Treatment Research
In a new study in mice, researchers showed they could enhance radiation therapy by boosting levels of the BAMBI protein in MDSC immune cells in the tumor microenvironment. After radiation, T cells flooded into the tumor and killed tumors elsewhere in the body.
In a clinical trial, people being treated for cancer who participated in virtual mind–body fitness classes were less likely to be hospitalized, and had shorter stays when they were hospitalized, than people who did not take the classes.
NCI’s James H. Doroshow, M.D., reflects on the accomplishments of NCI-MATCH, a first-of-its-kind precision medicine cancer trial, and gives an overview of three new successor trials: ComboMATCH, MyeloMATCH, and iMATCH.
A new study, conducted largely in mice, may help explain why a currently used molecular marker—called mismatch repair deficiency—doesn’t always work to predict which patients will respond to immunotherapies called immune checkpoint inhibitors.
New approach may increase the effectiveness of T-cell-based immunotherapy treatments against solid tumors.
A cancer-infecting virus engineered to tamp down a tumor’s ability to suppress the immune system shrank tumors in mice, a new study shows. The modified oncolytic virus worked even better when used along with an immune checkpoint inhibitor.
Despite recommendations, a new analysis shows few people with cancer undergo germline testing to learn if their cancer may have been caused by gene changes inherited from a parent. Germline testing can help doctors determine the best treatments for a patient and help identify people whose family members may be at higher risk of cancer.
ComboMATCH will consist of numerous phase 2 cancer treatment trials that aim to identify promising drug combinations that can advance to larger, more definitive clinical trials.
A new study has compared three formulations of an mRNA vaccine designed to treat cancers caused by human papillomavirus (HPV) infections. All three vaccines showed promise in mice.
Researchers have identified a mechanism by which cancer cells develop specific genetic changes needed to become resistant to targeted therapies. They also showed that this process, called non-homologous end-joining (NHEJ), can potentially be disrupted.
For some people with cancer, is 6 months of immunotherapy the only treatment they might ever need? Or 4 weeks of immunotherapy followed by minor surgery? Results from several small clinical trials suggest these scenarios may be bona fide possibilities.
Two research teams have developed ways of overcoming barriers that have limited the effectiveness of CAR T-cell therapies, including engineering ways to potentially make them effective against solid tumors like pancreatic cancer and melanoma.
In people with cancer treated with immune checkpoint inhibitors, a rare, but often fatal, side effect is inflammation in the heart, called myocarditis. Researchers have now identified a potential chief cause of this problem: T cells attacking a protein in heart cells called α-myosin.
Researchers have modified a chemo drug, once abandoned because it caused serious gut side effects, so that it is only triggered in tumors but not normal tissues. After promising results in mice, the drug, DRP-104, is now being tested in a clinical trial.
Two research teams have developed a treatment approach that could potentially enable KRAS-targeted drugs—and perhaps other targeted cancer drugs—flag cancer cells for the immune system. In lab studies, the teams paired these targeted drugs with experimental antibody drugs that helped the immune system mount an attack.
Inflammation is considered a hallmark of cancer. Researchers hope to learn more about whether people with cancer might benefit from treatments that target inflammation around tumors. Some early studies have yielded promising results and more are on the horizon.
NCI researchers are developing an immunotherapy that involves injecting protein bits from cytomegalovirus (CMV) into tumors. The proteins coat the tumor, causing immune cells to attack. In mice, the treatment shrank tumors and kept them from returning.
FDA has approved the combination of the targeted drugs dabrafenib (Tafinlar) and trametinib (Mekinist) for nearly any type of advanced solid tumor with a specific mutation in the BRAF gene. Data from the NCI-MATCH trial informed the approval.
People with cancer who take immunotherapy drugs often develop skin side effects, including itching and painful rashes. New research in mice suggests these side effects may be caused by the immune system attacking new bacterial colonies on the skin.
Researchers have developed tiny “drug factories” that produce an immune-boosting molecule and can be implanted near tumors. The pinhead-sized beads eliminated tumors in mice with ovarian and colorectal cancer and will soon be tested in human studies.
Women are more likely than men to experience severe side effects from cancer treatments such as chemotherapy, targeted therapy, and immunotherapy, a new study finds. Researchers hope the findings will increase awareness of the problem and help guide patient care.
Research to improve CAR T-cell therapy is progressing rapidly. Researchers are working to expand its use to treat more types of cancer and better understand and manage its side effects. Learn how CAR T-cell therapy works, which cancers it’s used to treat, and current research efforts.
Experts say studies are needed on how to best transition telehealth from a temporary solution during the pandemic to a permanent part of cancer care that’s accessible to all who need it.
Removing immune cells called naive T cells from donated stem cells before they are transplanted may prevent chronic graft-versus-host disease (GVHD) in people with leukemia, a new study reports. The procedure did not appear to increase the likelihood of patients’ cancer returning.
A specific form of the HLA gene, HLA-A*03, may make immune checkpoint inhibitors less effective for some people with cancer, according to an NCI-led study. If additional studies confirm the finding, it could help guide the use of these commonly used drugs.
The success of mRNA vaccines for COVID-19 could help accelerate research on using mRNA vaccine technology to treat cancer, including the development of personalized cancer vaccines.
Aneuploidy—when cells have too many or too few chromosomes—is common in cancer cells, but scientists didn’t know why. Two new studies suggest that aneuploidy helps the cells survive treatments like chemotherapy and targeted therapies.
New research suggests that fungi in the gut may affect how tumors respond to cancer treatments. In mice, when bacteria were eliminated with antibiotics, fungi filled the void and impaired the immune response after radiation therapy, the study found.
FDA has approved belumosudil (Rezurock) for the treatment of chronic graft-versus-host disease (GVHD). The approval covers the use of belumosudil for people 12 years and older who have already tried at least two other therapies.
In lab studies, the antibiotic novobiocin showed promise as a treatment for cancers that have become resistant to PARP inhibitors. The drug, which inhibits a protein called DNA polymerase theta, will be tested in NCI-supported clinical trials.
A drug called avasopasem manganese, which has been found to protect normal tissues from radiation therapy, can also make cancer cells more vulnerable to radiation treatment, a new study in mice suggests.
While doctors are familiar with the short-term side effects of immune checkpoint inhibitors, less is known about potential long-term side effects. A new study details the chronic side effects of these drugs in people who received them as part of treatment for melanoma.
Cholesterol-lowering drugs known as PCSK9 inhibitors may improve the effectiveness of cancer immune checkpoint inhibitors, according to studies in mice. The drugs appear to improve the immunotherapy drugs’ ability to find tumors and slow their growth.
Researchers have developed a nanoparticle that trains immune cells to attack cancer. According to the NCI-funded study, the nanoparticle slowed the growth of melanoma in mice and was more effective when combined with an immune checkpoint inhibitor.
A comprehensive analysis of patients with cancer who had exceptional responses to therapy has revealed molecular changes in the patients’ tumors that may explain some of the exceptional responses.
Researchers are developing a new class of cancer drugs called radiopharmaceuticals, which deliver radiation therapy directly and specifically to cancer cells. This Cancer Currents story explores the research on these emerging therapies.
FDA has recently approved two blood tests, known as liquid biopsies, that gather genetic information to help inform treatment decisions for people with cancer. This Cancer Currents story explores how the tests are used and who can get the tests.
Cancer cells with a genetic feature called microsatellite instability-high (MSI-high) depend on the enzyme WRN to survive. A new NCI study explains why and reinforces the idea of targeting WRN as a treatment approach for MSI-high cancer.
Efforts to contain the opioid epidemic may be preventing people with cancer from receiving appropriate prescriptions for opioids to manage their cancer pain, according to a new study of oncologists’ opioid prescribing patterns.
The gene-editing tool CRISPR is changing the way scientists study cancer, and may change how cancer is treated. This in-depth blog post describes how this revolutionary technology is being used to better understand cancer and create new treatments.
FDA’s approval of pembrolizumab (Keytruda) to treat people whose cancer is tumor mutational burden-high highlights the importance of genomic testing to guide treatment, including for children with cancer, according to NCI Director Dr. Ned Sharpless.
Patients with acute graft-versus-host disease (GVHD) that does not respond to steroid therapy are more likely to respond to the drug ruxolitinib (Jakafi) than other available treatments, results from a large clinical trial show.
NCI is developing the capability to produce cellular therapies, like CAR T cells, to be tested in cancer clinical trials at multiple hospital sites. Few laboratories and centers have the capability to make CAR T cells, which has limited the ability to test them more broadly.
An experimental drug may help prevent the chemotherapy drug doxorubicin from harming the heart and does so without interfering with doxorubicin’s ability to kill cancer cells, according to a study in mice.
In people with blood cancers, the health of their gut microbiome appears to affect the risk of dying after receiving an allogeneic hematopoietic stem cell transplant, according to an NCI-funded study conducted at four hospitals across the globe.
A novel approach to analyzing tumors may bring precision cancer medicine to more patients. A study showed the approach, which analyzes gene expression using tumor RNA, could accurately predict whether patients had responded to treatment with targeted therapy or immunotherapy.
Bone loss associated with chemotherapy appears to be induced by cells that stop dividing but do not die, a recent study in mice suggests. The researchers tested drugs that could block signals from these senescent cells and reverse bone loss in mice.
Some experts believe that proton therapy is safer than traditional radiation, but research has been limited. A new observational study compared the safety and effectiveness of proton therapy and traditional radiation in adults with advanced cancer.
In people with cancer, the abscopal effect occurs when radiation—or another type of localized therapy—shrinks a targeted tumor but also causes untreated tumors in the body to shrink. Researchers are trying to better understand this phenomenon and take advantage of it to improve cancer therapy.
An experimental drug, AMG 510, that targets mutated forms of the KRAS protein completely shrank tumors in cancer mouse models and data from a small clinical trial show that it appears to be active against different cancer types with a KRAS mutation.
Researchers have engineered an oncolytic virus to kill cancer cells and boost the immune response against tumors. In a new study, the virus provided T cells around tumors with a hormone they need for their own cell-killing functions.
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
A new NCI-supported study showed that altering cancer cell metabolism by feeding mice a diet very low in the nutrient methionine improved the ability of chemotherapy and radiation therapy to shrink tumors.
An NCI-funded clinical trial is testing the immunotherapy drug nivolumab (Opdivo) in people who have advanced cancer and an autoimmune disease, such as rheumatoid arthritis, lupus, or multiple sclerosis, who are often excluded from such trials.
Researchers have identified a protein called CD24 that may be a new target for cancer immunotherapy. The protein is a ‘don’t eat me’ signal that prevents immune cells called macrophages from engulfing and eating cells.
Injecting cells undergoing necroptosis, a form of cell death, into tumors in mice kickstarted an immune response against the tumors, researchers have found. When combined with immunotherapy, the treatment was effective at eliminating tumors in mice.
Researchers have identified proteins that may play a central role in transforming T cells from powerful destroyers to depleted bystanders that can no longer harm cancer cells. The findings could lead to strategies for boosting cancer immunotherapies.
Did you know that NCI supports clinical trials of new treatments for pet dogs with cancer? Learn more about NCI’s comparative oncology studies and how they may also help people with cancer.
Researchers have discovered a potential way to turn on one of the most commonly silenced tumor-suppressor proteins in cancer, called PTEN. They also found a natural compound, I3C, that in lab studies could flip the on switch.
New findings from a clinical trial suggest that a single dose of radiation therapy may control painful bone metastases as effectively as multiple lower doses of radiation therapy.
The expanding use of cancer immunotherapy has revealed a variety of side effects associated with this treatment approach. Researchers are now trying to better understand how and why these side effects occur and develop strategies for better managing them.
The investigational immunotherapy drug bintrafusp alfa (also called M7824), a bifunctional fusion protein, shrank the tumors of some patients with advanced HPV-related cancers, according to results from a phase 1 clinical trial.
A new study provides insight into how cancer immunotherapy works and suggests ways to enhance the treatment’s effectiveness. The NCI-led study, published in Science, examined the effect of high potassium levels on T cells.
Pain is a common and much-feared symptom among people with cancer and long-term survivors. As more people survive cancer for longer periods, there is a renewed interest in developing new, nonaddictive approaches for managing their chronic pain.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source

