelling in both her legs (Cengiz et al. 2006 ). The species of the parasite has not been reported. Her family were reported to be free from the disease.
Japan is another country in Asia that has successfully eliminated LF (Ichimori et al. 2007 ). The initiative to combat the infection was started in the 1970s and ended in 1999. The high level of cooperation among the population was an important factor in eliminating not only LF but also many other parasitic diseases.
In Oman, the LF situation presents no serious concerns. Only sporadic cases have been reported, most of which were imported. A study of 250 children aged 17 to 18 years in 2004 detected no positive cases (Al Awaidy et al. 2010 ), and the authors concluded that LF is nonendemic in Oman. In another study from 1999 to 2013, 5 cases of filariasis were reported, of which 4 cases were travel-associated infections. The type of filariasis was not reported in this study (Al-Abri et al. 2015 ).
An important factor which has a decisive influence on the prognosis of LF is climate change. As a vector-borne infectious disease, LF is considered among the parasitic diseases affected by climate change. Accordingly, it is expected that LF could readily spread to new areas and worsen the situation (Short et al. 2017 ). Soil and plant canopy moisture levels are factors which directly influence the distribution of LF because they affect mosquito larvae breeding sites (Thompson et al. 1996 ). Changing temperature and precipitation patterns will thus affect soil moisture levels and mosquito populations. In Africa, it is reported that based on the level of climate change, the population at risk of LF may increase from 543 to 804 million to as much as 1.65–1.86 billion by 2050 (Slater and Michael 2012 ).
A limitation of our study was that some articles not accessible. Although we used some abstracts to obtain a clear-cut picture of the LF situation in Asia, the lack of access to information in some full texts may somewhat compromise the integrity of the output. To offset this drawback as far as possible, we tried to review all 48 countries individually to ensure reliability.
We found no cases of LF in other countries in Asia, although other kinds of filariasis were reported (Negahban et al. 2007 ; Parsa et al. 2020 ; Reddy 2013 ; Rokni 2008 ; Simón et al. 2012 ).
After the WHO announced a major initiative to eliminate LF in 2000, considerable progress has been made. Many countries have succeeded in eliminating the disease in accordance with the goals set by the WHO, while other countries should still take additional steps. Our study shows that in general, the outcomes have been satisfactory, and measures recommended by the WHO were observed to an adequate extent. One disappointing aspect is that unfortunately the region encompasses many political and social issues in some countries, including immigration, disruption, and poverty. These problems hinder efforts to attain all the aims proposed by the WHO to eliminate the disease in all countries.
According to evaluations conducted by the WHO and some governments in the region, LF is on track to be controlled and eliminated, yet some important factors including climate changes and especially the deadly new disease caused by SARS-CoV-2 will undoubtedly affect future efforts. Increasing declines in financial and economic resources may foreseeably prevent further efforts to control and eradicate filariasis. In order to continue the fight against the disease and prevent its spread, it is necessary for health authorities to consider the following points:
- Regular training for health workers
- Systematic surveillance management
- Direct Network Report system
- Establishing new and effective diagnostic methods
- TAS and morbidity datasets should be developed for post-elimination surveillance strategies
- Long-term reporting of new cases
- Patient access to care (lymphedema management and hydrocele surgery)
- Patient outreach and identification activities
- Integration of LF clinical care into the primary health care system
- Establishing a system of travel health service, e.g., increasing physicians’ awareness of travel-associated infections and passenger inspection programs for countries with high LF endemicity due to high numbers of immigrants
- Eventually, five public health strategies recommended by the WHO to monitor neglected tropical diseases should be implemented by all involved countries: expansion of preventive chemotherapy, intensified case detection and case management, improved vector control, appropriate veterinary public health measures, and provision of safe water, sanitation, and hygiene.
Acknowledgments
The sincere cooperation of Dr A Alizadeh, is highly appreciated. We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for editing the English in the manuscript.
Availability of data and material
Authors’ contribution.
All authors contributed to the study conception and design. Material preparation and data collection were performed by Negar BIZHANI, Saeideh HASHEMI HAFSHEJANI1, and Mohammad Bager ROKNI. Analysis and graph preparation were performed by Neda MOHAMMADI and Mehdi Rezaei. The first draft of the manuscript was written by Mohammad Bager ROKNI and Negar BIZHANI. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The study was funded by a grant awarded by the National Institute for Medical Research Development, Ministry of Health and Medical Education, Iran (No. 977187).
Compliance with ethical standards
The authors declare that they have no conflicts of interest.
Not applicable
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Abdulcader MHM, Rajakone P, Rajendran K, Aponso L. Age, sex, and house distribution of Wuchereria bancrofti Microfilaremia in Ceylon. Am J Trop Med Hyg. 1966; 15 :519–522. doi: 10.4269/ajtmh.1966.15.519. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Addiss DG. Global elimination of lymphatic filariasis: addressing the public health problem. PLoS Negl Trop Dis. 2010; 4 :e741. doi: 10.1371/journal.pntd.0000741. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Agrawal VK, Sashindran VK. Lymphatic filariasis in India: problems, challenges and new initiatives. Med J Armed Forces India. 2006; 62 :359–362. doi: 10.1016/s0377-1237(06)80109-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmad N. Problems of filariasis with reference to post-war planning in India. J Indian Med Assoc. 1945; 14 :306–309. [ PubMed ] [ Google Scholar ]
- Al Awaidy ST, Bawikar S, Patel PK, Kurup P, Sonal GS, Al Mahrooqi S, Ramzy R. Absence of lymphatic filariasis infection among secondary-school children in Oman. East Mediterr Health J. 2010; 16 :1059–1063. doi: 10.26719/2010.16.10.1059. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Al-Abd NM, Nor ZM, Ahmed A, Al-Adhroey AH, Mansor M, Kassim M. Lymphatic filariasis in Peninsular Malaysia: a cross-sectional survey of the knowledge, attitudes, and practices of residents. Parasit Vectors. 2014; 7 :545. doi: 10.1186/s13071-014-0545-z. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Al-Abri SS, Abdel-Hady DM, Al Mahrooqi SS, Al-Kindi HS, Al-Jardani AK, Al-Abaidani IS. Epidemiology of travel-associated infections in Oman 1999-2013: a retrospective analysis. Travel Med Infect Dis. 2015; 13 :388–393. doi: 10.1016/j.tmaid.2015.08.006. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Al-Shaham AA, Sood S. Recurrent furunculosis as a cause of isolated penile lymphedema: a case report. J Med Case Rep. 2010; 4 :196. doi: 10.1186/1752-1947-4-196. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anonymous Major achievements and experience in filariasis control in the People’s Republic of China. National Technical Steering Group for Filariasis Control and Research. Chin Med J (Engl) 1991; 104 :446–453. [ PubMed ] [ Google Scholar ]
- Ashrafi K, Golchai J, Geranmayeh S. Human subcutaneous dirofilariasis due to Dirofilaria ( Nochtiella ) repens : clinically suspected as cutaneous fascioliasis. Iran J Public Health. 2010; 39 :105–109. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aye NN, Lin Z, Lon KN, Linn NYY, Nwe TW, Mon KM, Ramaiah K, Betts H, Kelly-Hope LA. Mapping and modelling the impact of mass drug adminstration on filariasis prevalence in Myanmar. Infect Dis Poverty. 2018; 7 :56. doi: 10.1186/s40249-018-0420-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cane L (2020) A bright future: eliminating lymphatic filariasis in Vietnam. https://medium.com/@RTI_INTL_DEV/a-bright-future-eliminating-lymphatic-filariasis-in-vietnam-776bae245c8c . Accessed 11 June 2020
- Cengiz N, Savaş L, Uslu Y, Anarat A. Filariasis in a child from southern Turkey: a case report. Turk J Pediatr. 2006; 48 :152–154. [ PubMed ] [ Google Scholar ]
- Chandrasena NT, Premaratna R, Samarasekera DS, de Silva NR. Surveillance for transmission of lymphatic filariasis in Colombo and Gampaha districts of Sri Lanka following mass drug administration. Trans R Soc Trop Med Hyg. 2016; 110 :620–622. doi: 10.1093/trstmh/trw067. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheun HI, Kong Y, Cho SH, Lee JS, Chai JY, Lee JS, Lee JK, Kim TS. Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol. 2009; 47 :323–335. doi: 10.3347/kjp.2009.47.4.323. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheun HI, et al. Follow-up study of patients previously diagnosed with lymphatic filariasis in Korea. Osong Public Health Res Perspect. 2017; 8 :421–424. doi: 10.24171/j.phrp.2017.8.6.10. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chu BK, Deming M, Biritwum NK, Bougma WR, Dorkenoo AM, el-Setouhy M, Fischer PU, Gass K, Gonzalez de Peña M, Mercado-Hernandez L, Kyelem D, Lammie PJ, Flueckiger RM, Mwingira UJ, Noordin R, Offei Owusu I, Ottesen EA, Pavluck A, Pilotte N, Rao RU, Samarasekera D, Schmaedick MA, Settinayake S, Simonsen PE, Supali T, Taleo F, Torres M, Weil GJ, Won KY. Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis. 2013; 7 :e2584. doi: 10.1371/journal.pntd.0002584. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Collaborators GDaH Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390 :1260–1344. doi: 10.1016/s0140-6736(17)32130-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Meillon B, Grab B, Sebastian A. Evaluation of Wuchereria bancrofti infection in Culex pipiens fatigans in Rangoon, Burma. Bull World Health Organ. 1967; 36 :91–100. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- De-Jian S, Xu-Li D, Ji-Hui D. The history of the elimination of lymphatic filariasis in China. Infect Dis Poverty. 2013; 2 :30. doi: 10.1186/2049-9957-2-30. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Department of Health Services (DoHS) T (2020) Kathmandu Lymphatic filariasis elimination. https://www.mohp.gov.np/eng/program/communicable-disease/lymphatic-fliariasis#:~:text=Lymphatic%20Filariasis%20(LF)%20is%20a,%3C1%25%20to%2039%25 . Accessed 7 June 2020
