Clinical Researcher

Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
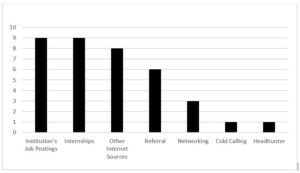
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
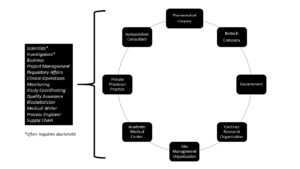
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Embracing the Future: Opportunities and Challenges of AI Integration in Healthcare

Utilizing Cultural Humility as a Tool to Support Diversity in Clinical Research

Looking at Clinical Trial Technology Through a Site Lens
How to Become a Clinical Research Specialist?

Clinical research specialists are leaders in their field, often directing teams of professionals as they plan and coordinate projects that advance medicine and treatment. The broad aim of clinical research specialists is to improve human health, often through clinical research trials.
Generally, clinical research specialists conduct their work in laboratories and offices. This is a high-level position that requires advanced communication skills, significant professional knowledge, the ability to direct others, and the expertise to manage and analyze clinical data. Those who explore how to become a clinical research specialist can find themselves on a rewarding path in the evolving field of medicine.
What Does a Clinical Research Specialist Do?
Before a new treatment can reach the masses, it must go through clinical research and trials. Clinical research specialists not only develop these new medicines and treatments, but also advance our understanding of how treatments impact illness and disease, and identify potential side effects.
In addition to strategizing procedures, this position requires the ability to shape and communicate protocols, work with patients in a sensitive manner, and evaluate and analyze the wealth of data generated through clinical trials.
There are many facets of what a clinical research specialist does, but some chief responsibilities include:
- Develop Protocols and Research Tools. To ensure that researchers and healthcare professionals conduct trials ethically and accurately, clinical research specialists determine the research methods, including how to collect and catalog data. This vital piece of the research process determines the scope and procedure for the entire trial. The clinical research specialist not only determines these protocols and methods, but also conveys them to everyone on the team, ensuring compliance until the research is complete.
- Coordinate, Manage, and Oversee a Team. To become a clinical research specialist, it is necessary to be an effective verbal and written communicator. In this leadership position, individuals must explain procedures to their team and any patients involved in the research. Additionally, strong interpersonal skills are a must to ensure that research is being conducted safely, legally, and consistently. The ability to manage others, establish procedures, and work well with a diverse group of people makes for insightful, accurate research.
- Analyze Data and Prepare Reports. Clinical research specialists not only prepare reports behind the scenes through budgets and operational records, but are also involved in analyzing the data yielded by research. Later, they are responsible for organizing their results into reports and presentations. This aspect of clinical research disseminates findings to the larger medical community, making it a critical part of the job.
Steps to Become a Clinical Research Specialist
There is some variation in possible routes to becoming a clinical research specialist. The responsibilities and requirements of individual companies may differ considerably and, as such, the specific skills required may also vary. However, there are some common professional and academic milestones — as well as a baseline of skill sets — that most employers require.
There are numerous potential job opportunities in clinical research, and the field is growing. That said, many employers require an advanced degree in nursing, life science, medical science, or a related course of study before they will consider an applicant. In addition to obtaining both a bachelor’s and a master’s degree, it is also recommended that aspiring clinical research specialists have specialized knowledge in a specific subject, such as biology, toxicology, pharmacology, microbiology, or anatomy.
While the process of becoming a clinical research specialist may differ from person to person, some basic steps often include:
Step One: Earn a Bachelor’s Degree in a Health-Related Field
The first step to becoming a clinical research specialist is to earn a bachelor’s degree from an accredited university. The course of study may vary, but degrees related to health science provide a robust starting point. Many individuals who obtain clinical research specialist jobs begin their journey with a bachelor’s degree in nursing, health science, biology, or a similar major. A four-year degree lays the foundation for further study and entry-level experience. It also strengthens vital skills that a clinical research specialist must obtain, such as communication, research and analysis, organization, and the ability to work as part of a team.
Step Two: Earn a Postgraduate Degree
To advance into a leadership role such as clinical research specialist, it is almost always necessary to obtain a postgraduate degree. Through advanced study, individuals interested in conducting clinical research strengthen their knowledge of implementation and assessment, and often have the opportunity to apply these skills during research phases. For example, an online Master of Science in Nursing provides students with a variety of advanced skills in research, analysis, leadership, and more.
Step Three: Gain Experience in the Field
Many job-seekers who wish to become clinical research specialists spend several years in related jobs, where they gain relevant experience. Aspiring professionals often begin their career in a clinical research assistant role. Working in a research setting under the leadership of a specialist provides great insight that is useful later on in a clinical research role.
Clinical Research Specialist Salaries
The many steps to becoming a clinical research specialist, and the high-profile work that they perform, often translates into competitive pay. According to Glassdoor, clinical research specialist salaries average around $82,000 annually, though pay can vary tremendously across organizations.
Depending on responsibilities, hours, and abilities, the salary for this position can range from $65,000 to more than $100,000 in a year, according to Glassdoor. Such well-compensated work often requires a graduate degree and at least three to five years of experience in the field. The variation in clinical research specialist salaries is reflective of the many different kind of research opportunities afforded to those in this position.
Future Growth of Clinical Research Specialists
Thanks to innovations in healthcare, a growing market for personalized medicine, and continual innovations in medical technologies, experts predict faster than average growth in the number of job opportunities for clinical research specialists. According to the U.S. Bureau of Labor Statistics, the field is expected to grow 13 percent from 2016 to 2026.
How a Master of Science in Nursing Can Help
Due to the growth potential of the field, as well as the ability to make meaningful contributions to human health, CNN declared clinical research associate to be one of the most desirable jobs in America. By applying advanced knowledge and ample experience to impactful research, clinical research specialists often find opportunities to advance in the field, as well as great satisfaction in their work.
The Maryville University online Master of Science in Nursing program provides individuals who are ready to play a lead role in advancing medical research with an exciting opportunity to pursue a career this field. Step into the evolving world of clinical research by learning more about how to earn your MSN today.
- Master of Science in Nursing
- Doctor of Nursing Practice
CNN Money, “Best Jobs in America”
Glassdoor. “Clinical Research Specialist Salaries”
Maryville University, “Career Opportunities for MSN Graduates”
New Scientist, “A Career in Clinical Research”
U.S. Bureau of Labor Statistics, “Medical Scientists”

Learn more about our programs.

Take your next step.
Stay in the know
Privacy and cookies.
We use cookies to give you the best experience on our website. By continuing, you're agreeing to use of cookies. We have recently updated our policy.

Clinical Project Manager
Clinical trial management certification.
A Clinical Project Manager ensures large-scale clinical studies are carried out properly, within budget, and on time by overseeing compliance, protocol development, data collection, trial sites, and quality control. Get clinical trial management certification to become a clinical project manager.

The clinical trial project manager is responsible for different aspects of the clinical trial process, such as setting timelines, developing budgets, and overseeing data analysis. To become a clinical trial manager, you can gain experience in project management or clinical research roles. Clinical trial manager certification will increase your chances of getting hired.
- To become a clinical project manager, one must first obtain a Bachelor's degree in a health-related field. Additionally, developing skills for project management and participating in relevant courses are necessary.
- Experience can be gained by volunteering or interning in clinical trials with pharmaceutical companies or medical research centers.
- Pursuing an advanced degree or getting certified as a Clinical Project Manager with open up more opportunities.
Clinical Trial Manager
Clinical Trial Managers are responsible for planning and overseeing all aspects of clinical research projects. This includes making sure the project is conducted according to regulations and best practices. They also manage budgets, timelines, and resources to ensure the project is completed successfully.
Clinical Project Managers plan and execute clinical research projects by coordinating with internal and external stakeholders, regulatory authorities, and by develop key documents like protocols, consent forms, investigator brochures, budget sheets, study reports, and final reports.
Clinical Research Managers make sure that data is being collected and analyzed correctly, and that everyone is compliant. Clinical Project Managers design research studies and monitoring projects in terms of cost, budgeting, quality assurance, and risk assessment.
The Clinical Trials Management Certificate program is designed to provide students with the knowledge and skills necessary to design, implement, and manage clinical trial protocols.
- This Clinical Trials Design & Management Certificate Program introduces learners to the fundamentals of clinical trials design and management.
- The program covers principles and regulations of clinical trial design, analysis techniques for statistical analysis, quality control and assurance, data management and reporting.
- Students will also gain an understanding of risk assessment strategies, study site selection, protocol implementation and monitoring, resource management as well as safety requirements for conducting clinical trials.
Clinical Trial Manager Salary
Clinical trial manager salaries vary based on experience, location, and company size. The average hourly rate for a clinical trial manager is $30-$60 per hour.
The monthly salary of a clinical trial manager typically ranges from $5,000 to $10,000. Those with more experience or who work at well-known organizations can earn up to $20,000 per month.
Annual clinical project manager salary ranges between $60,000 and $120,000 dollars per year. The median salary for a clinical trial manager is approximately $82,500 across all industries and geographies
Clinical Project Managers are responsible for creating project plans, timelines, budgets, and communication with vendors and stakeholders. They also provide training to personnel involved in the project, establish systems to track project progress, and identify risks associated with the project.
- Clinical Project Managers make sure that clinical research projects go well by working groups like contract research organizations (CROs), internal departments, and external vendors. Clinical Project Managers make sure that projects follow the research protocols, good clinical practices (GCPs), applicable regulations, and standards
- Research Project Managers also develop protocols for data collection and analysis, prepare reports for regulatory submissions, coordinate activities related to safety monitoring, and provide support to staff during project-related training sessions or workshops.
- Trial Project Managers make sure that data is collected accurately according to guidelines from the FDA or EMA. This includes finding risks associated with the project; assessing their impacts; planning ways to reduce the risks; and also planning how to use resources so that everything runs smoothly.