Investigators develop novel treatment for T-cell leukemias and lymphomas
by Johns Hopkins University School of Medicine
A novel treatment for leukemias and lymphomas that arise from immune system T cells, developed by investigators at the Johns Hopkins Kimmel Cancer Center and its Ludwig Center and Lustgarten Laboratory, was found to be effective at killing these cancers in mice bearing human T-cell tumors.
The therapy, an antibody-drug conjugate (ADC), combines an antibody that targets a protein called TRBC1 expressed on the surface of T-cell cancers with an anti-cancer drug called SG3249. The ADC works by using the antibody to seek out the cancer cells that express TRBC1. Then, those cancer cells ingest the ADC, where SG3249 is released and kills the cancer cells . A description of the work was published in Nature .
Each year, about 100,000 patients worldwide are affected by T-cell leukemias and lymphomas. Adults with relapsed T-cell cancers have limited therapeutic options and five-year survival rates of 7–38%.
"Developing treatments for T-cell leukemias and lymphomas is much more difficult than for leukemias and lymphomas arising from immune system B cells," explains senior study author Suman Paul, M.B.B.S., Ph.D., an assistant professor of oncology at the Johns Hopkins University School of Medicine.
Effective therapies for B-cell cancers wipe out both cancerous and noncancerous B cells, but patients still do well even without the immune system B cells that help fight infections. However, if similar approaches are used and a treatment wipes out both normal and cancerous T cells, it would leave patients without a functioning immune system and at high risk of dying from infections.
"Not much drug development has happened in this space of T-cell leukemias and lymphomas," Paul says. "We need new therapies for these cancers, but whatever therapies we develop in the space have to be cancer-specific. We have to preserve some of the normal T cells and wipe out cancerous T cells at the same time."
T-cell cancers express either TRBC1 or TRBC2, while normal T-cells express a mix of TRBC1 and TRBC2. Therefore, selective targeting of TRBC1 can potentially eradicate the normal and cancerous T cells expressing TRBC1 while preserving normal T cells expressing TRBC2. A recent clinical trial conducted elsewhere attempted to target TRBC1 cancers using chimeric antigen receptor (CAR) T-cell therapy.
These CAR T cells are genetically engineered T cells that bind to and kill TRBC1 cells. CAR T-cell therapies are FDA-approved treatment options used in several B-cell cancers. However, after administering the TRBC1-targeting CAR T cell therapy in human patients , trial investigators reported that the CAR T cells were not persisting inside the patients.
Such persistence is required for effective cancer cell-killing. Interested to understand why, Paul and colleagues found that the CAR T cells targeting TRBC1 could be killed by normal T cells, limiting their persistence.
This lack of CAR T-cell persistence led the team to try TRBC1 targeting with the use of antibody-drug conjugates. Paul and colleagues tried two different formulations of ADCs in mouse models of T-cell cancers. After a single injection of one formulation of the treatment, the cancers initially regressed but then recurred. After a single treatment with the anti-TRBC1-SG3249 ADC combination, investigators observed signs of cancer elimination within seven days, and the cancers were eventually undetectable, with no recurrences.
"The tumors didn't come back, and we followed the mice for more than 200 days," Paul explains.
The treatment was able to eliminate the cancer while preserving half of the remaining normal T cells. "The residual normal T cells should be sufficient to maintain some immune system protection against infectious diseases ," Paul says.
"Witnessing the successful elimination of T-cell cancers while sparing normal T cells in preclinical models was truly gratifying," adds Jiaxin Ge, a co-author of the study and third-year Ph.D. student in the Ludwig Center. "We believe this approach has the potential to address a critical unmet need in oncology, and we're committed to advancing it through further research."
Tushar Nichakawade, first author on the study and a fourth-year Ph.D. student at the Ludwig Center, says, "There are so many lessons to learn from the clinic, and it has been exciting to be a part of the iterative process of drug discovery. Every therapy has its pros and cons, but the preclinical efficacy of our ADC gives me hope that it can make a difference for patients suffering from these terrible cancers."
Investigators are now working with an industry partner to conduct early-phase safety and efficacy trials in human patients.
Explore further
Feedback to editors