- Dickson BFR, Graves PM, Aye NN, Nwe TW, Wai T, Win SS, Shwe M, Douglass J, Bradbury RS, McBride WJ. The prevalence of lymphatic filariasis infection and disease following six rounds of mass drug administration in Mandalay Region, Myanmar. PLoS Negl Trop Dis. 2018; 12 :e0006944. doi: 10.1371/journal.pntd.0006944. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Moamly AA, El-Sweify MA, Hafez MA. Using the AD12-ICT rapid-format test to detect Wuchereria bancrofti circulating antigens in comparison to Og4C3-ELISA and nucleopore membrane filtration and microscopy techniques. Parasitol Res. 2012; 111 :1379–1383. doi: 10.1007/s00436-012-2870-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Epidemiology and Disease Control Division Teku K, Nepal (2018) Lymphatic filariasis elimination program Annual report (2017/18). http://www.edcd.gov.np/resources/download/lymphatic-filariasisannual-report-201718 . Accessed 4 June 2020
- Fang Y, Zhang Y. Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China. Infect Dis Poverty. 2019; 8 :66. doi: 10.1186/s40249-019-0578-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foo PK, Tarozzi A, Mahajan A, Yoong J, Krishnan L, Kopf D, Blackburn BG. High prevalence of Wuchereria bancrofti infection as detected by immunochromatographic card testing in five districts of Orissa, India, previously considered to be non-endemic. Trans R Soc Trop Med Hyg. 2011; 105 :109–114. doi: 10.1016/j.trstmh.2010.10.006. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Galvez Tan JZ. The elimination of lymphatic filariasis: a strategy for poverty alleviation and sustainable development - perspectives from the Philippines. Filaria J. 2003; 2 :12. doi: 10.1186/1475-2883-2-12. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- George S, Joy TM, Kumar A, Panicker KN, George LS, Raj M, Leelamoni K, Nair P. Prevalence of neglected tropical diseases (leishmaniasis and lymphatic filariasis) and malaria among a migrant labour settlement in Kerala, India. J Immigr Minor Health. 2019; 21 :563–569. doi: 10.1007/s10903-018-0767-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghasemi E, Shamsinia S, Taghipour A, Anvari D, Bahadory S, Shariatzadeh SA, Kordi B, Majidiani H, Borji H, Chaechi Nosrati M, Yousefi A, Shams M. Filarial worms: a systematic review and meta-analysis of diversity in animals from Iran with emphasis on human cases. Parasitology. 2020; 147 (9):909–921. doi: 10.1017/S003118202000058X. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ginandjar P, Saraswati LD, Suparyanto D, Sakundarno M, Supali T. The prevalence of lymphatic filariasis in elementary school children living in endemic areas: a baseline survey prior to mass drug administration in Pekalongan District-Indonesia. Iran J Public Health. 2018; 47 :1484–1492. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gutierrez Y, Misselevich I, Fradis M, Podoshin L, Boss JH. Dirofilaria repens infection in northern Israel. Am J Surg Pathol. 1995; 19 :1088–1091. doi: 10.1097/00000478-199509000-00014. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hafiz I, Graves P, Haq R, Flora MS, Kelly-Hope LA. Clinical case estimates of lymphatic filariasis in an endemic district of Bangladesh after a decade of mass drug administration. Trans R Soc Trop Med Hyg. 2015; 109 :700–709. doi: 10.1093/trstmh/trv084. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haleem A, Al Juboury M, Al Husseini H. Filariasis: a report of three cases. Ann Saudi Med. 2002; 22 :77–79. doi: 10.5144/0256-4947.2002.77. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hotez PJ, Ehrenberg JP. Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv Parasitol. 2010; 72 :31–53. doi: 10.1016/s0065-308x(10)72002-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ichimori K, Graves PM, Crump A. Lymphatic filariasis elimination in the Pacific: PacELF replicating Japanese success. Trends Parasitol. 2007; 23 :36–40. doi: 10.1016/j.pt.2006.11.005. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Iddawela D, Ehambaram K, Wickramasinghe S. Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka. Asian Pac J Trop Med. 2015; 8 :1022–1026. doi: 10.1016/j.apjtm.2015.11.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Irvine MA, Kazura JW, Hollingsworth TD. Understanding heterogeneities in mosquito-bite exposure and infection distributions for the elimination of lymphatic filariasis. Proc Biol Sci. 2018; 285 (1871):20172253. doi: 10.1098/rspb.2017.2253. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Iyengar MO. Filariasis in Thailand. Bull World Health Organ. 1953; 9 :731–766. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jamail M, Andrew K, Junaidi D, Krishnan AK, Faizal M, Rahmah N. Field validation of sensitivity and specificity of rapid test for detection of Brugia malayi infection. Trop Med Int Health. 2005; 10 :99–104. doi: 10.1111/j.1365-3156.2004.01334.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jamshidi A, Jamshidi M, Mobedi I, Khosroara M. Periocular dirofilariasis in a young woman: a case report. Korean J Parasitol. 2008; 46 :265–267. doi: 10.3347/kjp.2008.46.4.265. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH. How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis. 2010; 4 :e755. doi: 10.1371/journal.pntd.0000755. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karim MJ, Haq R, Mableson HE, Sultan Mahmood ASM, Rahman M, Chowdhury SM, Rahman A, Hafiz I, Betts H, Mackenzie C, Taylor MJ, Kelly-Hope LA (2019) Developing the first national database and map of lymphatic filariasis clinical cases in Bangladesh: another step closer to the elimination goals. PLoS Negl Trop Dis 13(7):e0007542. 10.1371/journal.pntd.000754213:e0007542 [ PMC free article ] [ PubMed ]
- Khan AM, Dutta P, Sarmah CK, Baruah NK, Das S, Pathak AK, Sarmah P, Hussain ME, Mahanta J. Prevalence of lymphatic filariasis in a tea garden worker population of Dibrugarh (Assam), India after six rounds of mass drug administration. J Vector Borne Dis. 2015; 52 :314–320. [ PubMed ] [ Google Scholar ]
- Khieu V, Or V, Tep C, Odermatt P, Tsuyuoka R, Char MC, Brady MA, Sidwell J, Yajima A, Huy R, Ramaiah KD, Muth S. How elimination of lymphatic filariasis as a public health problem in the Kingdom of Cambodia was achieved. Infect Dis Poverty. 2018; 7 :15. doi: 10.1186/s40249-018-0394-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kron M, Walker E, Hernandez L, Torres E, Libranda-Ramirez B. Lymphatic filariasis in the Philippines. Parasitol Today. 2000; 16 :329–333. doi: 10.1016/S0169-4758(00)01705-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leang R, Socheat D, Bin B, Bunkea T, Odermatt P. Assessment of disease and infection of lymphatic filariasis in Northeastern Cambodia. Trop Med Int Health. 2004; 9 :1115–1120. doi: 10.1111/j.1365-3156.2004.01311.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee J, Ryu JS. Current status of parasite infections in Indonesia: a literature review. Korean J Parasitol. 2019; 57 :329–339. doi: 10.3347/kjp.2019.57.4.329. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leonardo L, Hernandez L, Magturo TC, Palasi W, Rubite JM, de Cadiz A, Moendeg K, Fornillos RJ, Tabios IK, Mistica M, Fontanilla IK. Current status of neglected tropical diseases (NTDs) in the Philippines. Acta Trop. 2020; 203 :105284. doi: 10.1016/j.actatropica.2019.105284. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li CY, Chang YL, Lee YC. Human pulmonary dirofilariasis coexisting with intercostal neurilemmoma: a case report and literature review. J Formos Med Assoc. 2013; 112 :644–647. doi: 10.1016/j.jfma.2012.07.016. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mallawarachchi CH, Nilmini Chandrasena TGA, Premaratna R, Mallawarachchi S, de Silva NR. Human infection with sub-periodic Brugia spp. in Gampaha District, Sri Lanka: a threat to filariasis elimination status. Parasit Vectors. 2018; 11 :68. doi: 10.1186/s13071-018-2649-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mandal NN, Bal MS, Das MK, Achary KG, Kar SK. Lymphatic filariasis in children: age dependent prevalence in an area of India endemic for Wuchereria bancrofti infection. Trop Biomed. 2010; 27 :41–46. [ PubMed ] [ Google Scholar ]
- Meyrowitsch DW, Toan ND, Hao HT, Dan NT, Michael E. A review of the present status of lymphatic filariasis in Vietnam. Acta Trop. 1998; 70 :335–347. doi: 10.1016/S0001-706X(98)00037-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mukhopadhyay AK. Lymphatic filariasis in Andhra Pradesh Paper Mill Colony, Rajahmundry, India after nine rounds of MDA programme. J Vector Borne Dis. 2010; 47 :55–57. [ PubMed ] [ Google Scholar ]
- Mukhopadhyay AK, Patnaik SK, Babu PS. Status of lymphatic filariasis in parts of east Godavari district of Andhra Pradesh, India. J Vector Borne Dis. 2007; 44 :72–74. [ PubMed ] [ Google Scholar ]
- Negahban S, Daneshbod Y, Atefi S, Daneshbod K, Sadjjadi SM, Hosseini SV, Bedayat GR, Abidi H. Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates: a case report. Acta Cytol. 2007; 51 :567–570. doi: 10.1159/000325796. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nithikathkul C, Wannapinyosheep S, Saichua P, Nithikathkul M. Filariasis: the disease will come to be the problem of Thailand. JSHMR. 2006; 24 :6. [ Google Scholar ]
- Noordin R, Mohd Zain SN, Yunus MH, Sahimin N. Seroprevalence of lymphatic filariasis among migrant workers in Peninsular Malaysia. Trans R Soc Trop Med Hyg. 2017; 111 :370–372. doi: 10.1093/trstmh/trx062. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Omar MS. A survey of bancroftian filariasis among South-East Asian expatriate workers in Saudi Arabia. Trop Med Int Health. 1996; 1 :155–160. doi: 10.1111/j.1365-3156.1996.tb00021.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000; 5 :591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Parsa R, Sedighi A, Sharifi I, Bamorovat M, Nasibi S. Molecular characterization of ocular dirofilariasis: a case report of Dirofilaria immitis in south-eastern Iran. BMC Infect Dis. 2020; 20 :520. doi: 10.1186/s12879-020-05182-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pradhan SP, Shrestha I, Palikhey N, Uprety RP. Epidemiological study of lymphatic filariasis in Gokarna village development committee of Kathmandu valley during August and Septem. J Nepal Hlth Res Council. 1998; 2 :13–17. [ Google Scholar ]