Clinical Research Project Manager Training
Advanced Clinical Research Associate Certification (ACRAC)
Introduction
CME Handout
Common Terminology Used In Clinical Research - Reference Glossary
Commonly Used Abbreviations and Terms in Clinical Research
An Overview of ICH GCP
CFR 21 Part 11
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated
Ethics of Research Involving Pregnant Women and Fetuses
Fundamentals of Project Management
Project Management Fundamentals
PMBOK Summary - Mandatory Project Management Review
Clinical Trial Project Management
Importance of Project Management
Roles and Relationships in Clinical Trials
Role of a Project Sponsor
ICH GCP E6 Section 5 - Sponsor/CRO Responsibilities
Institutional Review Board/Ethics Committee (IRB/EC) (Requirements, sIRB, Application, Exemptions, Expedited Review, Continuation, and Reporting)
Data Safety Monitoring board- DSMB
Stakeholders in Clinical Trials (Sponsor, Project Manager, IRB, PI, CRA, CRC, Site Staff, Data Team/DSMB, Patients)
Contract Research Organizations (Delegation, Responsibilities, Management )
ICH GCP E6 Sections 2-4 Principles, IRB, & Investigator Roles
ICH GCP E6 Section 4 - Reporting Responsibilities of the Investigators
Skills of a Project Manager
Essential skills of a Project Manager
Technical skills for Project Management
Project Team
Managing a Project Team
Project Management Documents
Regulatory Documents
Regulatory Documents in Clinical Trials
Delegation of Authority Log – DOAL
Investigators Brochure (IB)
Trial Master File
Essential Regulatory Documents Binder Tab Organization (Trial Master File)
Trial Master File Reference Guide
New Drug Application
The Investigational New Drug (IND) & New Drug Application (NDA) Process
Investigator Initiated Multi-Center Trials
IND and IDE AE Reporting
Safety Reporting Requirements for Sponsor Investigators of An IND
Problem Solving in Project Management
Problem Solving as a Project Manager
Project Failures and Statistics
Project Reporting Styles
Avoiding Project Failure
Budgeting for Clinical Trials and Projects
Project Budgeting
Payments and Budgeting for Investigators and Site
Advertisement Aid in Subject Recruitment and Retention
Clinical Trial Design
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
Randomized Controlled Trials (Randomization, Allocation Concealment, Validity, Blinding, Controls, Outcomes, Fidelity)
Blinding and Unblinding in Clinical Trials
The Clinical Trial Protocol - Advanced Mastery Review
Inclusion and Exclusion Criteria in Clinical Research (Writing, Assessing for Broad vs. Narrow, Organ Dysfunction, Older Adults, Pediatrics, Pregnant Women)
Protocol Deviations and Violations (Major, Minor, Exceptions, Resolution)
Project Management Scheduling and Tracking
Basics of Project Scheduling
Project Progress Tracking
Project Management Planning Process
Project Management Plan
Closing a Project
Project Delays
Process Mapping
Metric Tracking
Duties of a Successful Project Manager
Roles and Responsibilties of a Project Manager
Project Management Success Factors
Adverse Events
Advanced Review of Adverse Events
Site Selection and Visits
Types of Monitoring Visits (Selection, Initiation, Routine, Close-Out)
Site and Investigator Selection Criteria (Process, Criteria, Investigator Selection, Agreements, Decision-Making)
Site Selection/Qualification Pre-Study Visit (SSV/SQV) (Before, During, After, Letters, Checklists, and Report)
Audit and Inspections
Audits and Inspections in Clinical Trials
Clinical Trial Data Audits
FDA Warning Letter
Quality Control and Safety
Quality Control in Clinical Trials ( QC/QA, KQI, QMS, Checklist)
ICH GCP - Safety of Human Subjects in Clinical Research
Technology in Trials (IVRS, CTMS, EDC)
Clinical Trial Management System-CTMS
ICH GCP - Trial Management, Data Handling, and Record Keeping
An Overview of Remote Monitoring - COVID-19 Update
Centralized Monitoring
Interactive Response Technologies in Clinical Trials (IVRS, IWRS, IRT, RTSM)
Pharmacovigilance and Regulatory Affairs
Advanced Practice of Pharmacovigilance
Regulatory Affairs for Clinical Trials
Investigational Product and Labs
Investigational Product Storage and Dispensing
Investigational Product Accountability in Clinical Trials
Local and Central Labs in Clinical Trials (Local, Regional, Central, GLCP, CLIA Cert, Lab Audit Checklist)
Patient Recruitment, Retention, and Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Project Manager Job Readiness
Project Manager Skills Interview Questions
Interview Questions
Competency Examination
Competency Exam

About this course
- 17 hours of video content
The most advanced clinical trial project management training available
Take the fast track
Take the fast track to a lucrative career as a Clinical Research Project Manager to start earning salaries of $100k+
Get advanced training
Get the most advanced training - ACRPM is recognized as a gold standard by many CROs in the industry thanks to its comprehensive training
Work at your own pace
Work at your own pace from wherever you are with flexible online training. The 100+ modules included can be completed in as little as 2 weeks
Requirements
Designed for those holding a minimum of a BA in Science, ACRPM is internationally accredited to ACCRE, ACCME, ACPE, ANCC, and Transcelerate Biopharma. In other words, upon completion of the course and the final exam, you will have a level of knowledge equivalent to (and beyond!) that of a senior CRA.
ACRPM features 100+ modules, or 250 hours, of on-demand online training (worth 17.5 CME credits). The course has been put together by clinical trial project managers, enabling students to build a deep knowledge of the industry.
Certification
This course can be completed in as little as two weeks, with certification and a letter of recommendation awarded after completing a final exam. ACRPM also provides you with tools to help you find a job, including resume and interview guides, giving you a further edge over other applicants for the same position.
Clinical Project Manager Guide

Clinical Research Project Manager / Lead / Senior
How to apply.
A cover letter is required for consideration for this position and should be attached as the first page of your resume. The cover letter should address your specific interest in the position and outline skills and experience that directly relate to this position.
The University of Michigan Congenital Heart Center is seeking a Clinical Research Project Manager to serve as project manager and study coordinator for a pediatric, multi-center, investigator-initiated, FDA-regulated device clinical trial led by the University of Michigan. The device is patient-specific and the IDE is held by a University of Michigan investigator.
This position may provide study coordination and/or project management for multiple clinical research studies of any complexity. Coordinator experience and mastery of all job duties from Clinical Research Coordinator - Senior and/or CRC Lead is required. This position has achieved mastery in all aspects of clinical research management. This position has oversight for clinical research projects and has responsibility for ensuring that they are completed within specifications. This position establishes operational objectives and assignments and defines and manages project resource needs including project staff. Employees in this classification typically analyze, compare, and evaluate various courses of action and have the authority to make independent decisions on matters of significance, free from immediate direction, within the scope of their responsibilities. Primary activities and decision-making authority are predominantly performed independently affecting business operations to a substantial degree.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Responsibilities*
The selected candidate will have both Project Management and Study Coordinator duties. These specific duties include:
Project Management.
The selected candidate will:
- Serve as primary contact for sites participating in the multi-center clinical trial. Along with the UM team, the selected candidate will guide each site through study start up, enrollment, follow-up, and close out, and answer any questions that arise.
- Write clinical trial documentation as needed.
- Work with the device manufacturer to ensure the patient-specific devices are manufactured, sterilized, and delivered on time to both UM and participating sites.
- Coordinator monitoring visits for participating sites.
- Work with the SABER team at UM to ensure the trial database is designed and operates per specifications.
- Work with MICHR IND/IDE Investigator Assistance Program (MIAP) to ensure compliance with all applicable FDA regulations and reporting
- Work with liaison to create and update clinicaltrials.gov registration.
- Along with finance team, monitor the clinical trial budget.
- Maintain regulatory binder.
- Maintain IRB application.
- Support Expanded Access use of the device under the IDE for children who are ineligible for the trial
Study Coordination.
As UM is a participating site in the clinical trial, the selected candidate will:
- Ensure that subjects are consented, the protocol adhered to, and all regulatory documents are in order.
- Participate in all monitoring visits.
- Ensure data are entered, and queries answered, in a timely manner.
- Work within CTSU framework for initiating and carrying out the clinical triall, including enrolling and tracking patients in OnCore.
- Process patient incentive reimbursements.
The selected candidate will be embedded in the congenital heart center research team (M-CHORD), which consists of a dedicated team of study coordinators, project managers, and an analytic team, and faculty leaders. The selected candidate may work from home on days where in-person work is not required, as agreed upon with the selected candidate's manager and the PI of the trial. After the initial start-up phase of the clinical trial, additional clinical trials and studies may become the responsibility of the selected candidate. General responsibilities for this clinical trial, and future clinical trials and studies, are listed below.
Clinical Coordinator Responsibilities
- Demonstrates the ability to create and manage the logistics for a new clinical trial.
- Applies critical thinking and creative problem-solving skills in the development of new processes, procedures, tools, and training to enhance clinical research activities.
- Demonstrates the ability to evaluate a monitoring plan against protocol and relates the plan to study team members.
- Manage workloads and resources/procurement.
- Using available resources at the University, determines study site feasibility.
- Provides input on feasibility of new studies (workflow, resources, populations). Advises PI or clinical research teams of inconsistencies, errors or any issues of concern that may result in non-compliance or logistic issues.
- Assesses study budget for staff resource assessment.
- Proficient in study implementation.
- Knowledge of risk management strategies and principles.
- Responsible for workflow process and study planning.
- Develops tools and trainings to aid in the creation of study budgets and tracking of invoiceable items.
- May manage the research conduct at other sites if U of M is acting as a coordinating center for a multi-site study.
- Provide appropriate financial oversight
- Proficiency in budget and resource management
- Develops processes, tools, and training to enhance site compliance with the requirements necessary for the safe and effective development of investigational products.
- Demonstrated expert knowledge of clinical research systems.
- Assist in writing and review of protocols.