Dogs may provide new insights into human aging and cognition
14 hours ago
AI's ability to detect tumor cells could be key to more accurate bone cancer prognoses
15 hours ago
Novel pre-clinical models help advance therapeutic development for antibiotic-resistant bacterial infections

Far-UVC light can virtually eliminate airborne virus in an occupied room, study shows

Las Vegas mass shooting survivors continue to struggle with major depression, PTSD
16 hours ago

Combining food taxes and subsidies can lead to healthier grocery purchases for low-income households
New insights into muscle health: How innervation influences the recovery process
Simulations reveal mechanism behind protein buildup in Parkinson's disease

Increasing positive affect in adolescence could lead to improved health and well-being in adulthood
17 hours ago

Study suggests lung cancer does not decrease in line with reduced smoking
18 hours ago
Related Stories
New mechanism with potential to boost checkpoint-blocking cancer immunotherapies identified
Jan 15, 2024

CAR T cell therapy for T cell lymphoma shows promise in phase I trial
Jan 4, 2024

New CAR T-cell therapy research shows potential in solid tumors
Apr 7, 2023

Expanding the impact of CAR T cell therapy: An immunotherapy strategy against all blood cancers
Aug 31, 2023

CAR-T cell researchers optimistic about future of treating blood cancers
Sep 15, 2023

Researchers develop approach that could help supercharge T-cell therapies against solid tumors
Nov 1, 2023
Recommended for you

New insights into aggressive breast cancer and potential treatment options
19 hours ago

Study finds triple-negative breast cancer tumors with increased immune cells have lower risk of recurrence after surgery
20 hours ago
Study finds AI empowers patients before and after seeing physicians for radiation oncology treatment

New study seeks hereditary causes of childhood cancer
New insights into how tumors on adrenal glands develop
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Watch Now : CRI’s Patient Immunotherapy Summit
Cancer Research Institute Media Room

Cellular Cancer Immunotherapy Development Evolves, Expands with New Technologies and Targets
Latest analysis from the Cancer Research Institute of the global landscape of cellular immunotherapies, including R&D trends and real-world usage, highlights key challenges including effective solid tumor targeting, manufacturing complexities, and commercial access to approved therapies
The Cancer Research Institute (CRI), a nonprofit organization dedicated to the discovery and development of powerful immunotherapies for all types of cancer, announced today the publication of its newest analysis of the global landscape of cellular immunotherapies, including R&D trends and real-world usage data. The report, published today in Nature Reviews Drug Discovery , highlights trends in cellular immunotherapy for cancer including top modalities, targets, clinical development, and data from patients receiving CAR-T therapies in clinical practice. This report is an update to CRI’s prior cellular immunotherapy landscape analysis published in July 2021.
In this analysis, author Samik Upadhaya, PhD, assistant director of scientific affairs and member of the Anna-Maria Kellen Clinical Accelerator team at CRI, and colleagues provide an update on the overall status of the cellular cancer immunotherapy landscape as well as observations on key changes within the field including clinical practice. Findings include:
- As of April 15, 2022, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape analysis that identified 2,031 such agents, but also a modest deceleration compared to 43% growth in the prior year
- CAR-T therapeutics continue to dominate the cell therapy pipeline with growth of 24% since 2021
- Development continues for non-T cell therapies including NK cell, dendritic cell, stem cell, and other myeloid-derived cell therapies, with the greatest growth in NK cell therapy, up 55% over the prior year
- Clinical usage of cell therapy for cancer treatment is not keeping pace with regulatory approvals, with clinicians citing cost, travel, and supply limitations as key barriers to patient access
This latest report from the Cancer Research Institute, titled, “Landscape of cancer cell therapies: trends and real-world data,” was generated in collaboration with IQVIA, a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, which provided the authors with access to IQVIA’s proprietary clinical trials database. The report is part of a suite of CRI-owned immuno-oncology landscape analyses that includes reports on cell therapy drug development and the broader IO landscape including clinical development of checkpoint inhibitors, cancer vaccines, and oncolytic viruses in addition to bispecific antibodies and other immunomodulators.
To access an interactive dashboard of the Cancer Research Institute’s cancer cell immunotherapy report, visit the CRI website at cancerresearch.org/cell-therapy .
About the Cancer Research Institute The Cancer Research Institute (CRI), established in 1953, is a highly rated U.S. nonprofit organization dedicated exclusively to saving more lives by fueling the discovery and development of powerful immunotherapies for all cancers. Guided by a world-renowned Scientific Advisory Council that includes four Nobel laureates and 27 members of the National Academy of Sciences, CRI has invested $474 million in support of research conducted by immunologists and tumor immunologists at the world’s leading medical centers and universities and has contributed to many of the key scientific advances that demonstrate the potential for immunotherapy to change the face of cancer treatment. To learn more, go to cancerresearch.org .
About the Anna-Maria Kellen Clinical Accelerator CRI’s clinical program, the Anna-Maria Kellen Clinical Accelerator is a unique academic-nonprofit-industry collaboration model that serves an as “incubator” that delivers multicenter clinical trials of promising new immunotherapy combinations. CRI’s venture philanthropy fund supports clinical trials within the program, which fosters a collaborative environment that enables scientists to advance their most ambitious research ideas by accelerating studies that one group or company could not do alone. To learn more, go to cancerresearch.org/clinical-accelerator .
Let's spread the word about Immunotherapy! Click to share this page with your community.
This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy .

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Cancer research highlights from 2023
Share this:.
By Mayo Clinic staff
Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and in the future. Here are some highlights from their research over the past year:
Mayo Clinic researchers link ovarian cancer to bacteria colonization in the microbiome.
A specific colonization of microbes in the reproductive tract is commonly found in people with ovarian cancer, according to a study from the Mayo Clinic Center for Individualized Medicine . Published in Scientific Reports and led by Marina Walther-Antonio, Ph.D. , a Mayo Clinic researcher, and Abigail Asangba, Ph.D., the discovery strengthens the evidence that the bacterial component of the microbiome — a community of microorganisms that also consists of viruses, yeasts and fungi — is an important indicator for early detection, diagnosis and prognosis of ovarian cancer . The study also suggests that a higher accumulation of pathogenic microbes plays a role in treatment outcomes and could be a potential indicator for predicting a patient's prognosis and response to therapy. Read more .
Artificial intelligence is forging a new future for colorectal cancer and other digestive system diseases.
Colonoscopy remains the gold standard in detecting and preventing colorectal cancer , but the procedure has limitations. Some studies suggest that more than half of post-colonoscopy colon cancer cases arise from lesions missed at patients' previous colonoscopies. In 2022, Michael Wallace, M.D. , a Mayo Clinic gastroenterologist, published the results of an international, multicenter study testing the impact of adding artificial intelligence (AI) to routine colonoscopies. His team, including James East, M.D. , a Mayo Clinic gastroenterologist, and other researchers from the U.S., the U.K., Italy, Germany and Ireland, found that incorporating AI into colonoscopies reduced the risk of missing polyps by 50%. Read more .
A big step forward: Bringing DNA sequencing data to routine patient care.
The Tapestry study , an extensive genomic sequencing clinical research study, aims to complete exome sequencing (sequencing the protein-coding regions of a genome) for 100,000 Mayo Clinic patients. The results will be integrated into patients’ electronic health records for three hereditary conditions, and the amassed data will contribute to a research dataset stored within the Mayo Clinic Cloud on the Omics Data Platform. The overall hope of Tapestry is to accelerate discoveries in individualized medicine to tailor prevention, diagnosis and treatment to a patient's unique genetic makeup. It is poised to advance evidence that exome sequencing, when applied to a diverse and comprehensive general population, can proficiently identify carriers of genetic variants that put them at higher risk for a disease, allowing them to take preventive measures. Read more .
Patients with multiple tumors in one breast may not need a mastectomy.
Patients who have multiple tumors in one breast may be able to avoid a mastectomy if surgeons can remove the tumors while leaving enough breast tissue, according to research led by the Alliance in Clinical Trials in Oncology and Mayo Clinic Comprehensive Cancer Center . Patients would receive breast-conserving therapy — a lumpectomy followed by whole-breast radiation therapy — rather than mastectomy . The study is published in the Journal of Clinical Oncology . Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, says Judy Boughey, M.D. , lead author, Mayo Clinic breast surgical oncologist and the W.H. Odell Professor of Individualized Medicine. Read more .
Staging pancreatic cancer early with minimally invasive surgery shows positive results in patient prognosis.
A study published in the Journal of the American College of Surgeons reveals that performing a minor surgical procedure on patients newly diagnosed with pancreatic cancer helps to identify cancer spread early and determine the stage of cancer. The researchers add that the surgery ideally should be performed before the patient begins chemotherapy. "This is an important study because it supports that staging laparoscopy may help determine a patient's prognosis and better inform treatment so that patients avoid unhelpful or potentially harmful surgical therapy," says Mark Truty, M.D. , a Mayo Clinic surgical oncologist who led the research. Read more .
Mayo Clinic study reveals proton beam therapy may shorten breast cancer treatment.
In a trial published in The Lancet Oncology , Mayo Clinic Comprehensive Cancer Center researchers uncovered evidence supporting a shorter treatment time for people with breast cancer . The study compared two separate dosing schedules of pencil-beam scanning proton therapy , known for its precision in targeting cancer cells while preserving healthy tissue to reduce the risk of side effects. The investigators found that both 25-day and 15-day proton therapy schedules resulted in excellent cancer control while sparing surrounding non-cancerous tissue. Further, complication rates were comparable between the two study groups. "We can now consider the option of 15 days of therapy for patients based on the similar treatment outcomes observed," says Robert Mutter, M.D. , a Mayo Clinic radiation oncologist and physician-scientist. Read more .
Harnessing the immune system to fight ovarian cancer.
Mayo Clinic research is biomanufacturing an experimental, cell-based ovarian cancer vaccine and combining it with immunotherapy to study a "one-two punch" approach to halting ovarian cancer progression. This research begins with a blood draw from people with advanced ovarian cancer whose tumors have returned after standard surgery and chemotherapy. White blood cells are extracted from the blood, biomanufactured to become dendritic cells and returned to the patient. Dendritic cells act as crusaders that march through the body, triggering the immune system to recognize and fight cancer. "We're building on an earlier phase 1 clinical trial that showed promising results in terms of survival after the dendritic cell-based vaccine," says Matthew Block, M.D., Ph.D. , co-principal investigator and Mayo Clinic medical oncologist. "Of the 18 evaluable patients in the phase 1 study, 11 had cancer return, but seven of them — 40% — have been cancer-free for almost 10 years. We typically expect 90% of patients in this condition to have the cancer return." Read more .
New gene markers detect Lynch syndrome-associated colorectal cancer.
Researchers from Mayo Clinic Comprehensive Cancer Center and Mayo Clinic Center for Individualized Medicine have discovered new genetic markers to identify Lynch syndrome-associated colorectal cancer with high accuracy. Studies are underway to determine if these genetic markers are in stool samples and, if so, how this could lead to a non-invasive screening option for people with Lynch syndrome . The research was published in Cancer Prevention Research , a journal of the American Association for Cancer Research. "This is an exciting finding that brings us closer to the reality that clinicians may soon be able to offer a non-invasive cancer screening option to patients with the highest risk of getting cancer," says Jewel Samadder, M.D. , co-lead author of the paper and a Mayo Clinic gastroenterologist. Read more .
Mayo Clinic prepares to biomanufacture a new CAR-T cell therapy for B-cell blood cancers.
Mayo Clinic research has developed a new type of chimeric antigen receptor-T cell therapy (CAR-T cell therapy) aimed at killing B-cell blood cancers that have returned and are no longer responding to treatment. This pioneering technology, designed and developed in the lab of Hong Qin, M.D., Ph.D. , a Mayo Clinic cancer researcher, killed B-cell tumors grown in the laboratory and tumors implanted in mouse models. The preclinical findings are published in Cancer Immunology, Immunotherapy . "This study shows our experimental CAR-T cell therapy targets several blood cancers, specifically chronic lymphocytic leukemia," says Dr. Qin. "Currently, there are six different CAR-T cell therapies approved for treatment of relapsed blood cancers. While the results are impressive, not everyone responds to this treatment. Our goal is to provide novel cell therapies shaped to each patient's individual need." Read more .
Related Posts

After receiving CAR-T cell therapy at Mayo Clinic, Welsh-born John Cadwallader achieved remission and found new hope. He now receives care in the U.K. and is monitored by Mayo in the U.S. and London.

After undergoing genetic screening as part of a research study, Tammy LeDoux learned she had Lynch syndrome, which put her at high risk of cancer.