- Rahmah N, Lim BH, Azian H, Ramelah TS, Rohana AR. Short communication: use of a recombinant antigen-based ELISA to determine prevalence of brugian filariasis among Malaysian schoolchildren near Pasir Mas, Kelantan-Thailand border. Trop Med Int Health. 2003; 8 :158–163. doi: 10.1046/j.1365-3156.2003.01004.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rajagopalan PK, Das PK, Subramanian S, Vanamail P, Ramaiah KD. Bancroftian filariasis in Pondicherry, south India: 1. Pre-control epidemiological observations. Epidemiol Infect. 1989; 103 :685–692. doi: 10.1017/s0950268800031083. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raju K, Jambulingam P, Sabesan S, Vanamail P. Lymphatic filariasis in India: epidemiology and control measures. J Postgrad Med. 2010; 56 :232–238. doi: 10.4103/0022-3859.68650. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rana Krishna J (2003) A brief study on the epidemiology of filariasis in Nepal. J Nepal Med Assoc 11:155–168. 10.31729/jnma.1561
- Rao RU, Samarasekera SD, Nagodavithana KC, Dassanayaka TDM, Punchihewa MW, Ranasinghe USB, Weil GJ. Reassessment of areas with persistent Lymphatic Filariasis nine years after cessation of mass drug administration in Sri Lanka. PLoS Negl Trop Dis. 2017; 11 :e0006066. doi: 10.1371/journal.pntd.0006066. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rao RU, Samarasekera SD, Nagodavithana KC, Goss CW, Punchihewa MW, Dassanayaka TDM, Ranasinghe USB, Mendis D, Weil GJ. Comprehensive assessment of a hotspot with persistent Bancroftian filariasis in coastal Sri Lanka. Am J Trop Med Hyg. 2018; 99 :735–742. doi: 10.4269/ajtmh.18-0169. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reddy MV. Human dirofilariasis: an emerging zoonosis. Trop Parasitol. 2013; 3 :2–3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Research UNWBWSPf, Training in Tropical D, Mapping WUJPfH, World Health Organization. Division of Tropical D . Research on rapid geographical assessment of Bancroftian filariasis. Geneva: World Health Organization; 1998. [ Google Scholar ]
- Rojanapanus S, Toothong T, Boondej P, Thammapalo S, Khuanyoung N, Santabutr W, Prempree P, Gopinath D, Ramaiah KD. How Thailand eliminated lymphatic filariasis as a public health problem. Infect Dis Poverty. 2019; 8 :38. doi: 10.1186/s40249-019-0549-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008; 102 :283–295. doi: 10.1179/136485908x300805. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rubis P, Chang MS, Nagum AJ, Jau JL. Parasitological and entomological studies on filariasis in seven villages, Serian District, Sarawak, East Malaysia. Southeast Asian J Trop Med Public Health. 1981; 12 :30–35. [ PubMed ] [ Google Scholar ]
- Rubite JM (2018) Initiatives on NTDs in the Philippines. In: the First ASEAN LF Forum, Manila, Philippines, 11-12 July, 2018
- Sabesan S, Raju KH, Subramanian S, Srivastava PK, Jambulingam P. Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis. 2013; 13 :657–665. doi: 10.1089/vbz.2012.1238. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Senoo T. Detection of microfilaria malayi brug in Korea. Nippon Kiseichu Gakkai Kiji. 1943; 15 :36. [ Google Scholar ]
- Senoo T, Lincicome RD. Malayan filariasis; incidence and distribution in Southern Korea. U S Armed Forces Med J. 1951; 2 :1483–1489. [ PubMed ] [ Google Scholar ]
- Ruel E. Serrano, Tmong Udui, Vu Lam Binh, Morel E (2020) Three more countries eliminate lymphatic filariasis. https://www.who.int/westernpacific/news/detail/08-10-2018-three-more-countries-eliminate-lymphatic-filariasis . Accessed 11 June 2020
- Shamsuzzaman AK, et al. The significant scale up and success of Transmission Assessment Surveys ‘TAS’ for endgame surveillance of lymphatic filariasis in Bangladesh: one step closer to the elimination goal of 2020. PLoS Negl Trop Dis. 2017; 11 :e0005340. doi: 10.1371/journal.pntd.0005340. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sherchand JB, Obsomer V, Thakur GD, Hommel M. Mapping of lymphatic filariasis in Nepal. Filaria J. 2003; 2 :7. doi: 10.1186/1475-2883-2-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Short EE, Caminade C, Thomas BN. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis (Auckl) 2017; 10 :1178633617732296. doi: 10.1177/1178633617732296. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E, Montoya-Alonso JA. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev. 2012; 25 :507–544. doi: 10.1128/cmr.00012-12. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slater H, Michael E. Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS One. 2012; 7 :e32202. doi: 10.1371/journal.pone.0032202. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solgi R, Sadjjadi SM, Mohebali M, Djadid ND, Raz A, Zakeri S, Zarei Z. Susceptibility of Anopheles stephensi (Diptera: Culicidae) to Dirofilaria immitis (Spirurida: Onchocercidae) Russ J Nematol. 2017; 25 (2):121–127. doi: 10.24411/0869-6918-2017-00005. [ CrossRef ] [ Google Scholar ]
- Srividya A, Michael E, Palaniyandi M, Pani SP, Das PK. A geostatistical analysis of the geographic distribution of lymphatic filariasis prevalence in southern India. Am J Trop Med Hyg. 2002; 67 :480–489. doi: 10.4269/ajtmh.2002.67.480. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sun DJ, Chen PL. Filariasis surveillance at the post-control stage in China. Southeast Asian J Trop Med Public Health. 1992; 23 :369–376. [ PubMed ] [ Google Scholar ]
- Supali T, et al. High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. Am J Trop Med Hyg. 2002; 66 :560–565. doi: 10.4269/ajtmh.2002.66.560. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tavakolizadeh S, Mobedi I. Orbital dirofilariasis in Iran: a case report. Korean J Parasitol. 2009; 47 :397–399. doi: 10.3347/kjp.2009.47.4.397. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thompson DF, Malone JB, Harb M, Faris R, Huh OK, Buck AA, Cline BL. Bancroftian filariasis distribution and diurnal temperature differences in the southern Nile delta. Emerg Infect Dis. 1996; 2 :234–235. doi: 10.3201/eid0203.960313. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Toothong T, Tipayamongkholgul M, Suwannapong N, Suvannadabba S. Evaluation of mass drug administration in the program to control imported lymphatic filariasis in Thailand. BMC Public Health. 2015; 15 :975. doi: 10.1186/s12889-015-2325-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Torgerson PR, de Silva NR, Fèvre EM, Kasuga F, Rokni MB, Zhou XN, Sripa B, Gargouri N, Willingham AL, Stein C. The global burden of foodborne parasitic diseases: an update. Trends Parasitol. 2014; 30 :20–26. doi: 10.1016/j.pt.2013.11.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Triteeraprapab S, Karnjanopas K, Pruksakorn C, Sai-Ngam A, Yentakam S, Loymak S. Lymphatic filariasis caused by Brugia malayi in an endemic area of Narathiwat Province, southern of Thailand. J Med Assoc Thailand = Chotmaihet Thangphaet. 2001; 84 (Suppl 1):S182–S188. [ PubMed ] [ Google Scholar ]
- Upadhyayula SM, Mutheneni SR, Kadiri MR, Kumaraswamy S, Nagalla B. A cohort study of lymphatic filariasis on socio economic conditions in Andhra Pradesh, India. PLoS One. 2012; 7 :e33779. doi: 10.1371/journal.pone.0033779. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Urbani C (1997) Control of schistosomiasis and other helminthiasis in Cambodia. Me´decins Sans Frontie`res Switzerland, internal report n/d
- Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in Southeast Asia diversity, potential for control and prospects for elimination. Adv Parasitol. 2010; 72 :1–30. doi: 10.1016/s0065-308x(10)72001-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- WHO (2004) Report on the mid-term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec 79:457–468. http://www.who.int/neglected_diseases/resources/who_wer7940/en/ . Accessed 17 June 2020 [ PubMed ]
- WHO (2011) Global programme to eliminate lymphatic filariasis: a manual for National Elimination Programmes (Monitoring and Epidemiological Assessment of Mass Drug Administration). http://www.who.int/lymphatic_filariasis/resources/9789241501484/ . Accessed 14 Jun2 2020
- WHO (2013) Towards eliminating lymphatic filariasis: progress in the South-East Asia Region (2001–2011).
- WHO Global programme to eliminate lymphatic filariasis: progress report, 2013. Wkly Epidemiol Rec. 2014; 89 :409–418. [ PubMed ] [ Google Scholar ]
- WHO (2016) Maldives and Sri Lanka eliminate lymphatic filariasis. http://www.searo.who.int/mediacentre/releases/2016/1626/en/ . Accessed 3 June 2020
- WHO (2020) Neglected tropical diseases. https://www.who.int/neglected_diseases/diseases/en/ . Accessed 3 June 2020
- Wibawa T, Satoto TBT. Magnitude of neglected tropical diseases in Indonesia at postmillennium development goals era. J Trop Med. 2016; 2016 :5716785–5716789. doi: 10.1155/2016/5716785. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yap LF, Ramachandran CP, Balasingam E. A parasitological study of Pulau Pinang and Pulau Perhentian Kechil, off Trengganu, West Malaysia. I. Malaria and filariasis. Med J Malaya. 1968; 23 :118–122. [ PubMed ] [ Google Scholar ]
- Open access
- Published: 18 January 2022
Scaling-up filariasis lymphoedema management into the primary health care system in Kerala State, Southern India: a case study in healthcare equity
- Suma T. Krishnasastry ORCID: orcid.org/0000-0003-2305-3845 1 ,
- Charles D. Mackenzie 2 &
- Rajeev Sadanandan 3
Infectious Diseases of Poverty volume 11 , Article number: 9 ( 2022 ) Cite this article
3862 Accesses
2 Citations
5 Altmetric
Metrics details
Lymphatic filariasis (LF) remains one of the world’s most debilitating parasitic infections and is a major contributor to poor health in many endemic countries. The provision of continuing care for all those affected by LF and its consequences is an important component of the United Nations’ Sustainable Development Goals. The aim of this study is to integrate lymphedema care into the primary health care system of the State by developing lymphedema clinics at each district, through training of health personnel to fulfill WHO recommendation for morbidity management and disability prevention.
Selected health care providers from all the districts in Kerala State of India participated in intensive training sessions endorsed by the State’s health administration. The six training sessions (from 5 June 2017 to 25 May 2018) included appropriate self-care information and development of individual plans for each participating institution to provide instruction and care for their lymphoedema patients. The learning achieved by attendees was assessed by pre- and post-training tests. The number of lymphoedema patients receiving care and instruction from the post-training activities of each participating institution was assessed from local records, 6 months after the conclusion of the training sessions.