Data Coordinator Responsibilities
- Develops or contributes to the development of processes, tools, and trainings to ensure the accurate collection of data at the site level.
- Performs at the highest level of data management, including the monitoring of data collection and reporting to ensure compliance.
- Oversees study conduct by staff and ensures quality work is done.
- Reviews manuscripts/poster presentations/abstracts as appropriate.
- Proficient in data management and results reporting.
- Demonstrates ability to create and manage the logistics for a new clinical research trial.
- Responsible for implementing study assessment needs.
- Knowledge of the roles and responsibility of the various stakeholders in clinical research.
- Creates protocols for specimen collection, processing, shipping, etc., and implements procedures to maintain accuracy and efficiency.
Regulatory Coordinator Responsibilities
- Performs internal audits and adjusts SOPs for the management of IPs according to FDA regulations and GCPs.
- Knowledge of fraud and misconduct identification, reporting, and management.
- Responsible for workflow process and study planning.
- Facilitates internal and external audits.
- Ensure study compliance Provides oversight and follows FDA- Good Clinical Practices (GCP) or OHRP guidance for clinical research.
- Ensures ethical guidelines are reflected in SOPs by adapting any established procedures, processes, or workflows to reflect any new or updated regulations.
- Designs processes, tools, and trainings to guide study team members in the understanding, recognition, and documentation of subject protection and safety issues.
- Proficient in regulatory and compliance matters.
Administrative Responsibilities
- May run/delegate staff meetings
- May provide administrative supervision of a team > 2 employees
- May manage HR activities
- Knowledge of management concepts of and effective training methods to manage risk and improve quality in conduct of a clinical research study.
- Recruit and train appropriate personnel for clinical research
- Utilize staff performance metrics
- Knowledge of the principles and practices of leadership, management, and mentorship, and how to apply them within the working environment
Training
- All training requirements of the previous level.
- Specialty training or expertise is achieved at this level.
- Completes Michigan Medicine training for Managing Successfully and Leading Successfully.
- Attends and participates in all training assigned to this level.
Required Qualifications*
Clinical Resource Project Manager:
- CRC Governance Committee review and approval
- Master's Degree in Health Science or an equivalent combination of related education and experience is necessary.
- Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) or equivalent. Candidates must be eligible to register or take the exam at date of hire and the certification must be completed or passed etc. within six months of date of hire. (Please review eligibility criteria from SoCRA or ACRP prior to applying.)
- Minimum 6 years of directly related experience in clinical research and clinical trials is necessary. (Please review SoCRA's Definition of a Clinical Research Professional for qualifying experience prior to applying.)
- Excellent interpersonal, oral, and written communication skills Demonstrated knowledge of medical and research terminology. Ability to work with minimal supervision with diverse teams of people in a diplomatic, collaborative, and effective manner
- Exceptional organizational and computer skills are required with proficiency in Microsoft software applications
- Demonstrated problem solving and conflict resolution skills. Ability to multi-task, work well under time constraints and meet deadlines.
Clinical Research Coordinator Senior and Lead Required Qualifications:
- Bachelor's degree in Health Science or an equivalent combination of related education and experience is necessary.
- Minimum 5 years of directly related experience in clinical research and clinical trials is necessary.
Desired Qualifications*
- Previous experience with coordination and management of device trials (IDE)
- Experience leading a multi-site clinical trial
- Demonstrated understanding of FDA regulations
- Extensive experience with successful submissions to IRB
- Familiarity with congenital heart disease
Work Schedule
In this position you'll typically work Monday through Friday. Occasional weekend work may be required.
Work Locations
The in-person portion of this role will be at Mott Children's Hospital. The selected candidate may work from home on days where in-person work is not required, as agreed upon with the selected candidate's manager and the PI of the trial.
Underfill Statement
This position may be underfilled at a lower classification depending on the qualifications of the selected candidate.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Open access
- Published: 27 March 2024
Optimizing care for children with difficult-to-treat and severe asthma through specialist paediatric asthma centres: expert practical experience and advice
- M. W. Pijnenburg ORCID: orcid.org/0000-0003-0902-0860 9 na1 ,
- S. Rubak ORCID: orcid.org/0000-0001-7010-2350 14 na1 ,
- H. O. Skjerven 7 , 8 na1 ,
- S. Verhulst 18 , 19 na1 ,
- V. Elenius ORCID: orcid.org/0000-0001-9264-9904 1 na2 ,
- C. Hugen ORCID: orcid.org/0000-0002-9449-9679 2 na2 ,
- O. Jauhola 3 na2 ,
- C. Kempeneers 4 na2 ,
- E. Melén 6 na2 ,
- T. Reier Nilsen ORCID: orcid.org/0000-0001-8184-0607 12 , 13 na2 ,
- N. W. Rutjes ORCID: orcid.org/0000-0002-3996-1963 16 na2 ,
- M. Ruotsalainen 15 na2 ,
- H. Schaballie 17 na2 ,
- A. M. Zwitserloot 20 , 21 na2 ,
- M. Proesmans 10 , 11 &
- M. J. Mäkelä ORCID: orcid.org/0000-0002-2933-3111 5 , 22 na1
BMC Pediatrics volume 24 , Article number: 218 ( 2024 ) Cite this article
48 Accesses
Metrics details
Severe asthma in children carries an unacceptable treatment burden, yet its rarity means clinical experience in treating it is limited, even among specialists. Practical guidance is needed to support clinical decision-making to optimize treatment for children with this condition.
This modified Delphi convened 16 paediatric pulmonologists and allergologists from northern Europe, all experienced in treating children with severe asthma. Informed by interviews with stakeholders involved in the care of children with severe asthma (including paediatricians, nurses and carers), and an analysis of European guidelines, the experts built a consensus focused on the gaps in existing guidance. Explored were considerations for optimizing care for patients needing biologic treatment, and for selecting home or hospital delivery of biologics. This consensus is aimed at clinicians in specialist centres, as well as general paediatricians, paediatric allergologists and paediatric pulmonologists who refer children with the most severe asthma to specialist care. Consensus is based on expert opinion and is intended for use alongside published guidelines.
Our discussions revealed three key facets to optimizing care. Firstly, early asthma detection in children presenting with wheezing and/or dyspnoea is vital, with a low threshold for referral from primary to specialist care. Secondly, children who may need biologics should be referred to and managed by specialist paediatric asthma centres; we define principles for the specialist team members, tests, and expertise necessary at such centres, as well as guidance on when homecare biologics delivery is and is not appropriate. Thirdly, shared decision-making is essential at all stages of the patient’s journey: clear, concise treatment plans are vital for patient/carer self-management, and structured processes for transition from paediatric to adult services are valuable. The experts identified the potential for specialist paediatric asthma nurses to play a significant role in facilitating multidisciplinary working.
Through this project is agreed a framework of practical advice to optimize the care of children with severe asthma. We encourage clinicians and policymakers to implement this practical advice to enhance patient care.
Peer Review reports
Asthma is common in childhood, and most children achieve good symptom control with low-to-medium doses of inhaled corticosteroids. However, for some, symptoms remain problematic despite high treatment doses [ 1 , 2 ]. Asthma can be described as ‘difficult to treat’ when it is uncontrolled despite medium- or high-dose inhaled corticosteroids with a second controller and/or with maintenance systemic corticosteroids; or when high-dose treatment is needed to control symptoms and reduce the risk of exacerbations [ 3 ]. Difficult-to-treat asthma relates to modifiable factors such as poor adherence, incorrect inhaler technique, comorbidities or adverse exposures [ 1 , 4 ]. After addressing modifiable factors, children whose disease remains poorly controlled on high doses of medication form a heterogeneous group considered to have ‘severe’ asthma phenotypes [ 4 ], and are eligible for step-up therapies. These include long-acting muscarinic antagonists, oral corticosteroids, and most recently, biologics targeting immunoglobulin (Ig)E, interleukin (IL)-4Rα, IL-5, and IL-13 [ 1 , 5 ].
Severe asthma in children is a rare disease, accounting for less than 5% of all childhood asthma cases [ 4 , 6 ], yet it can cause frequent asthma attacks and hospital admissions, and its mortality is unacceptably high [ 4 ]. Furthermore, although biologics have been available for managing asthma in adults and adolescents for almost two decades, research on their efficacy and safety in children has lagged [ 7 ]. Accordingly, clinical experience in managing severe childhood asthma is limited, even among specialists.
This project convened paediatric pulmonologists and allergologists from northern Europe. Its initial aim was to give consensus-based practical advice on best practices to support clinical decision-making in two areas: first, considerations around biologic treatment for children aged 6–18 years with severe asthma; and second, on providing optimal care for these patients. We also considered how to disseminate these considerations throughout healthcare settings. As the project progressed, these objectives evolved with its emerging findings, and we refocused our aims on the considerations for selecting home or hospital delivery of biologics, as practical advice on this topic was found to be lacking.
This consensus is aimed at clinicians in specialist centres, as well as general paediatricians, paediatric allergologists and paediatric pulmonologists who refer children with the most severe asthma to specialist care. Consensus is based on expert opinion and is intended for use alongside published guidelines.
This was a modified Delphi, completed between June 2022 and May 2023. Delphi is a useful approach for gaining consensus when data are limited and expert opinion is needed to shape clinical judgements [ 8 , 9 , 10 ]. This two-round Delphi was done using online surveys.
Definition of consensus
Responses to survey statements were recorded using a 5-point Likert scale (1 [‘strongly disagree’] to 5 [‘strongly agree’]), similar to other consensus projects [ 9 ]. Experts could explain their responses to support statement modification between rounds.
Consensus was defined a priori when ≥ 75% of responses scored 4 or 5 (‘agree’ or ‘strongly agree’). Partial consensus occurred if some – but not all – parts of a multi-part survey item reached consensus.
Participants
The expert voting group comprised paediatric pulmonologists or allergologists working in a variety of secondary or tertiary settings across northern Europe (Sweden, Norway, the Netherlands, Finland, Denmark or Belgium; Table 1 ).