Mayo Clinic launched the Caregiver Support Program to help family caregivers in December 2021. This is its origin story.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
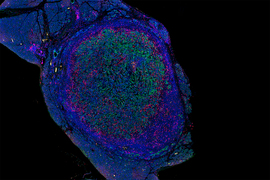
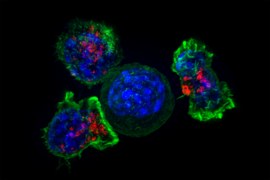
Biologists identify new targets for cancer vaccines
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Over the past decade, scientists have been exploring vaccination as a way to help fight cancer. These experimental cancer vaccines are designed to stimulate the body’s own immune system to destroy a tumor, by injecting fragments of cancer proteins found on the tumor.
So far, none of these vaccines have been approved by the FDA, but some have shown promise in clinical trials to treat melanoma and some types of lung cancer. In a new finding that may help researchers decide what proteins to include in cancer vaccines, MIT researchers have found that vaccinating against certain cancer proteins can boost the overall T cell response and help to shrink tumors in mice.
The research team found that vaccinating against the types of proteins they identified can help to reawaken dormant T cell populations that target those proteins, strengthening the overall immune response.
“This study highlights the importance of exploring the details of immune responses against cancer deeply. We can now see that not all anticancer immune responses are created equal, and that vaccination can unleash a potent response against a target that was otherwise effectively ignored,” says Tyler Jacks, the David H. Koch Professor of Biology, a member of the Koch Institute for Integrative Cancer Research, and the senior author of the study.
MIT postdoc Megan Burger is the lead author of the new study, which appears today in Cell .
T cell competition
When cells begin to turn cancerous, they start producing mutated proteins not seen in healthy cells. These cancerous proteins, also called neoantigens, can alert the body’s immune system that something has gone wrong, and T cells that recognize those neoantigens start destroying the cancerous cells.
Eventually, these T cells experience a phenomenon known as “T cell exhaustion,” which occurs when the tumor creates an immunosuppressive environment that disables the T cells, allowing the tumor to grow unchecked.
Scientists hope that cancer vaccines could help to rejuvenate those T cells and help them to attack tumors. In recent years, they have worked to develop methods for identifying neoantigens in patient tumors to incorporate into personalized cancer vaccines. Some of these vaccines have shown promise in clinical trials to treat melanoma and non-small cell lung cancer.
“These therapies work amazingly in a subset of patients, but the vast majority still don’t respond very well,” Burger says. “A lot of the research in our lab is aimed at trying to understand why that is and what we can do therapeutically to get more of those patients responding.”
Previous studies have shown that of the hundreds of neoantigens found in most tumors, only a small number generate a T cell response.
The new MIT study helps to shed light on why that is. In studies of mice with lung tumors, the researchers found that as tumor-targeting T cells arise, subsets of T cells that target different cancerous proteins compete with each other, eventually leading to the emergence of one dominant population of T cells. After these T cells become exhausted, they still remain in the environment and suppress any competing T cell populations that target different proteins found on the tumor.
However, Burger found that if she vaccinated these mice with one of the neoantigens targeted by the suppressed T cells, she could rejuvenate those T cell populations.
“If you vaccinate against antigens that have suppressed responses, you can unleash those T cell responses,” she says. “Trying to identify these suppressed responses and specifically targeting them might improve patient responses to vaccine therapies.”
Shrinking tumors
In this study, the researchers found that they had the most success when vaccinating with neoantigens that bind weakly to immune cells that are responsible for presenting the antigen to T cells. When they used one of those neoantigens to vaccinate mice with lung tumors, they found the tumors shrank by an average of 27 percent.
“The T cells proliferate more, they target the tumors better, and we see an overall decrease in lung tumor burden in our mouse model as a result of the therapy,” Burger says.
After vaccination, the T cell population included a type of cells that have the potential to continuously refuel the response, which could allow for long-term control of a tumor.
In future work, the researchers hope to test therapeutic approaches that would combine this vaccination strategy with cancer drugs called checkpoint inhibitors, which can take the brakes off exhausted T cells, stimulating them to attack tumors. Supporting that approach, the results published today also indicate that vaccination boosts the number of a specific type of T cells that have been shown to respond well to checkpoint therapies.
The research was funded by the Howard Hughes Medical Institute, the Ludwig Center at Harvard University, the National Institutes of Health, the Koch Institute Support (core) Grant from the National Cancer Institute, the Bridge Project of the Koch Institute and Dana-Farber/Harvard Cancer Center, and fellowship awards from the Jane Coffin Childs Memorial Fund for Medical Research and the Ludwig Center for Molecular Oncology at MIT.
Share this news article on:
Press mentions.
Writing for the NIH Director’s Blog, Dr. Francis Collins highlights how Prof. Tyler Jacks and research scientist Megan Burger’s work exploring T cell exhaustion led to the creation of a “strategy for developing cancer vaccines that can ‘awaken’ T cells and reinvigorate the body’s natural cancer-fighting abilities.” Collins writes that “the researchers hope to learn if this approach to cancer vaccines might work even better when used in combination with immunotherapy drugs, which unleash the immune system against cancer in other ways.”
Previous item Next item
Related Links
- Koch Institute
- Department of Biology
Related Topics
- National Institutes of Health (NIH)
Related Articles
New drug combo shows early potential for treating pancreatic cancer
Unmasking mutant cancer cells
Biologists identify key step in lung cancer evolution
More mit news.
MIT researchers discover “neutronic molecules”
Read full story →
A new computational technique could make it easier to engineer useful proteins

Designing solutions to ensure equity in health care

Training manufacturing technologists to be future shop floor leaders
Characterizing social networks
MIT economics to launch new predoctoral fellowship program
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
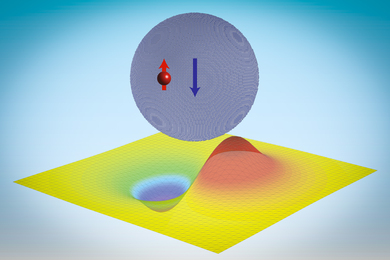
Immune cells that fight cancer become exhausted within hours of first encountering tumors – new research
Assistant Professor of Medicine and Pathology, Vanderbilt University
MD-Ph.D. Candidate in Molecular Pathology and Immunology, Vanderbilt University
Disclosure statement
The authors do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
Vanderbilt University provides funding as a founding partner of The Conversation US.
View all partners
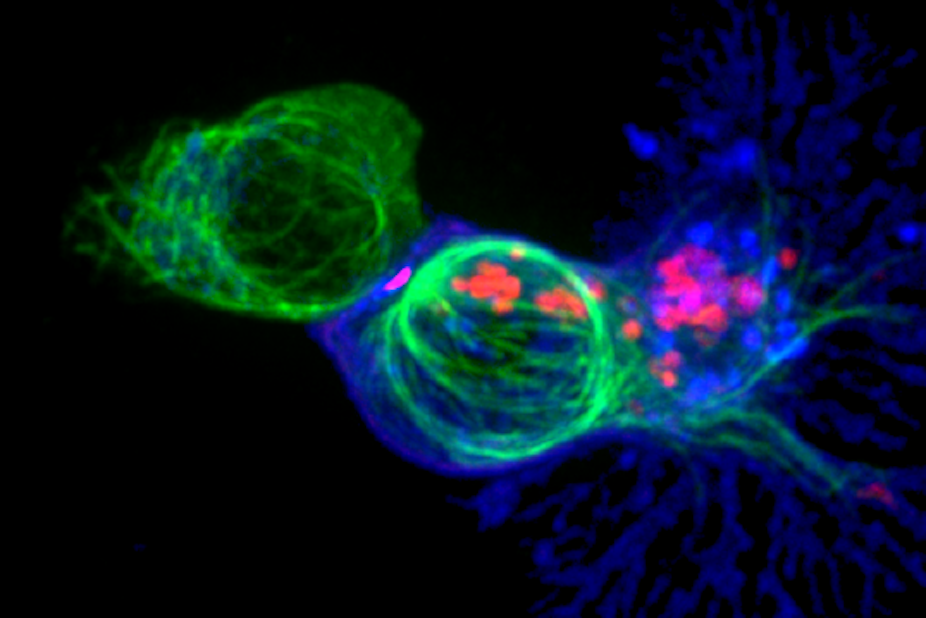
A key function of our immune system is to detect and eliminate foreign pathogens such as bacteria and viruses. Immune cells like T cells do this by distinguishing between different types of proteins within cells, which allows them to detect the presence of infection or disease.
A type of T cell called cytotoxic T cells can recognize the mutated proteins on cancer cells and should therefore be able to kill them. However, in most patients, cancer cells grow unchecked despite the presence of T cells.
The current explanation scientists have as to why T cells fail to eliminate cancer cells is because they become “exhausted.” The idea is that T cells initially function well when they first face off against cancer cells, but gradually lose their ability to kill the cancer cells after repeated encounters.
Cancer immunotherapies such as immune checkpoint inhibitors and CAR-T cell therapy have shown remarkable promise by inducing long-lasting remission in some patients with otherwise incurable cancers. However, these therapies often fail to induce long-term responses in most patients, and T cell exhaustion is a major culprit.
We are researchers who study ways to harness the immune system to treat cancer. Scientists like us have been working to determine the mechanisms controlling how well T cells function against tumors. In our newly published research, we found that T cells become exhausted within hours after encountering cancer cells.
Timing T cell exhaustion
By the time most patients are diagnosed with cancer, their immune system has been interacting with developing cancer cells for months to years . We wanted to go back earlier in time to figure out what happens when T cells first encounter tumor cells.
To do this, we used mice genetically engineered to develop liver cancers as they age, similarly to how liver cancers develop in people. We introduced trackable cytotoxic T cells that specifically recognize liver cancer cells to analyze the T cells’ function and monitor which of the genes are activated or turned off over time.
We also used these same trackable T cells to study their response in mice infected with the bacteria Listeria . In these mice, we found that the T cells were highly functional and eliminated infected cells. By comparing the differences between dysfunctional T cells from tumors and highly functional T cells from infected mice, we can home in on the genes that code for critical proteins that T cells use to regulate their function.
In our previous work , we found that T cells become dysfunctional with dramatically altered genetic structure within five days of encountering cancer cells in mice. We had originally decided to focus on the very earliest time points after T cells encounter cancer cells in mice with liver cancer or metastatic melanoma because we thought there would be fewer genetic changes. That would have allowed us to identify the earliest and most critical regulators of T cell dysfunction.
Instead, we found multiple surprising hallmarks of T cell dysfunction within six to 12 hours after they encountered cancer cells, including thousands of changes in genetic structure and gene expression.
We analyzed the different regulatory genes and pathways in T cells encountering cancer cells compared to those of T cells encountering infected cells. We found that genes associated with inflammation were highly activated in T cells interacting with infected cells but not in T cells interacting with cancer cells.
Next, we looked at how the initial early changes to the genetic structure of T cells evolved over time. We found that very early DNA changes were stabilized and reinforced with continued exposure to cancer cells, effectively “imprinting” dysfunctional gene expression patterns in the T cells. This meant that when the T cells were removed from the tumors after five days and transferred to tumor-free mice, they still remained dysfunctional.
Boosting T cell killing
Altogether, our research suggests that T cells in tumors are not necessarily working hard and getting exhausted. Rather, they are blocked right from the start. This is because the negative signals cancer cells send out to their surrounding environment induce T cell dysfunction, and a lack of positive signals like inflammation results in a failure to kick T cells into high gear.
Our team is now exploring strategies to stimulate inflammatory pathways in T cells encountering cancer cells to make them function as though they are encountering an infection. Our hope is that this will help T cells kill their cancer targets more effectively.
- Inflammation
- Immune cells
- Immunotherapy
- Cancer immunotherapy
- Killer T cells
- T cell exhaustion
- Cytotoxic T cells