One hundred and eighty-four medical personnel (91 doctors and 93 nurses) from 82 medical institutions were trained which quickly led to the establishment of active lymphoedema clinics providing the essential package of care (EPC) for lymphoedema patients at all the participating institutions. Six months after the training sessions the number of previously unidentified lymphoedema patients registered and receiving care at these clinics ranged from 296 to almost 400 per clinic, with a total of 3,477 new patients receiving training in EPC.
Conclusions
Generalist health personnel, when appropriately trained, can provide quality lymphoedema care in public health settings and patients when provided services close to their home, are willing to access them. This is a feasible strategy for integrating long term care for LF patients into the national health system, and is a clear example of moving towards equity in health care for the medically underserved, and thus successfully addresses a major goal of the global program to eliminate lymphatic filariasis.
Graphical Abstract
Lymphatic filariasis (LF) remains one of the world’s most debilitating parasitic infections and is a major contributor to poor health as the second most common global cause of physical disability . It is estimated that there are 450 million infected and affected people in India, accounting for 40% of the global human LF infections, with a further estimated 450 million population still ‘at risk’of infection [ 1 , 2 , 3 , 4 ] . In 2000, in India around 7.44 million were suffering from LF-induced lymphoedema and approximately 12.88 million were suffering from hydrocele [ 5 ]. In 1997 the World Health Assembly targeted LF for global elimination of infection as a public health problem [ 6 ]. This initiative includes alleviating the suffering in those who already have the disease through Morbidity Management and Disability Prevention (MMDP). In addition to providing an “essential package of care” (EPC) to those suffering from LF [ 7 , 8 ], there is the important additional recommendation for the integration of this medical support into the public health system of the endemic country. Such a goal is also in line with the global goals set out by the United Nations’ Sustainable Develop Goals (SDG) [ 9 ]. Such integration has often been a challenge for many LF endemic countries but has been an important goal for the filariasis program in highly endemic Kerala State, Southern India.
The major aim in the integration efforts in Kerala was to ensure the availability of the WHO recommended EPC for LF patients in all areas of the State, with the aim of providing 100% geographical coverage of the EPC for LF patients, and that at least one health facility designated for MMDP services per global program to eliminate lymphatic filariasis (GPELF) implementation unit (IU). In addition, as advised by WHO, these services should be provided at the appropriate level of the government health system, and be of good quality. This current communication describes the activities carried to fulfill this integration efforts and the successes achieved in addressing this important health initiative in this filariasis endemic region of India.
Study population
Kerala State in south India has a population of approximately 34.7 million people and 11/14 districts are known to be endemic for lymphatic filariasis (all districts except Pathanamthitta, Idukki and Wyanad). Although GPELF was officially launched by WHO in 2000, MDA was first started in Alappuzha, Kozhikode and Kannur districts in 1997. Subsequently, the MDA activities were extended to all 11 endemic districts with diethylcarbamazine and albendazole being given in a total of eight annual rounds of MDA, the last being carried out in 2012. Currently MDA is continuing only in certain areas of one district (Malappuram) where the infection has persisted.
Training procedures
The Director of Health Services for Kerala State selected specific health facilities in every district (i.e., 14 districts), which included the three non-endemic districts as patients with LF clinical disease have been reported to reside in these districts; doctors and nurses from these health facilities were then selected for the training. The training was given to a total of 184 health care providers, in 6 sessions of 3 days each, starting on 5 June 2017 till 25 May 2018. The training was carried out by the staff of the Filariasis Research Unit at the Government TD Medical College Hospital, Alappuzha, Kerala. The number of health care providers trained and the dates of the training events are given in Table 1 .
The training content followed the WHO Certified Training Module which encompasses components detailing the general background, as well as clinical and programmatic, aspects of LF. The learning objectives of the training sessions for the attendees were to: (1) to understand the background, the requirements and current status of GPELF; (2) to understand LF clinical disease focusing on lymphoedema, hydrocele, and acute attacks [adenolymphangitis (ADL)]; also to be aware of the recommended essential package of care; and to (3) learn details concerning the implementation of GPELF including situation analysis, selection and development of health facility to impart MMDP services, the documentation and reporting of services provided, and assessment of quality of services given by the health facility.
The longstanding WHO Filariasis Research Center in the Government TD Medical College of Alappuzha with considerable past experience in training, worked with Kerala’s health Administration to develop a comprehensive solid, state wide, system of specialized care for LF lymphoedema patients. The training of the medical staff occurred through organized, structured sessions over 6 months and included information, hands-on demonstrations and the development of specific LF lymphoedema care plans for the trainees’ hospitals (Figs. 1 , 2 ).
Flow diagram of procedures, major activities and outcomes activities in the Kerala Program

Training of medical staff in lymphoedema care. A Opening of training session. B Staff developing lymphoedema care plans for their individual institutions. C Direct hands-on experience for the medical staff in providing care to lymphoedema affected individuals
Assessment of activities and outcome
Each training session included interactive sessions, photo quizzes, as well as direct interaction with lymphoedema patients. Participants were instructed in taking clinical history, carrying out a physical examination, and given hands-on-training on limb hygiene and other details of lymphedema management. The participants were divided into groups according to their institutions and each group given the task of developing a proposal for initiating MMDP services at their own hospitals. The institutional plans that each group of participants developed during the training sessions were assessed for quality and content.
At the beginning of each of the six training sessions every attendee was administered a twenty-item questionnaire with questions covering both programmatic and clinical elements. The same questions were asked again at the conclusion of each training session. A few attendees (an overall total of 8 across all sessions) did not complete both tests. Throughout the training period regular conversations were held with the participants to obtain their opinions of the training and on any challenge, they might have had in implementing the instructions and advice they received.
The number of medical facilities that instituted a lymphoedema care system as result of attending the training sessions was assessed 1 year after the end of training sessions. The number of patients who received instruction in the package of self-care from these clinics at 6 months after the training session was also determined by survey of all participating institutions.
Training sessions
One hundred and eighty-four medical personnel (91 doctors and 93 staff nurses) from 82 medical institutions were trained which led to the immediate establishment of lymphoedema clinics at all these institutions to provide the EPC for these patients (Tables 1 , 2 ). Interviews with all the participants in all the six different sessions found that they were very enthusiastic about the training sessions and the included activities; participants were especially satisfied with the development, as part of the training program, of quality institutional proposals for starting and carrying out MMDP services for their own patients suffering from LF.
Feed back on the effectiveness of sessions
The structured questionnaire asked of the 144 attendees included questions related to the instructors/facilitators, to the organisation, and as well the opportunity to suggest changes/improvements in the format of the training. Comments about the instructors were all positive and no negative comments were made; those regarding the organisation were almost exclusively positive with an only a few minor comments related to logistic issues (e.g., “dinner should be provided”); a range of useful comments were made regarding improving the training session (e.g., providing certificates, involving the participants more actively, better infrastructural facilities). In summary, the vast majority of comments responding to the post-training interviews underscored the positive nature of the training sessions.
Development of proposals for starting lymphedema clinics
A key component of the training sessions was the development of specific proposal for training and patient care in the respective home bases of the participants. These proposals included appraising their superintendents and district medical officers (DMOs) about the need for initiating the MMDP services, the training of the other health care providers in their own hospitals, carrying out infrastructure modifications, procuring the necessary materials for management of lymphedema and acute attacks, i.e., antibiotics and other drugs, antiseptic, anti-fungal ointments, and the acquisition of the necessary materials for limb hygiene measures. These plans also included introduction of appropriate information, education and communication (IEC) activities and maintenance of records and documents, as well planning for regular documentation and reporting on their ongoing activities.
Support from State Government medical administration
An important factor in the success of these training sessions was the support from the State Health Administration. A policy decision was taken by the government that Kerala state should achieve LF elimination and MMDP in addition to MDA, was to be a key component of that strategy. To provide this in all parts of the State a team of health care providers—a doctor and staff nurse—from each Taluk Head Quarters hospitals should be given training in LF MMDP. These teams would then train others in their respective facilities, and thus ensure a wide distribution of the needed services across the state. The second example of important administrative support for the training activities was the attendance of high-level government officials at the opening of each session. This underscored the importance placed by the State Government in these training sessions.
Assessment of learning
A comparison of the pre-training tests with the post training ones was completed, showed a very high degree of learning achieved by virtually all attendees. Figure 3 shows the pre-training and post-training scores obtained by the attendees at each session who completed both the tests. Before training the number of questions answered correctly across the six sessions ranged from an average of 9.4 (with a range between groups of 8.6–10.6 of the 20 questions answered correctly; after training this improved to 16.7 (16.1–20.0). Thus, across all the training sessions there was almost twice as many questions being answered correctly after training, and in each group, there were attendees who got all questions correct or only answered one or two questions incorrectly. The institutional planning projects were all examined and found to be of high quality and to contain all the major needed components for implementation.
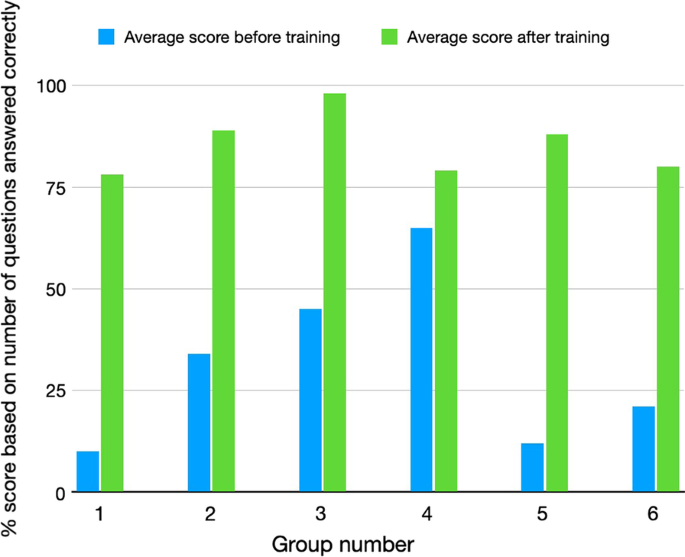
Comparison of the pre-test and post-test scores for 20 questions asked of each participant in each of the 6 different training session groups
Post training activities
Mmdp clinics.