These clinicians were invited based on their practical expertise, publishing records, national and regional standing, and interest. A subset of five experts formed a steering committee.
The sponsor abstained from discussions and had no input on the surveys or consensus. The views reported are the authors’ alone.
Stakeholder interviews, survey development and voting
The steering committee agreed the project’s scope, target patients and topics for consideration, and was informed by a gap analysis of guidelines [ 3 , 5 , 11 , 12 , 13 , 14 , 15 , 16 ]; this project focused on the gaps.
To explore these gaps, further inform the survey and reflect the needs and experience of patients, carers and relevant clinicians, we gathered insights from a stakeholder group. This comprised three secondary care/private paediatricians, two specialist paediatric asthma nurses, two pulmonologists specializing in adult care, one representative of a patient organization, and one carer of a child with severe asthma. Individual exploratory 60-min Zoom interviews were conducted in English by an independent facilitator and an observer, assisted by a translator when necessary. Recordings and contemporaneous notes were analysed to identify themes.
Using these inputs, draft statements were developed by the Delphi facilitator, refined and agreed by the steering committee, then voted on by the entire expert group. The steering committee reviewed the Round 1 results and developed the Round 2 survey, focusing on topics not at consensus. A virtual meeting elaborating results allowed the experts to contextualize their opinions; no further voting occurred.
Delphi results
After both rounds, 14 items were at full consensus, 7 at partial consensus and 3 at no consensus (Fig. 1 ). All data can be found in the Supplementary data file .
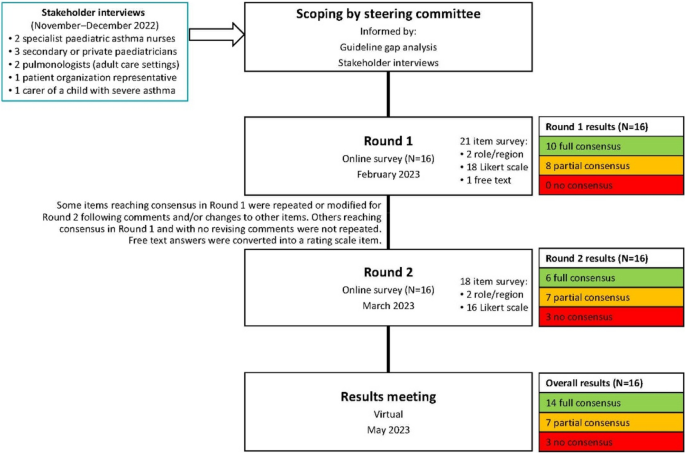
Project flow chart
The survey evolved to have several overarching themes. Notably, following the direction of the discussions informed by the gap analysis, the initial goal to explore the considerations around biologic treatment for children aged 6–18 years with severe asthma was refocused on the considerations for selecting home or hospital delivery of biologics. This was because practical advice on this topic was found to be lacking, and an educational need, while detailed recommendations on biologics have already been published by Global Initiative for Asthma (GINA) [ 5 ] and European Academy of Allergy & Clinical Immunology (EAACI) [ 14 ].
Diagnostic considerations in primary care
Stakeholder interviews highlighted that accessing clinical teams with experience of managing children with severe or difficult-to-treat asthma can be difficult, because such children are so rare that few experts have extensive clinical experience of treating them. The stakeholders noted that lacking access to such specialists is a barrier to patients’ optimal holistic care. There was also a concern that such children may reach the most specialist centres only after experiencing a severe exacerbation.
The expert group therefore agreed that detecting asthma early in children presenting with respiratory symptoms (e.g. wheeze, dyspnoea) – regardless of severity – is vital. They agreed that the minimum diagnostic workup for suspected asthma should evaluate signs and symptoms from both upper and lower airways, lung function, exacerbations, risk factors, medication compliance, atopic sensitization and blood eosinophil levels (Fig. 2 ). The experts agreed on the importance of assessing atopic sensitization and blood eosinophil levels, but advocated a stepwise testing approach, reserving these assessments until after other tests have been done. Fractional exhaled nitric oxide (FeNO) testing did not reach consensus as part of the initial minimum diagnostic workup, but was considered useful for differential diagnosis or as an additional diagnostic tool, and possibly later when choosing which biologic to use.
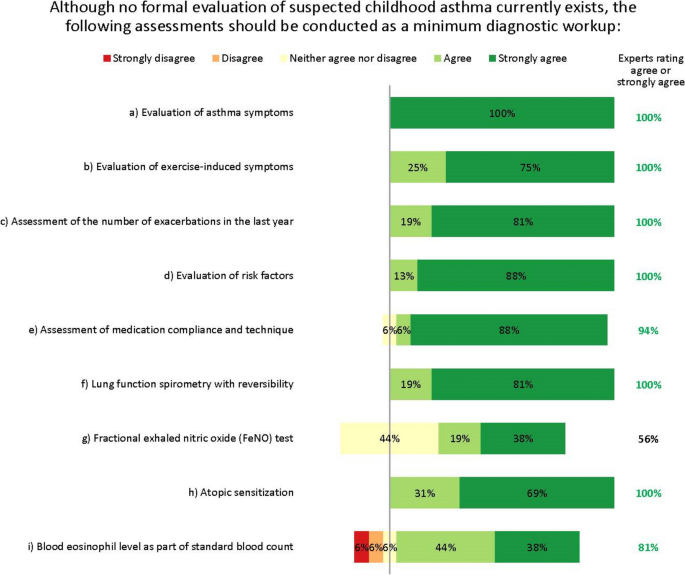
Minimum diagnostic workup for childhood asthma. Numbers are shown in green for those statements at consensus
When to refer to a specialist paediatrician or specialist paediatric asthma centre
The clinical settings in which these tests should be done and diagnosis made was explored. Stakeholder interviews and steering committee discussions revealed that the differentiation between primary, secondary and tertiary care for children with asthma is misleading, as some specialist paediatric asthma centres do not sit in tertiary settings, and some patients may be managed in tertiary settings that are not specialist paediatric asthma centres. We therefore differentiate between specialist care (secondary and tertiary care) and care in specialist paediatric asthma centres (which have the highest level of expertise and experience).
The experts suggested that the adequate tests, as available, should be done in primary care, with referral to specialist care or even a specialist paediatric asthma centre for further testing and confirmation of an asthma diagnosis in case of doubt or complicating factors (Fig. 3 ). However, they recognize a ‘one-size-fits-all’ approach would be undesirable. For example, referral to a specialist is unnecessary if a correct diagnosis and control can be achieved in primary or secondary care. Conversely, limitations of resources or expertise may prevent some general practitioners (GPs) from diagnosing accurately without specialist assistance. Indeed, the availability of diagnostic tests varies considerably by setting and region.
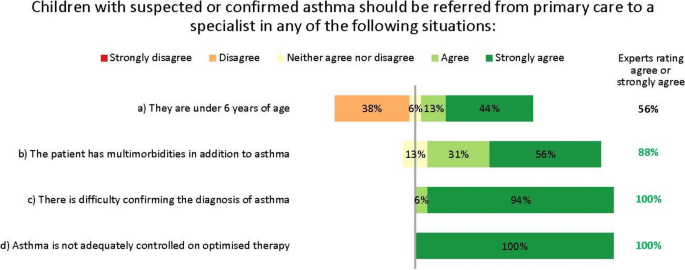
Referral from primary to specialist care. Numbers are shown in green for those statements at consensus
In discussions, the experts strongly agreed that the threshold for referral to specialist care should be low, to reduce the risk that children with potentially serious disease are misdiagnosed or undertreated. Notably, the experts disagreed that children aged under 6 years need automatic referral. In many cases, a decision to refer will depend less on age than on comorbidities and severity, as well as the facilities and expertise available in primary care. Further, viral-induced (‘preschool’) wheeze is so common in children of this age that automatic specialist care is almost always unwarranted in the absence of other risk factors. The experts unanimously agreed that children with difficult-to-treat or severe asthma who may need biologic treatment should be referred to and managed by specialist paediatric asthma centres. This referral may be direct from primary or secondary care, depending on local practices.
Appropriate diagnostic tests for specialist paediatric asthma centres
The experts did not fully agree which lung function tests are appropriate for the differential diagnosis of severe asthma in children aged over 6 years. The differences in opinion largely result from regional differences in practices and resources, as well as the wide variation in patients’ characteristics, meaning general recommendations are difficult to give. A unanimous consensus was reached for spirometry with bronchodilator response testing, and most experts agreed on body plethysmography, provocation testing and FeNO testing. Others suggested oscillometry in cases where spirometry is insufficient, such as for younger children [ 17 ]. However, no agreement was reached on continuous laryngoscopy during exercise or on volume of oxygen (VO 2 ) max testing (Fig. 4 ). Further, the experts acknowledge that many more tests than these also exist for monitoring asthma in children, such as the lung clearance index and infant lung function test. These are not yet widespread in northern Europe and are not suitable for all children or centres, but the experts suggest they may be helpful in some clinical scenarios, as recently discussed by Pijnenberg, et al. [ 18 ].
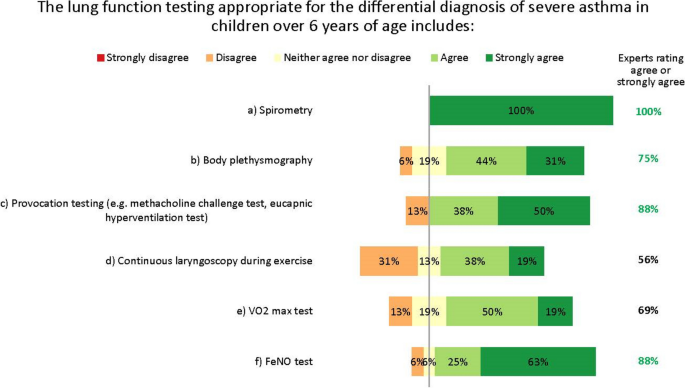
Lung function testing for diagnosis. Numbers are shown in green for those statements at consensus
Practical considerations for specialist paediatric asthma centres
The experts saw multiple reasons for managing children with difficult-to-treat or severe asthma who may need biologic treatment in specialist paediatric asthma centres (Fig. 5 ).