Events Officer

Lecturer (Hindi-Urdu)

Director, Defence and Security

Opportunities with the new CIEHF

School of Social Sciences – Public Policy and International Relations opportunities
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021
Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
- International edition
- Australia edition
- Europe edition

Cancer signs could be spotted years before symptoms, says new research institute
Tests that can identify early changes in cells would give doctors more time to offer treatment, say Cambridge researchers
Scientists at a recently opened cancer institute at Cambridge University have begun work that is pinpointing changes in cells many years before they develop into tumours. The research should help design radically new ways to treat cancer, they say.
The Early Cancer Institute – which has just received £11m from an anonymous donor – is focused on finding ways to tackle tumours before they produce symptoms. The research will exploit recent discoveries which have shown that many people develop precancerous conditions that lie in abeyance for long periods.
“The latency for a cancer to develop can go on for years, sometimes for a decade or two, before the condition abruptly manifests itself to patients,” said Prof Rebecca Fitzgerald, the institute’s director.
“Then doctors find they are struggling to treat a tumour which, by then, has spread through a patient’s body. We need a different approach, one that can detect a person at risk of cancer early on using tests that can be given to large numbers of people.”
One example of this is the cytosponge – a sponge on a string – which has been developed by Fitzgerald and her team. It is swallowed like a pill, expands in the stomach into a sponge and is then pulled up the gullet collecting oesophagus cells on the way. Those cells that contain a protein, called TFF3 – which is found only in precancerous cells – then provide an early warning that a patient is at risk of oesophageal cancer and needs to be monitored. Crucially, this test can be administered simply and on a wide scale.
This contrasts with current approaches to other cancers, added Fitzgerald. “At present, we are detecting many cancers late and are having to come up with medicines, which have become incrementally more expensive. We are often extending life by a few weeks at a cost of tens of thousands of pounds. We need to look at this from a different perspective.”
One approach being taken by the institute – which is to be renamed the Li Ka-shing Early Cancer Institute after the Hong Kong philanthropist who has supported other Cambridge cancer research – focuses on blood samples. Provided by women as part of past screening services for ovarian cancer and kept in special stores, these samples have now been repurposed by the institute. “We have around 200,000 such samples and they are a goldmine,” said Jamie Blundell, a research group leader at the institute.
Using these samples, researchers have identified changes that differentiate those donors who have subsequently been diagnosed with a blood cancer 10 or even 20 years after they provided samples, with those who did not develop such conditions.
“We are finding that there are clear genetic changes in a person’s blood more than a decade before they start to display symptoms of leukaemia,” said Blundell. “That shows there is a long window of opportunity that you could use to intervene and give treatments that will reduce the odds of going on to get cancer.”
Cancers grow in stages and by spotting those with cells that have taken an early step on this ladder, it should be possible to block or hamper further developments. The crucial point is that at this early stage there is time for doctors to take action and avoid them having to deal with a cancer at a late stage when it has spread.
A similar strategy is being taken by Harveer Dev, another group leader, who has investigated men who have had their prostates removed. His team are now developing biomarkers that will provide better ways to pinpoint those who are likely to suffer poor outcomes from prostate cancer, one of the most common tumours in the UK.
“Our pilot data suggests that these tests may be much better than existing PSA tests and will be crucial in spotting those who with prostate cancer that is likely to progress,” said Dev.
Pinpointing those at risk of cancer – for example, people from families who have an inherited predisposition to tumours – will form a key part of the institute’s strategy. In addition, it will focus on finding ways to reduce cancer risks, as well as ensuring treatments can be widely administered.
A woman had, in her 80s, decided to leave the university £1m for cancer research, Fitzgerald said. “However, she lived until she was over 100 and only died recently, so we only just got that donation. We want to understand what makes some live into very old age while others get cancer, so more people can live as long as she did.”
- Cancer research
- The Observer
- Medical research
- Health policy
- Prostate cancer
- University of Cambridge
Most viewed
- Português Br
- Journalist Pass
New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Kelley Luckstein
Share this:

ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.
"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."
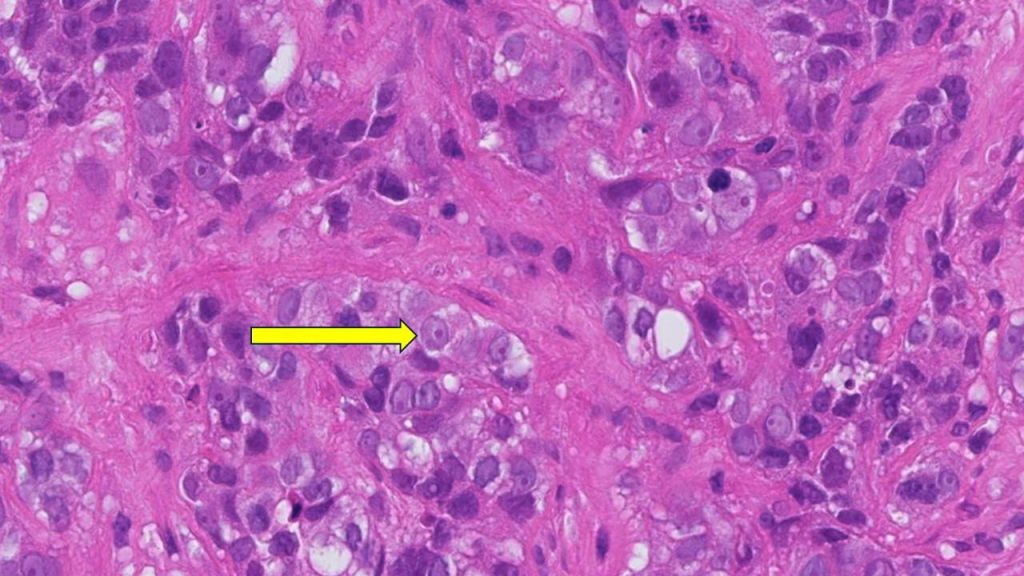
"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group , led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors taken into consideration to determine the course of chemotherapy treatment for each person are the tumor size and the presence of lymph node metastases. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
About Mayo Clinic Comprehensive Cancer Center Designated as a comprehensive cancer center by the National Cancer Institute , Mayo Clinic Comprehensive Cancer Center is defining new boundaries in possibility, focusing on patient-centered care, developing novel treatments, training future generations of cancer experts and bringing cancer research to communities. At Mayo Clinic Comprehensive Cancer Center, a culture of innovation and collaboration is driving research breakthroughs that are changing approaches to cancer prevention, screening and treatment, and improving the lives of cancer survivors.
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
About Gustave Roussy Ranked as the leading French and European Cancer Centre and fourth in the world, Gustave Roussy is a centre with comprehensive expertise and is devoted entirely to patients suffering with cancer. The Institute is a founding member of the Paris Saclay Cancer Cluster. It is a source of diagnostic and therapeutic advances. It caters for almost 50,000 patients per year and its approach is one that integrates research, patient care and teaching. It is specialized in the treatment of rare cancers and complex tumors and it treats all cancers in patients of any age. Its care is personalized and combines the most advanced medical methods with an appreciation of the patient’s human requirements. In addition to the quality of treatment offered, the physical, psychological and social aspects of the patient’s life are respected. 4,100 professionals work on its two campuses: Villejuif and Chevilly-Larue. Gustave Roussy brings together the skills, which are essential for the highest quality research in oncology: 40% of patients treated are included in clinical studies. For further information: www.gustaveroussy.fr/en , Twitter , Facebook , LinkedIn , Instagram
Media contact:
Kelley Luckstein, Mayo Clinic Communications, [email protected]
- CAR-T cell therapy helps man continue community advocacy
Related Articles

- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine scientists transform cancer cells into weapons against cancer
Researchers found that when they turned cancer cells into immune cells, they were able to teach other immune cells how to attack cancer.
March 1, 2023 - By Christopher Vaughan

Ravi Majeti and his colleagues programmed mouse leukemia cells so that some of them could be induced to transform themselves into cells that attacked the cancer from which the cells were derived. Steve Fisch
Some cities fight gangs with ex-members who educate kids and starve gangs of new recruits. Stanford Medicine researchers have done something similar with cancer — altering cancer cells so that they teach the body’s immune system to fight the very cancer the cells came from.
“This approach could open up an entirely new therapeutic approach to treating cancer,” said Ravi Majeti , MD, PhD, a professor of hematology and the study’s senior author. The research was published March 1 in Cancer Discovery . The lead author is Miles Linde, PhD, a former PhD student in immunology who is now at the Fred Hutchinson Cancer Institute in Seattle.
Some of the most promising cancer treatments use the patient’s own immune system to attack the cancer, often by taking the brakes off immune responses to cancer or by teaching the immune system to recognize and attack the cancer more vigorously. T cells, part of the immune system that learns to identify and attack new pathogens such as viruses, can be trained to recognize specific cancer antigens, which are proteins that generate an immune response.
For instance, in CAR T-cell therapy, T cells are taken from a patient, programmed to recognize a specific cancer antigen, then returned to the patient. But there are many cancer antigens, and physicians sometimes need to guess which ones will be most potent.
Like an immune response
A better approach would be to train T cells to recognize cancer via processes that more closely mimic the way things naturally occur in the body — like the way a vaccine teaches the immune system to recognize pathogens. T cells learn to recognize pathogens because special antigen presenting cells (APCs) gather pieces of the pathogen and show them to the T cells in a way that tells the T cells, “Here is what the pathogen looks like — go get it.”
Something similar in cancer would be for APCs to gather up the many antigens that characterize a cancer cell. That way, instead of T cells being programmed to attack one or a few antigens, they are trained to recognize many cancer antigens and are more likely to wage a multipronged attack on the cancer.
Now that researchers have become adept at transforming one kind of cell into another, Majeti and his colleagues had a hunch that if they turned cancer cells into a type of APC called macrophages, they would be naturally adept at teaching T cells what to attack.
“We hypothesized that maybe cancer cells reprogrammed into macrophage cells could stimulate T cells because those APCs carry all the antigens of the cancer cells they came from,” said Majeti, who is also the RZ Cao Professor, assistant director of the Institute for Stem Cell Biology and Regenerative Medicine and director of the Ludwig Center for Cancer Stem Cell Research and Medicine .
Cell conversion
The study builds on prior research from the Majeti lab showing that cells taken from patients with a type of acute leukemia could be converted into non-leukemic macrophages with many of the properties of APCs.
In the current study, the researchers programmed mouse leukemia cells so that some of them could be induced to transform themselves into APCs. When they tested their cancer vaccine strategy on the mouse immune system, the mice successfully cleared the cancer.
“When we first saw the data showing clearance of the leukemia in the mice with working immune systems, we were blown away,” Majeti said. “We couldn’t believe it worked as well as it did.”
Other experiments showed that the cells created from cancer cells were indeed acting as antigen-presenting cells that sensitized T cells to the cancer. “What’s more, we showed that the immune system remembered what these cells taught them,” Majeti said. “When we reintroduced cancer to these mice over 100 days after the initial tumor inoculation, they still had a strong immunological response that protected them.”
“We wondered, If this works with leukemias, will it also work with solid tumors?” Majeti said. The team tested the same approach using mouse fibrosarcoma, breast cancer, and bone cancer. “The transformation of cancer cells from solid tumors was not as efficient, but we still observed positive results,” Majeti said. With all three cancers, the creation of tumor-derived APCs led to significantly improved survival.
Lastly, the researchers returned to the original type of acute leukemia. When the human leukemia cell-derived APCs were exposed to human T cells from the same patient, they observed all the signs that would be expected if the APCs were indeed teaching the T cells how to attack the leukemia.
“We showed that reprogrammed tumor cells could lead to a durable and systemic attack on the cancer in mice and a similar response with human patient immune cells,” Majeti said. “In the future we might be able to take out tumor cells, transform them into APCs and give them back to patients as a therapeutic cancer vaccine.”
“Ultimately, we might be able to inject RNA into patients and transform enough cells to activate the immune system against cancer without having to take cells out first,” Majeti said. “That’s science fiction at this point, but that’s the direction we are interested in going.”
The work was supported by funding from the Ludwig Foundation for Cancer Research, the Emerson Collective Cancer Research Fund, the New York Stem Cell Foundation, the Stinehart-Reed Foundation, the Leukemia and Lymphoma Society, the J. Benjamin Eckenhoff Fund, the Blavatnik Family Fellowship, the Deutsche Forschungsgemainshaft, the Knut and Alice Wallenberg Foundation, the Stanford Human Biology Research Exploration Program, the National Institutes of Health (grant F31CA196029), the American Society of Hematology, the A.P. Giannini Foundation, and the Stanford Cancer Institute.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Collection 01 July 2020
Cancer at Nature Portfolio
The Nature Portfolio editors who handle cancer primary research, methods, protocols and reviews bring you the latest articles, covering all aspects from disease mechanisms to therapeutic approaches. Collected here you will also find specially curated content, such as collections, focus issues and animations, all ready to be used in presentations and educational materials. You can also find out about the editors handling cancer content, and the journals at Nature Portfolio that publish articles on this topic and how to submit to them.
- Collection content
- Collections
- PrimeViews and posters
Research Articles
Polygenic risk score for ulcerative colitis predicts immune checkpoint inhibitor-mediated colitis
Colitis is one of the most common immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors. Here the authors show that a polygenic risk score for ulcerative colitis can predict immune checkpoint inhibitor-mediated colitis in patients with cancer.
- Pooja Middha
- Rohit Thummalapalli
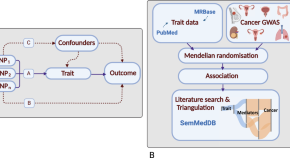