On returning to their posts after the training session, the participants had discussions with their superintendents and DMOs, organized training program for doctors, nurses and health care workers and carried out various IEC activities. Importantly the State Government allocated funds for the LF MMDP activities in every district as part of the SDG program and now all the districts with clinical LF have started MMDP clinics. Twelve months after the training sessions were completed a total of 82 MMDP clinics which had been started in the state as part of the overall SDG program with an average of 5 facilities per district.
Increases in patients receiving care
The training of the medical staff occurred through organized structured sessions over 6 months that included information, hands-on demonstrations and the development of specific LF lymphoedema care plans for the trainees’ home hospitals and medical centers (Fig. 1 ).
In the following 6 months following the training the number of previously unidentified lymphoedema patients registered and receiving care at these clinics ranged from 296 to almost 400 per clinic, with a total of 3,477 new patients receiving the EPC during this period.
The impairment and disability resulting from the LF-associated lymphoedema, elephantiasis and hydrocele cause a significant public health problem. However, there are relatively simple medical interventions that can address these problems and assist patients. These simple interventions that are central to the training and dissemination of care for those suffering from lymphoedema, and they form the central core of training systems described here. These interventions include treatment for acute attacks (ADL), the reduction in the frequency and severity of ADL with simple hygiene measures, such as washing and basic skin care; which assist in preventing progression of the lymphoedema to the stage of elephantiasis. The currently recommended treatment for bouts of ADL is administration of antibiotics and other supportive measures. The hygiene measures used include: washing the affected parts twice daily with soap and clean water at room temperature and drying carefully with a clean cotton cloth (especially between the toes); maintaining clean nails and treating interdigital lesions (usually with anti-fungal creams); avoiding ‘entry’ lesions through using proper footwear and the use of antiseptic or antibiotic creams to treat small wounds or abrasions. For management of hydrocele, surgical intervention with a hydrocelectomy is the standard option. This is usually a relatively uncomplicated surgical intervention if carried out using adequate surgical procedures and appropriate pre- and post-surgery management.
Prior to the initiation of the GPELF those affected by the lymphedema induced by this parasitic infection, and who suffer the consequent disability and compromised quality of life, have often been unable to obtain the needed care except in a few specialised clinics in concerned countries, such as India, Sri Lanka and Brazil [ 3 , 11 , 12 , 13 ]. The dissemination of the appropriate care to all patients affected, as the current description of the Kerala Program shows, can occur through well designed and implemented training activities. Success in fulfilling this requirement of the global LF Program by provide care for all those clinically affected with LF is an example of progress towards restoring health equity. This case study demonstrated how equity for a group of people who have, at least until recently, largely been ignored, can be achieved by a set of well designed, simple measures. It is vital that LF patients suffering from lymphoedema obtain the care as defined by GPELF so that these patients have a fair opportunity to reach their full health potential through the provision of care [ 6 ]. This requires that the appropriate care they need is available, not only through specialised clinics, but also thorough the national public health system of the endemic area, as has been achieved here in Kerala.
Arguably the two most important factors to the success of this overall venture were, firstly the policy decision (SDG activities) and support of government who saw this as an essential ingredient to eliminate LF and secondly augmenting the medical skills and capability of clinical staff. These two factors have often been difficult to achieve in many endemic countries, although those that have managed to incorporate these components have often achieved success. It is, in general, logically better to follow WHO recommendation to carry out a situational analysis and assessment of local disease LE burden before including specific health facilities in training sessions; however, here in Kerala this was not done, and the inclusion of a particular health facility in training sessions was based on local knowledge of the presence of these patients in the area. It is important to note that the attendees of the training sessions indicated that they had not previously been taking adequate care of LF patients due to a lack of awareness of the required procedures and a lack of facilities. Following the training, the attendees realised that with their newfound knowledge they are now capable of providing quality care to LF patents through simple and affordable measures, which actively improved the quality of life of their patients. It was reported that direct interaction with LF patients and the hands-on activities during training gave them confidence to manage these neglected patients, to become champions for LF care, and to be able to train other health workers in their medical institutions.
The activities presented in this paper, with the training of 184 medical personnel from 82 institutions, are an example of successful integration of healthcare into the public health system in an endemic area. Over 3 years the longstanding WHO Filariasis Center in the Government TD Medical College of Alappuzha, Kerala State, worked with Kerala state government medical officials to develop a solid state wide system of integrating specialised care for LF lymphoedema patients in a public health system, and provided a strong example of achieving equity in healthcare. This is one of first reports of such initiatives in the published literature. It is also a major step towards achieving the goals of GPELF for the people of Kerala, which became the first state in India to achieve this integration of LE care into the public health system, and arguably one of the first amongst all global endemic countries. The approaches taken here are likely to be useful in other countries and Indian states. Kerala’s success in imparting MMDP services to LF patients throughout Kerala is being recognized by the international NTD community as the “Kerala story”.
This Kerala approach to increasing the provision of support for lymphoedema self-care has demonstrated that generalist health personnel, when appropriately trained, can provide quality lymphoedema care in public health settings, and that patients, when provided services close to their home, are willing to access these services. The key factors in achieving this success in Kerala, which are likely applicable to many situations across the LF endemic world, were: (1) Engaging the support and involvement of senior government officials from the Health and Family Welfare Department. (2) Ensuring the engagement of medical staff from all endemic districts. (3) Participatory training sessions with both active practical and planning components.
This approach, a feasible strategy for integrating long term care for LF patients into a national health system, is an example of moving towards equity in health care for the medically underserved, and successfully addresses an essential target of GPELF.
Availability of data and materials
All data included in this manuscript is available from the primary institute.
Abbreviations
District medical officer
Information, education and communication
- Lymphatic filariasis
- Lymphoedema
Global program for the elimination of lymphatic filariasis
Morbidity management and disability prevention
Essential care package
Disease management and disability inclusion
Adenolymphangitis
Neglected tropical diseases
Sustainable development goals
World Health Organization. Global programme to eliminate lymphatic filariasis: progress report 2014. Wkly Epidemiol Rec. 2015;90(38):489–504.
Google Scholar
Shenoy RK, Suma TK, Rajan K, Kumaraswami V. Prevention of acute adenolymphangitis in brugian filariasis: comparison of the efficacy of ivermectin and diethylcarbamazine, each combined with local treatment of the affected limb. Ann Trop Med Parasitol. 1998;92(5):587–94. https://doi.org/10.1080/00034989859285 .
Article CAS PubMed Google Scholar
Suma TK, Shenoy RK, Kumaraswami V. Efficacy and sustainability of a foot-care programme in preventing acute attacks of adenolymphangitis in Brugian filariasis. Trop Med Int Health. 2002;7(9):763–6. https://doi.org/10.1046/j.1365-3156.2002.00914.x .
Cromwell EA, Schmidt CA, Kwong KT, Pigott DM, Mupfasoni D, Biswas G, et al. The global distribution of lymphatic filariasis, 2000–18: a geospatial analysis. Lancet Glob Health. 2020;8(9):e1186–94. https://doi.org/10.1016/S2214-109X(20)30286-2 .
Article Google Scholar
Ramaiah KD, Das PK, Michael E, Guyatt H. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16(6):251–3. https://doi.org/10.1016/s0169-4758(00)01643-4 .
World Health Assembly. WHA50.29: elimination of lymphatic filariasis as a public health problem. Geneva: World Health Organization, 1997.
Mackenzie CD, Mante S. Caring for patients in the global programme to eliminate lymphatic filariasis. Int Health. 2020;13(Suppl 1):S48–54. https://doi.org/10.1093/inthealth/ihaa080 .
Article PubMed PubMed Central Google Scholar
World Health Organisation. Lymphatic filariasis. An aide memoire for LF program managers. Second edition. WHO/HTM/NTD/PCT/2016. Web Annex A. Protocol for evaluating minimum package of care of morbidity management and disability prevention for lymphoedema management in designated health facilities. 2021 https://apps.who.int/iris/bitstream/handle/10665/339870/9789240017085-eng.pdf . Accessed on 03 Oct 2021.
WHO. Towards a monitoring framework with targets and indicators for the health goals of the post-2015 Sustainable Development Goals. Draft, WHO, 2015.
Addiss DG, Brady MA. Morbidity management in the global programme to eliminate lymphatic filariasis: a review of the scientific literature. Filaria J. 2007;6:2. https://doi.org/10.1186/1475-2883-6-2 .
Shenoy RK, Suma TK, Kumaraswami V. A qualitative study on the feasibility and benefits of foot hygiene measures practiced by patients with Brugian filariasis. J Commun Dis. 2003;35(1):9–16.
CAS PubMed Google Scholar
PAHO. Brazil moves towards the elimination of the transmission of Lymphatic Filariasis. https://www3.paho.org/hq/index.php?option=com_content&view=article&id=9399:2014-brasil-avanza-hacia-eliminacion-transmision-filariasis-linfatica&Itemid=40264&lang=en . Accessed on 03 Oct 2021.
Chandrasena N, Premaratna R, Gunaratna IE, de Silva NR. Morbidity management and disability prevention for lymphatic filariasis in Sri Lanka: current status and future prospects. PLoS Negl Trop Dis. 2018;12(5): e0006472. https://doi.org/10.1371/journal.pntd.0006472 .
Download references
Acknowledgements
We would like to thank all the different team members involved in this initiative, especially the government officials, the clinical officers, the nurses, and the patients.
All the Kerala based activities described in this manuscript (planning, advocacy, training and implementation) were supported by the Government of Kerala through the Department of Health and Family Welfare, and by the Govt. TD Medical College, Alappuzha. In addition, the publication of the completed study was supported by the Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD), which is funded at The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation (OPP1190754), by UK aid from the British government, and by the United States Agency for International Development through its Neglected Tropical Diseases Program. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Author information
Authors and affiliations.
Filariasis Research Unit, WHO Collaborating Center for LF MMDP, Lymphatic Filariasis Morbidity Management and Disability Prevention, Department of Internal Medicine, Govt. T. D. Medical College Hospital, Kerala University of Health Sciences, Alappuzha, 688005, India
Suma T. Krishnasastry
NTD Support Center, Task Force for Global Health, Atlanta, GA, 30030, USA
Charles D. Mackenzie
Health Systems Transformation Platform, New Delhi, 110070, India
Rajeev Sadanandan
You can also search for this author in PubMed Google Scholar
Contributions
SK carried out the planning and implementation of the overall activities, wrote the original draft of the manuscript, and co-authored the final manuscript. CM analysed data, formulated and co-authored the final manuscript. RS contributed to the design and implementation of the training programme, and contributed to the final manuscript from health policy maker’s perspective. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Suma T. Krishnasastry .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval is not required for medical trading activities and their assessment.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
Supplementary Information
Additional file 1:.