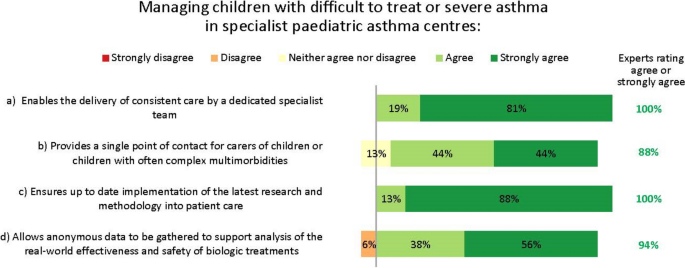
Importance of specialist paediatric asthma centres. Numbers are shown in green for those statements at consensus
Essential facilities and tests
Specialist paediatric asthma centres must have access to imaging facilities, bronchoscopy and lung function testing (spirometry, body plethysmography, provocation testing and FeNO testing), and have experience with biologic administration and monitoring in children. Having an intensive care unit in the same centre was not considered essential, but having access to such facilities elsewhere was deemed desirable (Fig. 6 ).
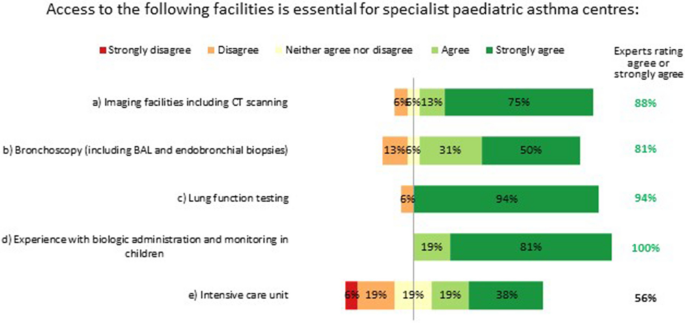
Essential facilities for specialist paediatric asthma centres. Numbers are shown in green for those statements at consensus. BAL: bronchoalveolar lavage; CT: computed tomography
Multidisciplinary staff and working practices
The experts stressed the importance of having care delivered by a multidisciplinary team working at the specialist paediatric asthma centre. In the interest of improving the standards of holistic care, the experts suggest that such teams should include the perspectives of a diverse range of specialists. For example, the following likely provide essential insights: a paediatric pulmonologist/allergologist or paediatrician; a paediatric pulmonary nurse or specialist asthma nurse; a pulmonary function analyst (or similar); a medical social worker or paediatric psychologist; and a physiotherapist with paediatric experience. Access to a dietician, a geneticist or an immunologist may be valuable should a patient’s individual circumstances warrant this. We suggest that the best composition of multidisciplinary teams will need tailoring within the constraints of existing healthcare systems and local practices, and warrants closer research.
Specialist paediatric asthma nurses have an influential role in coordinating and optimizing a patient’s care as part of the multidisciplinary team (Fig. 7 ). Interestingly, nurses’ importance and potential were recognized even though several experts work in settings where nurses play no key role at present. While recognizing the constraints that healthcare settings face, the experts unanimously agreed many essential facets of a nurse’s role (Fig. 7 ). Specialist – or possibly general – nurses could help administer biologics, according to local practices.
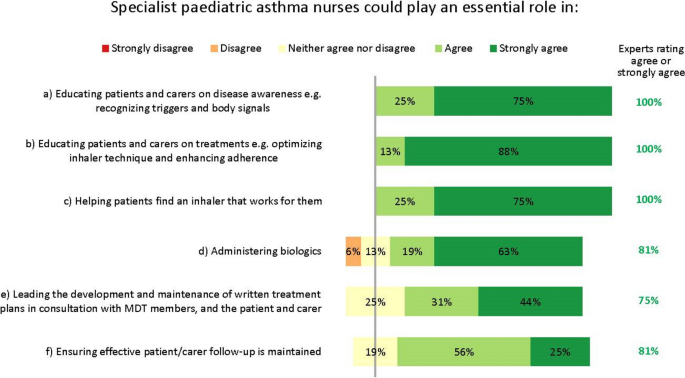
Roles of specialist paediatric asthma nurses. Numbers are shown in green for those statements at consensus. MDT: multidisciplinary team
The experts also agreed specialist nurses could lead the development and maintenance of written treatment plans, in consultation with the multidisciplinary team, the patient and their carer (Fig. 7 ). These plans should be concise and clear, and contain top-line information on the patient’s treatment goals, medications and emergency contacts, and advice on when and how to contact the medical team.
Shared decision-making and communication across clinical settings
The experts almost unanimously agreed that patients and their carers should be at the centre of decision-making on all aspects of their care and treatment; this requires joined-up management. However, stakeholder interviews revealed that collaboration between primary, specialist and specialist paediatric asthma care providers is inconsistent and uncommon, and from a carer/patient perspective, this can be a barrier to effective care.
The experts reached consensus that specialist paediatric asthma centres should keep primary care providers – and other services involved in a patient’s care – updated on treatment and management needs, especially for complex cases. However, the practicality of doing this was a concern, with some experts highlighting operational difficulties such as the administrative burden of communicating with multiple settings. Others suggested that electronic health records are sufficient to provide effective cross-setting communication, and that these are usually accessible to GPs. Further, almost all experts agreed that occasionally inviting representatives of other care settings to consultations is appropriate, but that resourcing challenges may preclude this.
Some experts suggested that specialist paediatric asthma nurses, given their oversight of treatment plans, could help bridge the gap between the specialist paediatric asthma centre and primary or secondary health providers.
Transition to adult services
The GINA guidelines state that the process of transition for paediatric to adult care should support the adolescent in gaining greater autonomy and responsibility for their own health and wellbeing [ 5 ]. However, despite its importance, stakeholder interviews revealed that this transition is inconsistent and can be poor [ 19 ]. Transition varies by country and clinical setting, and usually occurs at ages 15–18 years. Transition generally proceeds via an electronic referral only, although sometimes patients are handed over following telephone briefings or joint consultations between the paediatric and adult physicians, and often their primary care providers. The experts agreed several factors essential for achieving an effective transition (Fig. 8 ).
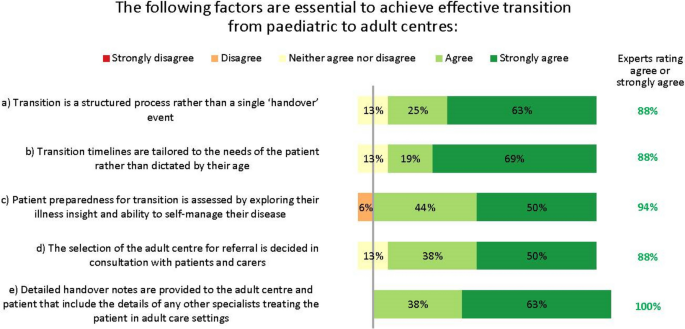
Factors needed for effective paediatric–adult services transition. Numbers are shown in green for those statements at consensus
Almost all clinical stakeholders interviewed said they would like to improve transition, and some had tried but encountered barriers, such as limited resources and complexity of scheduling joint consultations between paediatric and adult centres. However, many voting experts (63%) felt that having at least one joint consultation between the paediatric and adult specialists before formal transition would be desirable.
One major complexity associated with transition is that many patients have multimorbidities. In the paediatric setting, these are often managed by paediatricians, but in adult settings, multiple clinicians may be involved: patients report this to be overwhelming and confusing. Clinicians felt that patients prioritized the conditions of greatest impact to them and deprioritized others.
Homecare delivery of biologics
The experts unanimously agreed that when choosing a biologic, caregivers should consider clinical and practical factors and patient/carer preferences. However, in reality, cost and reimbursement also play a role in treatment decisions.
The experts agreed that for children, both hospital and homecare delivery of biologics can be appropriate, but in alignment with other Delphi groups considering adult patients, recognised that this is not suitable for all patients [ 20 ]. They therefore suggested some guidance on when to avoid homecare. Home delivery of biologics should be avoided for patients with complicated family or social circumstances, severe anxiety, lack of confidence in injection ability, or prior allergies to injections (Fig. 9 ). Despite the consensus, some suggested the injection confidence barrier could be overcome if injections were given by nurses instead of the patient or carer at the patient’s home, school or nearest healthcare centre.
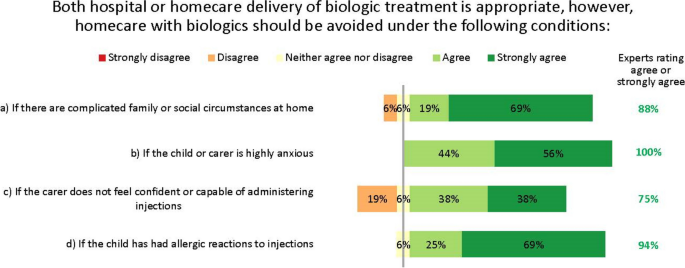
Circumstances in which homecare biologics delivery is inappropriate. Numbers are shown in green for those statements at consensus
The experts did not reach consensus on whether home spirometry may enable effective home delivery of biologics in patients with poor symptom perception. Most (56%) recognized that home spirometry can be useful to help patients recognize their symptoms, but that it can be unreliable in children because of a lack of cooperation or proficiency. Others suggested that if a patient’s symptom perception remains poor despite using home spirometry, then this would indicate biologics to be given at a clinical centre, not at home.
Research priorities for biologics
Agreed areas for further research on biologics relate mostly to treatment escalation/de-escalation, biomarkers and head-to-head comparisons between agents (Table 2 ). Where the experts did not reach consensus, it was because sufficient evidence already exists and not because the topic is of low priority.