Objectives and description of various sessions on LF MMDP training in Kerala.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Krishnasastry, S.T., Mackenzie, C.D. & Sadanandan, R. Scaling-up filariasis lymphoedema management into the primary health care system in Kerala State, Southern India: a case study in healthcare equity. Infect Dis Poverty 11 , 9 (2022). https://doi.org/10.1186/s40249-022-00936-6
Download citation
Received : 04 October 2021
Accepted : 04 January 2022
Published : 18 January 2022
DOI : https://doi.org/10.1186/s40249-022-00936-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health equity
- Global program to eliminate lymphatic filariasis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Nuanced interventions and new tools, transitioning to monitoring and surveillance, considering costs and other resources, new challenges and new opportunities, new tools and nuanced interventions to accelerate achievement of the 2030 roadmap for neglected tropical diseases.
Potential conflicts of interest. The authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
- Article contents
- Figures & tables
- Supplementary Data
Andreia Vasconcelos, Cláudio Nunes-Alves, T Déirdre Hollingsworth, New Tools and Nuanced Interventions to Accelerate Achievement of the 2030 Roadmap for Neglected Tropical Diseases, Clinical Infectious Diseases , Volume 78, Issue Supplement_2, 15 May 2024, Pages S77–S82, https://doi.org/10.1093/cid/ciae070
- Permissions Icon Permissions
The World Health Organization roadmap for neglected tropical diseases (NTDs) sets out ambitious targets for disease control and elimination by 2030, including 90% fewer people requiring interventions against NTDs and the elimination of at least 1 NTD in 100 countries. Mathematical models are an important tool for understanding NTD dynamics, optimizing interventions, assessing the efficacy of new tools, and estimating the economic costs associated with control programs. As NTD control shifts to increased country ownership and programs progress toward disease elimination, tailored models that better incorporate local context and can help to address questions that are important for decision-making at the national level are gaining importance. In this introduction to the supplement, New Tools and Nuanced Interventions to Accelerate Achievement of the 2030 Roadmap for Neglected Tropical Diseases, we discuss current challenges in generating more locally relevant models and summarize how the articles in this supplement present novel ways in which NTD modeling can help to accelerate achievement and sustainability of the 2030 targets.
Neglected tropical diseases (NTDs) are a group of 21 conditions caused by a range of bacteria, fungi, parasites, viruses, and toxins that affect more than 1 billion people worldwide [ 1 ]. The epidemiology of NTDs is diverse and complex, often characterized by intricate pathogen life cycles, vector-borne transmission and/or the involvement of intermediary hosts, and dependence on environmental conditions, all of which present substantial challenges to disease control and elimination [ 2 ]. Despite this heterogeneity, NTDs share common features in their geographical distribution and socioeconomic impact. Affecting predominantly tropical regions worldwide, 80% of the NTD burden is concentrated in just 16 countries [ 1 ]. Furthermore, these diseases disproportionately affect resource-poor communities, where they further accentuate economic hardship and perpetuate cycles of poverty.
Over the past decade, significant progress has been made in the control, elimination, and eradication of these diseases, driven by the 2012–2020 World Health Organization (WHO) roadmap for accelerating work to overcome the global impact of NTDs [ 3 ]. Notably, the number of people who require NTD interventions globally decreased by 600 million between 2010 and 2020, and 42 countries, areas, and territories successfully eliminated at least 1 NTD [ 1 ]. However, many of the 2020 targets were not achieved, prompting WHO to launch the 2021–2030 roadmap that revises disease-specific targets and outlines 3 pillars deemed essential for achieving them: accelerating programmatic action, intensifying cross-cutting approaches, and facilitating country ownership through changes in operating models and culture [ 4 ]. Importantly, the 2030 roadmap also aligns NTD targets with the United Nations’ Sustainable Development Goals, representing a step change in policy recognition toward the importance of eliminating these diseases.
To achieve the 2030 targets, national NTD programs require relevant and accurate information that can effectively guide decision-making. This is becoming increasingly important as national health ministries are faced with diverse challenges, such as locally increased transmission rates that resulted from program interruptions due to the coronavirus disease 2019 (COVID-19) pandemic, donor funding cuts, reduced domestic revenues, climate change, and, in some cases, geopolitical instability. In this context, quantitative analysis and epidemiological and economic modeling are important tools for better understanding and forecasting the dynamics of NTDs, informing the design of optimally effective intervention and surveillance strategies, and facilitating the evaluation and implementation of new tools. For example, the NTD modeling community was consulted during the formulation of the 2030 targets [ 5 ] and, more recently, partnered with WHO to estimate how disruptions due to the COVID-19 pandemic impacted NTD programs and assess how remedial strategies could help program recovery [ 6 , 7 ].
Despite these successes, the NTD modeling community is facing new challenges. As the 2021–2030 roadmap emphasizes a push toward country ownership of NTD programs, there is a growing need for models to incorporate local context and focus on priority questions that are most relevant for informing decisions of national and subnational programs [ 8 ]. While these priority questions vary across disease and location, they can be summarized into 3 main areas. The first is in understanding how best to achieve the 2030 targets. Here, models are important to determine whether programs are on track and to explore nuanced interventions and/or new tools that can accelerate progress to achieve different end points: disease control, elimination of transmission, elimination as a public health problem (EPHP), or eradication. The second area is in planning for what to do once targets are achieved. The continued success of control programs means that there is a growing emphasis on planning the next stages, focusing on eliminating disease in low-prevalence settings and designing post-validation surveillance strategies to ensure the sustainability of NTD programs and avoid disease resurgence [ 8 ]. Models can provide insights into the optimal strategies for stopping interventions and designing monitoring and surveillance after disease elimination. Importantly, such decisions have economic implications due to the need to balance the costs involved in monitoring and surveillance with the benefits of maintaining NTDs under control and avoiding resurgence. This is the third priority area where modeling can help inform decision-making by better incorporating economic parameters.
Here, we focus on these issues by discussing the challenges associated with the transition to more locally relevant models with a stronger emphasis on the final stages of elimination and surveillance. We also summarize how the studies in this supplement demonstrate the ability of NTD models to aid in the design of nuanced interventions tailored to specific contexts and the use of new tools to accelerate achievement of the 2030 targets ( Figure 1 ).
Moving from global scenarios to local projections. Schematic representation of the main concepts discussed, including how models with increased geographical resolution and incorporating local data, information on costs and resources, and considerations of new and nuanced interventions can support programmatic decisions. Credits: Neighborhood image (below) by Freepik, global map (above): image by macrovector on Freepik .
A crucial way in which NTD models can help accelerate progress toward the 2030 targets is by estimating the likely impact of nuanced or new interventions on disease elimination. For example, models can be used to estimate the likely impact of different numbers of treatment rounds, increasing or expanding coverage, reaching “never-treated” individuals, or deploying new interventions, such as new drugs or vaccines.
Extending Coverage
Models can be useful in informing decisions on whether to extend current interventions to additional groups. For example, while schistosomiasis interventions have primarily focused on school-aged children (SAC), new guidelines recommend that treatment be expanded to include preschool-aged children (pre-SAC), women of reproductive age, and adults [ 9 ]. Individual-based stochastic models that simulate the impact of mass drug administration (MDA) and include these additional groups can help estimate the number of treatment rounds required to meet the 2030 schistosomiasis target of EPHP [ 10 ]. These scenario-based models reveal how the number of rounds needed to achieve EPHP depends on the baseline disease prevalence and the treatment coverage used. For example, EPHP can be achieved within 7 years in low-, medium-, and high-transmission areas. However, reaching the entire affected population is challenging for MDA programs, and so the modeling suggests that these results can only be achieved if the percentage of never-treated individuals (ie, those individuals who self-report that they have never ingested tablets during any round of MDA) [ 11 ] in these areas is less than 10%, 5%, and 1%, respectively. Therefore, the higher the intensity of transmission and the lower the treatment coverage, the lower the acceptable value of “never treatment” becomes, highlighting how efforts to increase coverage and/or minimize never treatment can shorten program duration.
The proportion of never treatment during MDA campaigns is also a key barrier to the elimination of lymphatic filariasis. Individual-based stochastic models of lymphatic filariasis transmission can be used to estimate the maximum level of never treatment for which lymphatic filariasis targets can be achieved within 10 years under different scenarios (with varying annual MDA coverage, drug combinations, and transmission settings) [ 12 ]. These models show that the proportion of never treatment has a strong impact on the achievement of elimination, which is greater in high-transmission areas, and for campaigns based on the use of ivermectin + albendazole (IA). For example, in Anopheles transmission settings where the baseline microfilaremia (mf) prevalence is 10%, treating 80% of the eligible population annually with IA can achieve the elimination threshold within 10 years of annual treatment as long as never treatment is lower than 10%. Higher proportions of never treatment are acceptable when lower baseline prevalence and/or more efficacious treatment regimens (such as diethylcarbamazine + albendazole [DA] or ivermectin + diethylcarbamazine + albendazole [IDA]) are used.
Identifying Target Areas
Another potential use for models is to aid in the identification of areas that may require enhanced treatment to meet WHO elimination criteria. For example, available survey data (on the trachomatous inflammation–follicular [TF] prevalence in children aged 1–9 years) can be used to build an ensemble of probabilistic models to forecast the prevalence of clinical trachoma across 11 760 districts in trachoma-endemic countries [ 13 ]. These models identified 172 districts that are likely to exceed the 5% TF control threshold in 2030 with the current interventions, suggesting that global EPHP of trachoma by 2030 may require enhanced intervention and/or surveillance of high-risk districts.
Similar efforts to account for spatial heterogeneities can help achieve the lymphatic filariasis goal of validating EPHP in 58 (81%) of the currently endemic countries, a challenging target particularly for programs in resource-limited countries. For this, a combination of disease transmission models with geospatial statistical modeling can help to estimate the expected progress toward the 2030 goals across 44 sub-Saharan African countries at a fine spatial scale [ 14 ]. The resulting projections suggest that although >80% of the endemic countries in Africa are on track to achieve EPHP, pockets of highly endemic locations are likely to miss this target unless they increase the frequency and/or coverage of current interventions.
This combination of geostatistical mapping with transmission modeling can also be useful in elucidating the progress toward lymphatic filariasis elimination at the subnational level, as demonstrated for Ethiopia [ 15 ]. While mainly serving as a proof-of-concept example based on historic data from the successful lymphatic filariasis program in Ethiopia, this analysis demonstrates that similar strategies could be implemented in other countries at earlier stages of their lymphatic filariasis programs to help identify areas where elimination is likely to be challenging, so that additional resources can be put in place to accelerate progress.
Once programs achieve their targets, emphasis needs to shift from how best to achieve elimination to how to optimally wind down interventions and move to post-validation monitoring and surveillance. Such decisions are usually based on predefined prevalence thresholds, as is the case for the stop-MDA decision in lymphatic filariasis programs. MDA of antifilarial drugs is usually based on 2-drug combinations (IA or DA), with stop-MDA decisions for these regimes depending on the prevalence of both mf and circulating filarial antigenemia. However, recent studies showing that the triple drug combination is more effective than dual drug combinations have accelerated the use of IDA for elimination of lymphatic filariasis. As antigen can persist for a long time in people treated with IDA and adults are most likely to be infected and usually participate in MDA at a lower rate than younger age groups, WHO intends to base the stop-MDA decision for IDA-treated areas on mf prevalence in adults. Modeling can help to assess how the probability of reaching elimination depends on this critical threshold used in transmission assessment surveys (TASs) to determine whether transmission was successfully suppressed and whether triple-drug MDA can be stopped [ 16 ]. Based on analyses in treatment-naive Indian settings, a single TAS 1 year after the last MDA round provides limited predictive value of having achieved suppressed transmission, while additional surveys conducted in later years (3 and 5 years post-MDA) provide further insights on the prospect of elimination. For example, the predictive value of a single TAS is always <95% for threshold values ≥0.5% mf prevalence, whereas with 2 additional TASs, predictive values of ≥95% are possible for the same threshold, even when MDA coverage is as low as 65%. Therefore, such studies can provide important insights into the best strategies for post-MDA surveillance.
Models can also be used to improve the design of elimination surveys, as in the case of soil-transmitted helminths (STHs). Control of these infections involves the delivery of preventive chemotherapy to SAC through schools. Progress of STH control programs is currently monitored using periodical school-based prevalence surveys, known as impact assessment surveys (IASs). IASs are typically carried out after 5 years of preventive chemotherapy delivery. When the prevalence of STH in the target population falls below 2%, WHO recommends suspending preventive chemotherapy. Given the high cost associated with conducting an IAS, it is important that such surveys are optimally designed and enable the identification of disease hot spots that can be targeted for preventive chemotherapy delivery. Using prevalence data collected in Kenya, the integration of geostatistical methods with a Markov model or a mechanistic transmission model could be used for forecasting STH prevalence, although prediction accuracy was lower in areas with high prevalence hot spots [ 17 ].
Insights from modeling can also help to optimize surveillance strategies, including those based on passive surveillance. Using a generic model of disease transmission with slow epidemic growth rates and cases detected through severe symptoms and passive detection, it is possible to identify scenarios under which passive surveillance is sufficient to control disease transmission [ 18 ]. These models show that reducing the period of infectiousness by decreasing time to treatment has only a small effect on reducing transmission, implying that passive surveillance needs to be very efficient to prevent resurgence. These data suggest that passive surveillance alone is unlikely to be enough to maintain elimination goals for many “case-finding” NTDs.
NTD programs also need to carefully consider costs and other resources during decision-making. Therefore, there is a growing need for models to incorporate these factors into projections to inform decision-making more effectively. Economic models can help guide decisions related to new treatments, as in the case of onchocerciasis. For this disease, ongoing concerns that annual MDA of ivermectin may not lead to elimination of parasite transmission in all endemic areas have stressed the importance of considering alternative treatments, particularly the use of moxidectin [ 19 ]. However, it is important to consider the economic implications of these alternative strategies. An updated economic assessment of moxidectin- versus ivermectin-based strategies across a range of scenarios (with varied disease prevalence and treatment coverage) suggests that moxidectin-based strategies could not only accelerate progress toward elimination of onchocerciasis transmission but are also likely to reduce programmatic delivery costs compared with ivermectin-based strategies [ 20 ].
For helminth infections, including schistosomiasis, there is concern that current diagnostic tools are inadequate for detecting low-intensity infections, highlighting the need for better diagnostics. Transmission models for schistosomiasis, coupled with statistical analysis of Schistosoma mansoni egg counts from Burundi, can be used to probe how more sensitive diagnostics can improve decision-making regarding stopping or continuing interventions [ 21 ]. These models show that more sensitive diagnostics have a reduced impact on improving health outcomes and are associated with increased costs related to the continuation of MDA interventions unless the stop-MDA threshold is revised. However, if this threshold is set too high, treatment may be stopped too early, resulting in a rebound of infection levels.
Similar trade-offs between additional rounds of treatment and rebounds apply to the design of surveillance strategies for lymphatic filariasis [ 22 ]. In this context, modeling can help us to understand how adjusting the threshold used in TASs impacts decisions about the stop of interventions and at what cost. For many settings, a reduction in the threshold increases the probability of elimination, decreases the number of treatment rounds required, and reduces costs. Importantly, however, in certain circumstances (eg, when coverage is lower), lower thresholds can imply an increase in the number of rounds of treatment required to reach that threshold (with increased costs) but help mitigate chronic conditions (such as lymphoedema and hydrocele) and result in longer sustained elimination with fewer future rebounds.
The continued development of increasingly complex NTD models has led to new challenges. While NTD modeling has historically relied on adapting existing models for other diseases, current models are at the forefront of the inference field. As a result, NTD modelers face unique problems, including the need to understand the role of asymptomatic infections in hindering progress to control and eliminate case-finding NTDs [ 23 ].
Another significant challenge lies in transitioning from scenario-based global simulations to more local models that can be used to project outcomes and inform decisions at the national and subnational levels. However, to generate meaningful national forecasts, these models require the input of complete and accurate data that reflect local contexts, which are currently limited. One recent example of progress in this area is the advent of new data streams, including from surveys that capture the frequency of never-treated persons in MDA campaigns [ 11 ]. Such data, which shed light on previously underrepresented aspects of NTD epidemiology, serve as invaluable input for epidemiological models, heightening their accuracy and potential impact on NTD control, elimination, and surveillance strategies. An additional challenge in this transition to more relevant local models is the need to strengthen in-country capacity and ownership [ 4 ], including by training and supporting national modelers and modeling programs.
While limitations regarding locally relevant data persist, scenario-based models built on extensive global datasets will remain important for policy-making, even though they may not always include the exact conditions that reflect individual countries. Furthermore, given that closely tailored analyses are, in general, still reliant on fewer, lower-quality data, they are characterized by high levels of uncertainty, which limit their utility. As data availability and quality increase, local models will become increasingly accurate and relevant, enabling better-informed decisions, including on cost-effectiveness of interventions and surveillance strategies, by incorporating national perspectives on economic analyses. For this, modelers will need to continue to carefully consider what information is needed versus what is available and to work closely with national programs and relevant stakeholders to ensure that models are built on the best possible data and address relevant questions that will aid in making decisions that accelerate achievement of the 2030 targets. Until then, model interpretation and communication of results to different audiences need to be done carefully while acknowledging existing limitations and uncertainty.
Acknowledgments. The authors are grateful, as always, to the academic partners, field staff, and neglected tropical diseases (NTDs)–affected populations who generate primary data and invaluable insights that are generously made available to facilitate modeling work.
Disclaimer. The authors alone are responsible for the views expressed, which do not necessarily represent the views, decisions, or policies of the institutions with which the authors are affiliated.
Supplement sponsorship. This article appears as part of the supplement “New Tools and Nuanced Interventions to Accelerate Achievement of 2030 Roadmap for Neglected Tropical Diseases,” sponsored by funding of Professor T. Déirdre Hollingsworth's research by the Li Ka Shing Foundation at the Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford; and funding of the NTD Modelling Consortium by the Bill & Melinda Gates Foundation (INV-030046).
World Health Organization . Global report on neglected tropical diseases 2023 . Geneva, Switzerland : World Health Organization , 2023 . Licence: CC BY-NC-SA 3.0 IGO. Available at: https://apps.who.int/iris/rest/bitstreams/1489232/retrieve . Accessed 25 January 2024.
Google Scholar
Google Preview
Liese B , Rosenberg M , Schratz A . Programmes, partnerships, and governance for elimination and control of neglected tropical diseases . Lancet 2010 ; 375 : 67 – 76 . doi: 10.1016/S0140-6736(09)61749-9 .
World Health Organization . Accelerating work to overcome the global impact of neglected tropical diseases—a roadmap for implementation . Geneva, Switzerland : World Health Organization , 2012 . Available at: https://apps.who.int/iris/handle/10665/70809 . Accessed 25 January 2024.
World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030 . Geneva : World Health Organization , 2022 . Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.who.int/publications/i/item/9789240010352 . Accessed 25 January 2024.
Clark J , Stolk WA , Basáñez M-G , et al. How modelling can help steer the course set by the World Health Organization 2021–2030 roadmap on neglected tropical diseases . Gates Open Res 2021 ; 5 : 112 . doi: 10.12688/gatesopenres.13327.2 .
World Health Organization . Impact of the COVID-19 pandemic on seven neglected tropical diseases: a model-based analysis . Geneva, Switzerland : World Health Organization , 2021 . Licence: CC BY-NC-SA 3.0 IGO. Available at: https://apps.who.int/iris/handle/10665/343993 . Accessed 25 January 2024.
Borlase A , Le Rutte EA , Castaño S , et al. Evaluating and mitigating the potential indirect effect of COVID-19 on control programmes for seven neglected tropical diseases: a modelling study . Lancet Glob Health 2022 ; 10 : e1600 – 11 .
Vasconcelos A , King JD , Nunes-Alves C , et al. Accelerating progress towards the 2030 neglected tropical diseases targets: how can quantitative modeling support programmatic decisions? Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S83 – 92 .
Faust CL , Osakunor DNM , Downs JA , et al. Schistosomiasis control: leave no age group behind . Trends Parasitol 2020 ; 36 : 582 – 91 .
Kura K , Mutono N , Basáñez MG , Coffeng LE , Thumbi SM , Anderson RM . How does treatment coverage and proportion never treated influence the success of Schistosoma mansoni elimination as a public health problem by 2030? Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S126 – 30 .