This project convened northern European specialists in managing children with severe and difficult-to-treat asthma. While rare, these patients carry a substantial treatment burden [ 1 ]. The advent of biologic treatment has provided much-needed treatment, but questions remain unanswered about the practicalities of optimal clinical management. As a result of this project, we have agreed a framework of practical advice to optimize the care of children with difficult-to-treat and severe asthma. We encourage clinicians and policymakers to implement this practical advice to enhance patient care.
Key suggestions
Our experts’ key recommendations centre on three facets, which are summarized in Fig. 10 for quick reference: 1) early detection of asthma in children presenting with wheezing and/or dyspnoea is vital and the threshold for referral from primary to specialist care should be low; 2) children who may need biologics should be managed by specialist paediatric asthma centres, for which we have defined facilities, staff and responsibilities; and 3) shared decision-making and inter-setting communication is vital at all stages of the patient journey, up to and including transition to adult services.
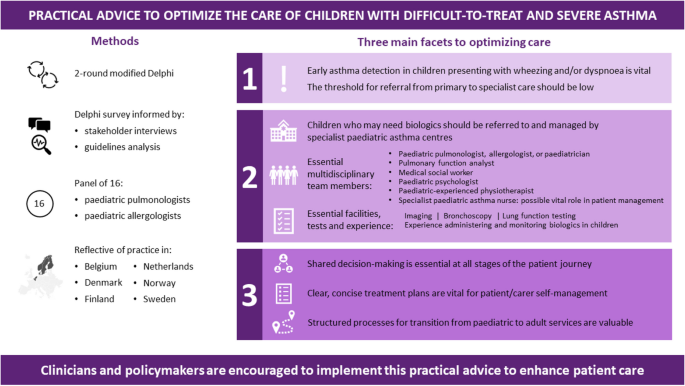
Practical advice to optimize the care of children with difficult-to-treat and severe asthma
Early detection of asthma and referral to specialist care
In some cases, diagnosing asthma in children can be done in primary care, without referring to secondary or tertiary care. However, in some countries including Belgium, primary care providers lack access to the essential diagnostic tests to make such a diagnosis. Further, some guidelines recommend that diagnostic tests for children should be done by a specialist [ 16 ]. Since the differential diagnosis can be complicated by comorbidities or non-specific symptoms, and asthma may be severe or refractory, some cases warrant referral to centres with more centralized expertise, i.e. specialist paediatric asthma centres. Crucially, the threshold for such referrals should be low [ 21 ]. This aligns with recommendations from other groups [ 1 ], including those of the UK National Working Group of asthma experts, who further suggest that timely consultation after referral is vital [ 20 ]. Our interviews and Delphi showed the triggers for referral to specialist care are unified, and align with recognized treatment-escalation criteria such as those in the GINA guidelines [ 5 ].
Interestingly, while many experts favoured its use, we did not reach consensus that FeNO testing should be mandatory for the minimum diagnostic workup for children with suspected childhood asthma, mostly because it is inconsistently available and reimbursed throughout Europe. However, its use may become more widespread now that FeNO testing is recommended by the ERS as part of the diagnostic workup for children aged 5–16 years [ 12 ]. Nevertheless, most experts agreed FeNO is useful as an add-on test for differential diagnosis, endotyping, or for more advanced diagnostics in specialist settings, and that together with measuring total/specific IgE, could be most helpful after diagnosis has been made; for example when choosing which biologic to use. These suggestions reflect what is possible and practicable within standard clinical care [ 12 ].
Specialist paediatric asthma centres
Our experts felt that providing care in specialist paediatric asthma centres could have many benefits for children with severe asthma. Indeed, the need for, and value of such centres has been shown in multiple studies [ 21 ]. Such benefits include providing patients with access to consistent care by a dedicated specialist team (something our interviewees suggested is currently lacking); giving patients a single point of contact, which may be especially important for those with complex comorbidities; and delivering optimal care informed by the latest research and clinical advances.
These benefits may be particularly acute for patients receiving biologic treatment for the most severe asthma. As our discussions and uncertainties evolved, it became clear that, while detailed clinical recommendations on biologics use have been published by GINA [ 5 ] and EAACI [ 14 ], there is little practical advice on the holistic aspects of biologics use, including the considerations for selecting home or hospital delivery of biologics, although recent Delphi projects have begun to address this [ 20 ]. Reflecting uncertainties in the literature, the experts did not reach consensus on whether home spirometry has a role in providing optimal treatment for patients on biologics, with some experts concerned about its reliability in children. For example, a 2006 retrospective study in 36 children found poor concordance between asthma severity scores and home spirometry indices among children with severe asthma [ 22 ]; conversely, a 2019 interventional study in 77 children reported improved asthma control among those who used home spirometry combined with a self-management app compared with those using conventional treatment [ 23 ]. Other studies have similarly conflicting results [ 24 , 25 ]. Despite this uncertainty, over half our experts felt that home spirometry could be valuable in some situations. Postulated benefits include the potential to help patients better recognize their symptoms, and the ability to monitor symptoms in a patient’s home environment, not an artificial clinic environment. This could inform the decision on whether the biologic should be given at a clinical centre or at home, and this potential application has prompted research into improving the reliability of this technique in children [ 23 ].
Shared decision-making and inter-setting communication
Because severe and difficult-to-treat asthma is complex, heterogenous, and often occurs with comorbidities [ 26 ], it cannot be treated in isolation: asthma care must form part of a holistic care plan in conjunction with other specialties and specialist centres, and patients themselves. Care must be integrated vertically between primary, secondary and specialist paediatric asthma settings, as well as horizontally among health, education and social services, acknowledging the impact of constraints in each area upon the others [ 21 ]. The importance of shared care is underscored many times throughout this initiative and by other researchers [ 1 , 20 , 21 ], but the reality is often different: a source of frustration for clinicians and patients [ 27 ].
An area where improving shared care is particularly needed is around the longitudinal transition between paediatric and adult care. In recognition of this need, the EAACI published specific guidance on this topic in 2020 [ 28 ], and our discussions complement their recommendations. Transition needs advanced planning and should not be a one-time event, but barriers to successful transition are legion, including a lack of standardized processes, difficulties with advanced planning, and suboptimal communication [ 13 , 29 ]. GINA guidelines state that the process of transition should support the young person in gaining greater autonomy and responsibility for their own health and wellbeing [ 5 ]. This is an important yet mostly overlooked consideration, with only the Finnish and British Thoracic Society (BTS) guidelines addressing the subject in detail [ 13 , 16 ]. Our experts’ recommendations are in line with those of the BTS, and include the need for structure, individual tailoring and patient centricity [ 13 ], and recognise the essential role of primary care providers.
Further findings
One interesting theme from our consensus is that the experts believe specialist paediatric asthma nurses could help in achieving optimal care. In the Netherlands, for example, nurses have an important role in managing the care of children with asthma. Similarly, the Finnish Care Guideline recommends that specialist nurses be involved in the treatment and care of asthma in children under school age, and for those aged < 12 years if local practices allow [ 16 ]. Nevertheless, many of our experts work in settings where nurses are not available at all, and others that do have them explained they are not effectively used to deliver paediatric asthma care.
Enhancing nurses’ existing roles to include facilitation and care oversight could improve cross-specialty and cross-setting communication. For example, by overseeing treatment plans, performing diagnostic tests and coordinating care de-escalation/escalation when appropriate, nurses could form vital bridges among all specialists involved in a patient’s care. Also, by assisting patients to receive their biologic injections at home, rather than in clinics, they could help relieve the pressure on clinic times and improve patients’ perceptions of care. This is supported by a qualitative international study in adults with severe asthma, published in 2022, which showed that the benefits of home administration of biologics usually outweigh the inconvenience and side effects: a view echoed by our experts [ 30 ].
Several studies have shown that these types of nurse-led activity can improve the outcomes of children with asthma [ 31 , 32 ], as well as with other chronic conditions [ 33 , 34 , 35 ]. Those experts whose clinics employ nurses report that they are indispensable for helping patients manage their own asthma, for example by educating them on correct inhaler technique and avoiding exacerbating factors. Indeed, a Delphi consensus project from Canada supports this assertion, recognising the critical role nurses can play in patients’ education, empowerment and ongoing management [ 36 ]. We therefore suggest nurses may help manage the burgeoning costs of biologics, by ensuring patients only receive expensive biologic drugs when all other modifiable factors have been addressed [ 18 ]. Cost-effectiveness analyses are therefore warranted.
Coordinated, nurse-led communication could also improve the longitudinal transition from paediatric to adult services, which, as we have seen, is often haphazard or lacking. Indeed, having an identified coordinator who supports the young person throughout transition is recommended by the BTS, and nurses could fill this role [ 13 ].
Strengths and limitations
This study has limitations, mostly relating to the size and composition of our expert and stakeholder groups. When scoping this project, the steering committee discussed whether to include GPs as part of the expert group. It was felt that since the project’s focus was on children with severe asthma, who are almost universally managed by specialists in the countries we represent, GPs were not included. However, we of course recognise that GPs are experts in recognising patients needing referral, thus referral needs were discussed in that context. We particularly appreciate the essential role that GPs play in managing transitional care for adolescents, and indeed, this was acknowledged during discussions. Nevertheless, our key focus was on the considerations for providing specialist attention beyond that available in primary care, and our expert group reflected this need.
Overall, few stakeholders with experience of biologics for children with difficult-to-treat or severe asthma were able to participate, citing time limitations, lack of relevant expertise with such patients and conflicts of interest. However, those who did participate all practise across a small number of northern European countries, and this focus became one of this project’s key strengths: we were well placed to highlight the similarities and differences between these countries.
Disappointingly, we found no psychologists or adolescent patients willing to be interviewed, so their views are absent. This also means that the opinions are skewed in favour of treating physicians and may overlook some considerations important for patients’ holistic care. In terms of experts, the number of specialist paediatric asthma centres across the countries is low, and experts from these centres in some (but notably not all) countries were precluded from participating in the Delphi by conflicts of interest.
Future clinical and research implications
Throughout this project, the experts recognized that there is a disconnect between the ideal standard of care described in the consensus and what is feasible within the constraints of existing healthcare models. For example, the ability of primary care providers to perform diagnostic tests – even those noted here as being essential before onward referral – varies greatly among countries and settings, and depends heavily on local practices, expertise, funding and resources [ 16 ]. This disconnect is well understood and has inspired a call for a worldwide charter for asthma in children to better implement best practice recommendations [ 37 ]. Similarly, while our consensus defines the essential capabilities and personnel needed for specialist asthma centres, in alignment with suggestions from other countries [ 38 , 39 ], in practice, the provision of these is limited by the same constraints. We suggest that investing in specialist nurses is a desirable aim for policymakers and healthcare planners. Their activities may help alleviate many of the bottlenecks and frustrations that exist within existing healthcare frameworks, improving the outcomes for patients with severe and difficult-to-treat asthma without the need for unrealistic structural changes to existing healthcare models.
Conclusions
Specialist asthma centres are valuable for treating children with severe asthma, and the threshold for referral from primary to specialist care should be low, to optimize their diagnosis, treatment and quality of care. Especially, children who may need biologics should be managed by these specialist centres, for which we have defined facilities, staff and responsibilities. Lastly, shared decision-making and inter-setting communication is vital at all stages of the patient journey, up to and including transition to adult services.
This consensus reflects optimal care in an ‘ideal world’, and we encourage clinicians and policymakers in each country to consider how they could implement our suggestions to bring the reality closer to the ideal. We propose that investing in specialist nurses could overcome many difficulties resulting from poor cross-specialty and cross-setting communication, and could ease the burden on overstretched settings without necessitating unattainable structural changes.
Availability of data and materials
All data generated or analysed during this study are included in this published article and available as supplementary information.
Abbreviations
British Thoracic Society
European Academy of Allergy & Clinical Immunology
Fractional exhaled nitric oxide
Global Initiative for Asthma
General practitioner
Haktanir Abul M, Phipatanakul W. Severe asthma in children: Evaluation and management. Allergol Int. 2019;68(2):150–7.
Article PubMed Google Scholar
Porsbjerg C, Melén E, Lehtimäki L, Shaw D. Asthma Lancet. 2023;401(10379):858–73.
Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588.
Article CAS PubMed Google Scholar
Pijnenburg MW, Fleming L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir Med. 2020;8(10):1032–44.
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Available from: https://ginasthma.org/gina-reports/ .
Lang A, Carlsen KH, Haaland G, Devulapalli CS, Munthe-Kaas M, Mowinckel P, et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy. 2008;63(8):1054–60.
Bacharier LB, Jackson DJ. Biologics in the treatment of asthma in children and adolescents. J Allergy Clin Immunol. 2023;151(3):581–9.
Canonica GW, Spanevello A, de Llano LP, Domingo Ribas C, Blakey JD, Garcia G, et al. Is asthma control more than just an absence of symptoms? An expert consensus statement. Respir Med. 2022;202:106942.
Suehs CM, Menzies-Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma. A Delphi study. Am J Respir Crit Care Med. 2021;203(7):871–81.
Menzies-Gow A, Bafadhel M, Busse WW, Casale TB, Kocks JWH, Pavord ID, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145(3):757–65.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73.
Gaillard EA, Kuehni CE, Turner S, Goutaki M, Holden KA, de Jong CCM, et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J. 2021;58(5):2004173.
British Thoracic Society (BTS), Scottish InterCollegiate Guidelines Network (SIGN). SIGN158: British guideline on the management of asthma. 2019. Available from: http://www.sign.ac.uk/our-guidelines/british-guideline-on-the-management-of-asthma/ .
Agache I, Akdis CA, Akdis M, Canonica GW, Casale T, Chivato T, et al. EAACI biologicals guidelines-recommendations for severe asthma. Allergy. 2021;76(1):14–44.
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021. Executive summary and rationale for key changes. Arch Bronconeumol. 2022;58(1):35–51.
Suomalaisen Lääkäriseuran SK. Suomen Lastenlääkäriyhdistys. Astma. 2022. Available from: https://www.kaypahoito.fi/hoi06030 .
Melén E, Guerra S, Hallberg J, Jarvis D, Stanojevic S. Linking COPD epidemiology with pediatric asthma care: implications for the patient and the physician. Pediatr Allergy Immunol. 2019;30(6):589–97.
Pijnenburg MW, Baraldi E, Brand PLP, Carlsen K-H, Eber E, Frischer T, et al. Monitoring asthma in children. Eur Respir J. 2015;45(4):906–25.
Ödling M, Andersson N, Hallberg J, Almqvist C, Janson C, Bergström A, et al. A gap between asthma guidelines and management for adolescents and young adults. J Allergy Clin Immunol Pract. 2020;8(9):3056–65.e2.
Jackson DJ, Butler C, Chaudhuri R, Pink K, Niven R, Prigmore S, et al. Recommendations following a modified UK-Delphi consensus study on best practice for referral and management of severe asthma. BMJ Open Respir Res. 2021;8(1):e001057.
Article PubMed PubMed Central Google Scholar
Pike KC, Levy ML, Moreiras J, Fleming L. Managing problematic severe asthma: beyond the guidelines. Arch Dis Child. 2018;103(4):392–7.
Brouwer AF, Roorda RJ, Brand PL. Home spirometry and asthma severity in children. Eur Respir J. 2006;28(6):1131–7.
Ljungberg H, Carleborg A, Gerber H, Ofverstrom C, Wolodarski J, Menshi F, et al. Clinical effect on uncontrolled asthma using a novel digital automated self-management solution: a physician-blinded randomised controlled crossover trial. Eur Respir J. 2019;54(5):1900983.
van der Kamp MR, Klaver EC, Thio BJ, Driessen JMM, de Jongh FHC, Tabak M, et al. WEARCON: wearable home monitoring in children with asthma reveals a strong association with hospital based assessment of asthma control. BMC Med Inform Decis Mak. 2020;20(1):192.
Deschildre A, Béghin L, Salleron J, Iliescu C, Thumerelle C, Santos C, et al. Home telemonitoring (forced expiratory volume in 1 s) in children with severe asthma does not reduce exacerbations. Eur Respir J. 2012;39(2):290–6.
Guilbert TW, Bacharier LB, Fitzpatrick AM. Severe asthma in children. J Allergy Clin Immunol Pract. 2014;2(5):489–500.
Morrow S, Daines L, Wiener-Ogilvie S, Steed L, McKee L, Caress AL, et al. Exploring the perspectives of clinical professionals and support staff on implementing supported self-management for asthma in UK general practice: an IMP(2)ART qualitative study. NPJ Prim Care Respir Med. 2017;27(1):45.
Roberts G, Vazquez-Ortiz M, Knibb R, Khaleva E, Alviani C, Angier E, et al. EAACI guidelines on the effective transition of adolescents and young adults with allergy and asthma. Allergy. 2020;75(11):2734–52.
Willis LD. Transition from pediatric to adult care for young adults with chronic respiratory disease. Respir Care. 2020;65(12):1916–22.
PubMed Google Scholar
Flokstra-de Blok B, Kocks J, Wouters H, Arling C, Chatelier J, Douglass J, et al. Perceptions on home-administration of biologics in the context of severe asthma: an international qualitative study. J Allergy Clin Immunol Pract. 2022;10(9):2312–23.e2.
Griffiths C, Foster G, Barnes N, Eldridge S, Tate H, Begum S, et al. Specialist nurse intervention to reduce unscheduled asthma care in a deprived multiethnic area: the east London randomised controlled trial for high risk asthma (ELECTRA). BMJ. 2004;328(7432):144.
Seeleman C, Stronks K, van Aalderen W, Bot ML. Deficiencies in culturally competent asthma care for ethnic minority children: a qualitative assessment among care providers. BMC Pediatr. 2012;12:47.
Mackie AS, Islam S, Magill-Evans J, Rankin KN, Robert C, Schuh M, et al. Healthcare transition for youth with heart disease: a clinical trial. Heart. 2014;100(14):1113–8.
Tappin D, Nawaz S, McKay C, MacLaren L, Griffiths P, Mohammed TA. Development of an early nurse led intervention to treat children referred to secondary paediatric care with constipation with or without soiling. BMC Pediatr. 2013;13:193.
Christie D, Thompson R, Sawtell M, Allen E, Cairns J, Smith F, et al. Structured, intensive education maximising engagement, motivation and long-term change for children and young people with diabetes: a cluster randomised controlled trial with integral process and economic evaluation - the CASCADE study. Health Technol Assess. 2014;18(20):1–202.
Godbout K, Bhutani M, Connors L, Chan CKN, Connors C, Dorscheid D, et al. Recommendations from a Canadian Delphi consensus study on best practice for optimal referral and appropriate management of severe asthma. Allergy Asthma Clin Immunol. 2023;19(1):12.
Article CAS PubMed PubMed Central Google Scholar
Szefler SJ, Fitzgerald DA, Adachi Y, Doull IJ, Fischer GB, Fletcher M, et al. A worldwide charter for all children with asthma. Pediatr Pulmonol. 2020;55(5):1282–92.
McDonald VM, Vertigan AE, Gibson PG. How to set up a severe asthma service. Respirology. 2011;16(6):900–11.
Majellano EC, Clark VL, McLoughlin RF, Gibson PG, McDonald VM. Using a knowledge translation framework to identify health care professionals’ perceived barriers and enablers for personalised severe asthma care. PLoS One. 2022;17(6):e0269038.