Brady MA , Toubali E , Baker M , et al. Persons “never treated” in mass drug administration for lymphatic filariasis: identifying programmatic and research needs from a series of research review meetings 2020–2021 . Int Health 2023 : ihad091 . doi: 10.1093/inthealth/ihad091/7319430 .
Kura K , Stolk WA , Basáñez MG , et al. How does the proportion of never treatment influence the success of mass drug administration programs for the elimination of lymphatic filariasis? Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S93 – 100 .
Srivathsan S , Abdou A , Al-Khatib T , et al. District-level forecast of achieving trachoma elimination as a public health problem by 2030: an ensemble modelling approach . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S101 – 7 .
Touloupou P , Fronterre C , Cano J , et al. An ensemble framework for projecting the impact of lymphatic filariasis interventions across sub-Saharan Africa at a fine spatial scale . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S108 – 16 .
Prada JM , Touloupou P , Kebede B , et al. Subnational projections of lymphatic filariasis elimination targets in Ethiopia to support national level policy . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S117 – 25 .
James A , Coffeng LE , Blok DJ , King JD , de Vlas SJ , Stolk WA . Predictive value of microfilariae-based stop-MDA thresholds after triple drug therapy with IDA against lymphatic filariasis in treatment-naive Indian settings . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S131 – 7 .
Eyre MT , Bulstra CA , Johnson O , et al. A comparison of Markov and mechanistic models for soil-transmitted helminths prevalence projections in the context of survey design . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S146 – 52 .
Minter A , Medley GF , Hollingsworth TD . Using passive surveillance to maintain elimination as a public health problem for neglected tropical diseases: a model-based exploration . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S169 – 74 .
Kura K , Milton P , Hamley JID , et al. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos Trans R Soc Lond B Biol Sci 2023 ; 378 : 20220277 .
Turner HC , Kura K , Roth B , Kuesel AC , Kinrade S , Basáñez MG . An updated economic assessment of moxidectin treatment strategies for onchocerciasis elimination . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S138 – 45 .
Coffeng LE , Graham M , Browning R , et al. Improving the cost-efficiency of preventive chemotherapy: impact of new diagnostics on stopping decisions for control of schistosomiasis . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S153 – 9 .
Oliver MCA , Graham M , Gass KM , et al. Reducing the antigen prevalence target threshold for stopping and restarting mass drug administration for lymphatic filariasis elimination: a model-based cost-effectiveness simulation in Tanzania, India and Haiti . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S160 – 8 .
Rock KS , Chapman LAC , Dobson AP , Adams ER , Hollingsworth TD . The hidden hand of asymptomatic infection hinders control of neglected tropical diseases: a modeling analysis . Clin Infect Dis 2024 ; 78 ( Suppl 2 ): S175 – 82 .
Author notes
Email alerts, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform ... a case study. J Clin Epidemiol. 2020; 121: 81-90. doi: 10.1016/j.jclinepi.2020.01.008 ... A systematic review and an individual patient data meta-analysis of ivermectin use in children ...
Abstract. Filariasis is a disease group caused by filariae that affects humans and animals. Of the hundreds of described filarial parasites,only 8 species cause natural infections in humans. In this, repeated episodes of inflammation and lymphedema lead to lymphatic damage, chronic swelling and elephantiasis of the legs, arms, scrotum, vulva ...
Filariasis is a disease group caused by filariae that affects humans and animals. Of the hundreds of described filarial parasites, only 8 species cause natural infections in humans. In this, repeated episodes of inflammation and lymphedema lead to lymphatic damage, chronic swelling and elephantiasis of the legs, arms, scrotum, vulva and breasts.
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform, PLOS Neglected Tropical Diseases, 18, 1 ...
Three studies were carried out in Brazil focusing on sequelae in patients with chronic lymphatic filariasis. Dreyer, Norões, and Mattos, 2006 [ 18 ], developed a care program that could equip lymphoedema patients with the skills, motivation, and enthusiasm to sustain an effective, low-cost, and convenient self-care to prevent episodes of ADLA ...
Lymphatic filariasis (LF) remains one of the world's most debilitating parasitic infections and is a major contributor to poor health in many endemic countries. ... This case study demonstrated how equity for a group of people who have, at least until recently, largely been ignored, can be achieved by a set of well designed, simple measures ...
This was a case of lymphatic filariasis caused by Wuchereria bancrofti. Morphologic features presented in the images included: sheathed microfilaria within the size range for W. bancrofti (note: the sheath is unstained but still visible in the images). short head space. a tail that is tapered to a point.
Here we present a case of an 11-year-old female with no travel history who was seen in our clinic for a 1-year history of painless left cervical lymphadenopathy secondary to lymphatic filariasis. ... Examination of the NIS from 2000 to 2014 revealed 865 patients admitted with a diagnosis of lymphatic filariasis. Most patients are in the mid to ...
Key facts. Lymphatic filariasis impairs the lymphatic system and can lead to the abnormal enlargement of body parts, causing pain, severe disability and social stigma. Over 882 million people in 44 countries worldwide remain threatened by lymphatic filariasis and require preventive chemotherapy to stop the spread of this parasitic infection.
Filariasis is an infectious disease that spreads through mosquito bites. Some people have no symptoms. Others may have inflammation, swelling or fever. Filariasis can lead to lymphedema (fluid retention) or hydrocele (swelling in the scrotum). You can prevent filariasis by avoiding mosquito bites if you live in or travel to tropical climates.
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform Luzia T. Freitas1,2,3 ☯, Mashroor Ahmad Khan4☯, Azhar Uddin4, Julia B. Halder1,2,3,5, Sauman Singh-Phulgenda3,6, Jeyapal Dinesh Raja4, Vijayakumar Balakrishnan4,
Case #446 - June, 2017. Blood specimens were collected at night from members of a village in Myanmar endemic for lymphatic filariasis. Roughly one-third of the cases were symptomatic, with or without recurrent episodes of fever and various degrees of lymphedema. The objects in Figures A and B were observed on Giemsa-stained thick blood films ...
The number of lymphedema and hydrocele patients in lymphatic filariasis endemic communities are usually underreported. This consequently affects their management. ... Hoerauf A ea. Developing a community-led SMS reporting tool for the rapid assessment of lymphatic filariasis morbidity burden: case studies from Malawi and Ghana. BMC infectious ...
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform PLoS Negl Trop Dis. 2024 Jan 16 ... Background: Lymphatic filariasis (LF) is a neglected tropical disease (NTD) targeted by the World Health Organization for elimination as a public health ...
Timor-Leste has 435 confirmed LF patients who are currently on follow-up. The country has not detected a new LF case since 2019, and it has set focus on eliminating the disease by adopting two strategies under the WHO's Global Programme to Eliminate Lymphatic Filariasis (GPELF)- stopping the spread of infection through large-scale annual treatment of all eligible people in an area or region ...
Introduction. Filariasis is a vector borne disease especially prevalent in tropical and subtropical regions and caused by slender thread-like filarial worms from the superfamily of Filarioidea, which has an affinity towards skin and subcutaneous tissue or lymphatic system 1.While the nematode-like Onchocerca volvulus and Loa loa cause filariasis of the skin, Wuchereria bancrofti, Brugia malayi ...
Lymphatic filariasis (LF) is a major cause of acute and chronic morbidity manifested as lymphedema and hydrocele. ... Map of Sierra Leone Showing Location of Case Patient and Familial Relations. ... A study from the mid-1990s of 441 Indian children (ages 1-14 years) with LF found only 12 cases of lymphedema (Harinath et al., 2000).
Lymphatic filariasis (LF) is a mosquito-borne neglected tropical disease (NTD) caused by 3 parasites, namely, Wuchereria bancrofti, Brugia malayi, and Brugia timori . LF can cause chronic morbidity, such as hydrocele or lymphedema which are associated with disability, pain, mental health problems, reduced productivity, and social stigmatisation ...
Lymphatic filariasis (LF), a neglected tropical disease (NTD), is a leading cause of preventable morbidity and disability due to lymphedema, hydrocele, and acute inflammatory episodes with resultant fevers (acute dermatolymphangioadenitis) and still affects more than 50 million people worldwide [].The most common causative agent is the parasitic filarial nematode worm Wuchereria bancrofti.
Background. Fourteen years after its launch in 2000, the Global Programme to Eliminate Lymphatic Filariasis (GPELF) is well under way. For example, in 2012 (the last year with reported data), 56 of 73 LF-endemic countries provided mass drug administration (MDA) to approximately 425 million people [].Despite a number of challenges, GPELF is progressing in many areas in West and East Africa, and ...
The lymphatic filariasis treatment study landscape: A systematic review of study characteristics and the case for an individual participant data platform. ... A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? ...
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) aims to reduce and maintain infection levels through mass drug administr ... Effectiveness of a triple-drug regimen for global elimination of lymphatic filariasis: a modelling study. ... Positive-case follow up for lymphatic filariasis after a transmission assessment survey in Haiti.
Author summary Lymphatic filariasis (LF) is a debilitating parasitic disease that the World Health Organization (WHO) has earmarked for elimination by 2030 through a combination of mass distribution of anti-parasitic medicines and disease management approaches. Great strides have been made towards the elimination of LF as a public health problem, but nearly 900 million people still require ...
Lymphatic filariasis (LF) is an important neglected parasitic disease according to the World Health Organization. In this study, we aimed to determine the prevalence of human LF in Asia using a systematic review and meta-analysis approach. Records from 1990 to 2018 in reputable databases including PubMed, Science Direct, Embase, and Cochrane Library were searched using a panel of related ...
Lymphatic filariasis (LF) is an important neglected parasitic disease according to the World Health Organization. In this study, we aimed to determine the prevalence of human LF in Asia using a systematic review and meta-analysis approach. Records from 1990 to 2018 in reputable databases including PubMed, Science Direct, Embase, and Cochrane ...
Lymphatic filariasis (LF) remains one of the world's most debilitating parasitic infections and is a major contributor to poor health in many endemic countries. The provision of continuing care for all those affected by LF and its consequences is an important component of the United Nations' Sustainable Development Goals. The aim of this study is to integrate lymphedema care into the ...
Such decisions are usually based on predefined prevalence thresholds, as is the case for the stop-MDA decision in lymphatic filariasis programs. MDA of antifilarial drugs is usually based on 2-drug combinations (IA or DA), with stop-MDA decisions for these regimes depending on the prevalence of both mf and circulating filarial antigenemia.