Download references
Acknowledgements
The authors extend special thanks to each of the stakeholders provided their vital perspectives as patients, carers, and from other clinical specialties, and whose insights were invaluable in shaping our research: Peter Csonka, Christophe Goubau, Carina Larsson, Charlotte Suppli Ulrik, Yvonne den Boer, Kim van der Klei, and the others not named. The authors wish also to thank Keena McKillen PhD (CCN17 Ltd, Cambridge, UK) for project management and facilitation of the Delphi process, Janice Steed (Steed Consulting, Cambridge UK) for facilitating meetings, and Alice Kirk (Kirk MedComms Ltd, Cambridge, UK) for preparing the manuscript and providing editorial assistance. These services were funded by Sanofi (Cambridge, MA, USA), in accordance with Good Publication Practice guidelines ( www.ismpp.org/gpp-2022 ).
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This work was funded by Sanofi. The expert panel was invited by the sponsor but remained independent, with activities coordinated by an independent Delphi facilitator.
Author information
M. W. Pijnenburg, S. Rubak, H. O. Skjerven and S. Verhulst shared first authorship.
Elenius V., Hugen C., Jauhola O., Kempeneers C., Melén E., Reier Nilsen T., Rutjes N. W., Ruotsalainen M., Schaballie H. and Zwitserloot A. M. contributed equally to this work.
Authors and Affiliations
Department of Pediatrics, Turku University Hospital, Turku, Finland
Department of Pediatrics, Radboud University Medical Center, Nijmegen, The Netherlands
Department of Allergology, Helsinki University Hospital, Helsinki, Finland
Division of Respirology, Department of Pediatrics, University Hospital Liège, Liège, Belgium
C. Kempeneers
Department of Allergology, HUS, Helsinki University Hospital, Allergic Diseases and University of Helsinki, P.O. Box 160, FIN-00029, Helsinki, Finland
M. J. Mäkelä
Sachsska Children’s Hospital and Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Stockholm, Sweden
Division of Paediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway
H. O. Skjerven
Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Department of Paediatrics, Division of Respiratory Medicine and Allergology, Erasmus University Medical Centre – Sophia Children’s Hospital, Rotterdam, The Netherlands
M. W. Pijnenburg
Department of Pediatrics, University Hospital Leuven, Leuven, Belgium
M. Proesmans
Department of Development and Regeneration, Catholic University of Leuven, Leuven, Belgium
The Norwegian Olympic Sports Centre, Oslo, Norway
T. Reier Nilsen
Department of Sports Medicine, Oslo Sport Trauma Research Centre, Oslo, Norway
Danish Center of Pediatric Pulmonology and Allergology, Department of Pediatrics and Adolescent Medicine, Department of Clinical Medicine, University Hospital of Aarhus, Aarhus University, Aarhus, Denmark
Department of Pediatrics, Kuopio University Hospital, Kuopio, Finland
M. Ruotsalainen
Department of Pediatric Pulmonology and Allergy, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
N. W. Rutjes
Pediatric Pulmonology, Pediatric Department, Ghent University Hospital, Ghent, Belgium
H. Schaballie
Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Antwerp, Belgium
S. Verhulst
Department of Pediatrics, Antwerp University Hospital, Antwerp, Belgium
Beatrix Children’s Hospital Department of Pediatric Pulmonology and Pediatric Allergy, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
A. M. Zwitserloot
University of Groningen, University Medical Center Groningen, Groningen Research Institute for Asthma and COPD (GRIAC), Groningen, The Netherlands
Skin and Allergy Hospital, Meilahdentie 2, Helsinki, Finland
You can also search for this author in PubMed Google Scholar
Contributions
MJM, MWP, SR, HOS, and SV designed and guided the Delphi process. All authors participated in the Delphi process and read and approved the final manuscript.
Corresponding author
Correspondence to M. J. Mäkelä .
Ethics declarations
Ethics approval and consent to participate.
According to the Finnish Medical Research Act (488/1999) available at https://www.finlex.fi/fi/laki/ajantasa/1999/19990488 , ethics committee approval for this study was unnecessary. All participants gave their informed consent to participate in stakeholder interviews or the Delphi, with all contributions anonymised.
Consent for publication
All participants consented to their views' publication.
Competing interests
V Elenius has received lecture and/or advisory board fees from Glaxo, Novartis, Sanofi, Vertex Pharmaceuticals outside the present study. C Hugen has no competing interest to declare outside the present study. O Jauhola has received lecture and/or advisory fees from Airsonett and Astra Zeneca outside the present study. C Kempeneers has received research grant and travel funding from GSK, outside the submitted work. MJ Mika has no competing interest to declare outside the present study. E Melén has received lecture and/or advisory board fees from Airsonett, ALK, AstraZeneca, Chiesi and Sanofi outside the present study. HO Skjerven is participating in MSD and Boehringer Ingelheim studies, and has received advisory board fees from Boehringer Ingelheim and Sanofi outside the present study. M Pijnenburg is involved in a DMC of Novartis, is participating in studies of AstraZeneca and Sanofi and received advisory board fees from Sanofi outside the present study. M Proesmans has no competing interest to declare outside the present study. T Reier-Nilsen has received fees for lectures, advisory boards or consultant work from ALK, MediTuner and Sanofi outside the present study. S Rubak has no competing interest to declare outside the present study. M Ruotsalainen has no competing interest to declare outside the present study. NW Rutjes has received advisory board fees from GlaxoSmithKline and Sanofi, outside the submitted work. H Schaballie has received advisory board fees from Pfizer and Sanofi, outside the submitted work. S Verhulst has no competing interest to declare outside the present study. AM Zwitserloot reports a research grant (money to institution) from Vertex Pharmaceuticals, outside the submitted work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pijnenburg, M.W., Rubak, S., Skjerven, H.O. et al. Optimizing care for children with difficult-to-treat and severe asthma through specialist paediatric asthma centres: expert practical experience and advice. BMC Pediatr 24 , 218 (2024). https://doi.org/10.1186/s12887-024-04707-0
Download citation
Received : 02 January 2024
Accepted : 13 March 2024
Published : 27 March 2024
DOI : https://doi.org/10.1186/s12887-024-04707-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Difficult-to-treat asthma
- Severe asthma
- Asthma care
- Asthma nurses
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
3,696 Clinical Research Project Specialist jobs available on Indeed.com. Apply to Clinical Specialist, Clinical Nurse Specialist, Research Specialist and more!
Program Specialist - Clinical Research. Veterans Health Administration San Francisco, CA. $90,310 Annually. Full-Time. Employee will serve as a Program Specialist - Human Research Protection Program (HRPP) Coordinator ... Project Management IMPORTANT: A full year of work is considered to be 35-40 hours of work per week.
As a clinical research specialist, your job is to coordinate clinical research projects. Your duties involve planning and directing the projects by preparing each study, directing the team and participants, and maintaining detailed records of the findings. The career usually requires at least a bachelor's degree in a health care field and ...
Today's top 1,000+ Clinical Research Project Specialist jobs in United States. Leverage your professional network, and get hired. New Clinical Research Project Specialist jobs added daily.
The top companies hiring now for clinical research specialist jobs in United States are Black Hills Regional Eye Institute, Molecular Testing Labs, Flourish Research, Alliance of Multispeciality Research, MD Anderson Cancer Center, Emory University, American Society of Clinical Oncology, Alliance Clinical Network, Worldwide Clinical Trials ...
The estimated total pay for a Clinical Project Specialist is $96,073 per year in the United States area, with an average salary of $85,657 per year. These numbers represent the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users.
Analytical and writing skills are also very important, because a CPM job will involve developing documents such as protocols, informed consent documents, contracts, and grants. A skilled CPM should also be familiar with the financial aspects of a clinical study, including reviewing invoices, performing daily accounting tasks, and preparing budgets.
After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate). The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry.
Project Specialist At Syneos Health our Project Specialists are responsible for maintaining and coordinating the logistical aspects of clinical projects and provide overall support to functional leads to ensure the successful completion of project deliverables. As Project Specialists we ensure the contracted services and expectations of ...
The first step to becoming a clinical research specialist is to earn a bachelor's degree from an accredited university. The course of study may vary, but degrees related to health science provide a robust starting point. Many individuals who obtain clinical research specialist jobs begin their journey with a bachelor's degree in nursing ...
Whether you're a Clinical Research Associate, Clinical Trial Manager, Project Specialist, Project Manager or Director or work in Study Start Up; we all work in sync to deliver results and bring therapeutics to the patients that need them. ... and site management activities for Phase I-IV clinical research projects. Our job is to assess the ...
Clinical Project Managers make sure that projects follow the research protocols, good clinical practices (GCPs), applicable regulations, and standards Research Project Managers also develop protocols for data collection and analysis, prepare reports for regulatory submissions, coordinate activities related to safety monitoring, and provide ...
The University of Michigan Congenital Heart Center is seeking a Clinical Research Project Manager to serve as project manager and study coordinator for a pediatric, multi-center, investigator-initiated, FDA-regulated device clinical trial led by the University of Michigan. The device is patient-specific and the IDE is held by a University of ...
An Clinical Trials Manager at Qureight Ltd and a recent graduate from the University of Cambridge. Throughout my studies and work experience, I have demonstrated that I am a keen and quick learner and a highly motivated individual. I have experience in imaging research and in managing multicentral and multination imaging studies and working with both academic and industrial partners.
Alla Kholmogorova currently works at the Moscow State University of Psychology and Education (dean of the faculty of Counseling and Clinical Psychology). Alla does research in Health Psychology ...
Recruitment of volunteers will be competitive. A maximum of 450 children aged 12 to 17 years inclusive will be screened in the study, of which it is planned to include and randomize 300 children who meet the criteria for inclusion in the study and do not have non-inclusion criteria, data on which will be used for subsequent safety and immunogenicity analysis.
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
Severe asthma in children carries an unacceptable treatment burden, yet its rarity means clinical experience in treating it is limited, even among specialists. Practical guidance is needed to support clinical decision-making to optimize treatment for children with this condition.This modified Delphi convened 16 paediatric pulmonologists and allergologists from northern Europe, all experienced ...