Phenome-wide Mendelian randomisation analysis of 378,142 cases reveals risk factors for eight common cancers
Mendelian randomisation can identify potential risk factors from large populations. Here, the authors analyse 3000 traits across multiple cancer types to search for potential risk factors and molecular biomarkers.
- Richard Houlston
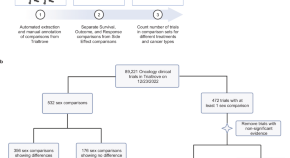
Outcome differences by sex in oncology clinical trials
The role of sex differences in response to cancer therapy remains unclear but this could be improved by reporting sex comparisons of outcomes in clinical trials. Here, the authors characterise the sex outcome comparisons in 89,221 interventional trials, finding that while comparisons were rare, important insights could be obtained.
- Ashwin V. Kammula
- Alejandro A. Schäffer
- Eytan Ruppin
FANCJ promotes PARP1 activity during DNA replication that is essential in BRCA1 deficient cells
Here the authors show that PARPi efficacy along with the fitness of BRCA1 deficient cells relies on FANCJ, which maintains S-phase PARP1 activity by preventing its sequestration with MSH2 on G-quadruplexes.
- Nathan MacGilvary
- Sharon B. Cantor
Threonine fuels glioblastoma through YRDC-mediated codon-biased translational reprogramming
Rich and colleagues show that glioblastoma stem cells have increased global protein translation, which is achieved via the tRNA modifier YRDC. They show that targeting it or reducing its substrate threonine suppresses tumor growth.
- Huairui Yuan
- Jeremy N. Rich
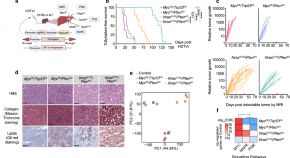
Cancer cell genetics shaping of the tumor microenvironment reveals myeloid cell-centric exploitable vulnerabilities in hepatocellular carcinoma
Distinct genetic mutations can shape the tumor immune microenvironment. Here the authors generate preclinical mouse models of hepatocellular carcinoma bearing clinically-relevant oncogenic driver combinations, identifying myeloid cell-centric exploitable therapeutic vulnerabilities.
- Christel F. A. Ramirez
- Daniel Taranto
- Leila Akkari
Multipeptide vaccines for melanoma in the adjuvant setting: long-term survival outcomes and post-hoc analysis of a randomized phase II trial
Peptide-based cancer vaccines require epitopes for both CD8+ and CD4+ T cells. Here the authors report the long-term outcomes of a randomized phase II trial (NCT00118274) in patients with melanoma designed to evaluate a class I MHC-restricted peptide vaccine plus one of two “helper” peptide preparations to stimulate CD4+ T cells, either non-specific help or melanoma-specific help.
- Emily K. Ninmer
- Craig L. Slingluff Jr
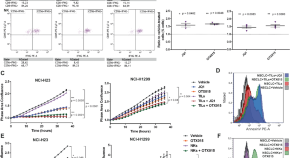
BET inhibitors drive Natural Killer activation in non-small cell lung cancer via BRD4 and SMAD3
Combination of BET inhibitors (BETi) with immunotherapy has been reported to be synergic for the treatment of non-small cell lung carcinoma (NSCLC). Here, the authors show that BETi-induced epigenetic reprogramming downregulates the expression of NK cell inhibitory receptors on NK cells, increasing their activation and cytotoxicity against NSCLC.
- Francesca Reggiani
- Giovanna Talarico
- Valentina Sancisi
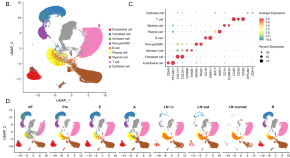
Single cell deciphering of progression trajectories of the tumor ecosystem in head and neck cancer
Head and neck squamous cell carcinoma (HNSCC) is characterised with high heterogeneity and unfavourable prognosis. Here, the authors perform single cell transcriptomics to investigate the tumour microenvironment features of HNSCC initiation, progression, lymph node metastasis and recurrence.
Spatial relationships in the urothelial and head and neck tumor microenvironment predict response to combination immune checkpoint inhibitors
Spatial positioning of cells within the tumour microenvironment may have a function in the success of immune checkpoint immunotherapy (ICI). Here the authors analyse spatial relationships from immunohistochemistry samples prior to ICI therapy and show that CD8 T cell or macrophage proximity to cancer cells is associated with better responses.
- Alberto Gil-Jimenez
- Nick van Dijk
- Lodewyk F. A. Wessels
The EIF3H-HAX1 axis increases RAF-MEK-ERK signaling activity to promote colorectal cancer progression
Eukaryotic initiation translation factor 3 subunit h (EIF3H) possesses an alternative “moonlighting” function of deubiquitinase, while its role in colorectal carcinogenesis remains to be explored. Here the authors show that EIF3H deubiquitinates and stabilizes HAX1, which enhances RAF-MEK-ERK signaling to promote colorectal tumor growth and metastasis.
- Xiaoling Huang
- Mong-Hong Lee
Tertiary lymphoid structures and B cells determine clinically relevant T cell phenotypes in ovarian cancer
Intratumoral tertiary lymphoid structure (TLS) density has been associated with better prognosis in several cancer types. Here the authors provide a comprehensive characterization of TLSs in patients with high-grade serous ovarian carcinoma.
- Lenka Kasikova
- Jana Rakova
- Jitka Fucikova

Aberrant non-canonical NF-κB signalling reprograms the epigenome landscape to drive oncogenic transcriptomes in multiple myeloma
The downstream molecular mechanisms following the activation of the NF-κB pathway in multiple myeloma (MM) remain to be characterised. Here, it is shown that aberrant non-canonical NF-κB signalling causes epigenomic reprogramming leading to transcriptional changes that favour MM progression.
- Daniel A. Ang
- Jean-Michel Carter
GAS41 modulates ferroptosis by anchoring NRF2 on chromatin
GAS41 is recognized as a histone reader and oncogene, but the mechanism by which GAS41 contributes to tumorigenesis is not well understood. Here, the authors discover that GAS41 is a ferroptosis repressor that anchors NRF2 to chromatin, promoting tumor growth.
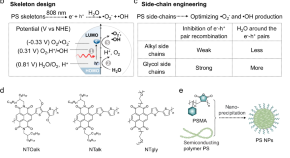
Oxygen-independent organic photosensitizer with ultralow-power NIR photoexcitation for tumor-specific photodynamic therapy
Conventional photodynamic therapy (PDT) is hindered by oxygen-dependent photosensitization pathways and high-power-density photoexcitation. Here, the authors develop polymer-based organic photosensitizers (PSs) through PS skeleton design and side-chain engineering to allow tumor-specific PDT under oxygen-free conditions using ultralow-power 808 nm photoexcitation.
- Yuanyuan Li
PIEZO1 mechanically regulates the antitumour cytotoxicity of T lymphocytes
Blocking the mechanical sensor PIEZO1 in cytotoxic T lymphocytes strengthens their traction forces and augments their cytotoxicity against tumour cells.
- Ruiyang Pang
Generation of synthetic whole-slide image tiles of tumours from RNA-sequencing data via cascaded diffusion models
Cascaded diffusion models can be used to synthesize realistic whole-slide image tiles from latent representations of RNA-sequencing data from human tumours.
- Francisco Carrillo-Perez
- Marija Pizurica
- Olivier Gevaert
Metabolic targeting of cancer associated fibroblasts overcomes T-cell exclusion and chemoresistance in soft-tissue sarcomas
Cancer associated fibroblasts can shape the tumor microenvironment (TME) and modulate immune infiltration. Here the authors characterize the TME in preclinical models of softtissue sarcomas, identifying a subset of “glycolytic” cancer-associated fibroblasts that inhibit cytotoxic T cell infiltration into the tumor parenchyma.
- Marina T. Broz
- Emily Y. Ko
- Jlenia Guarnerio
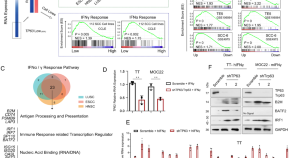
Reciprocal inhibition between TP63 and STAT1 regulates anti-tumor immune response through interferon-γ signaling in squamous cancer
TP63 is a master regulator transcription factor in squamous cell carcinomas (SCCs). Here the authors report that TP63 suppresses IFNγ signaling in SCC tumors and that its inhibition is associated with enhanced anti-tumor immunity and response to anti-PD1.
- Yueyuan Zheng
- Yan-Yi Jiang
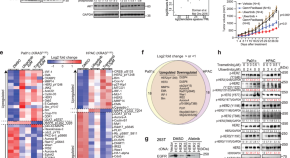
Combined KRAS-MAPK pathway inhibitors and HER2-directed drug conjugate is efficacious in pancreatic cancer
The MAPK pathway is an important therapeutic target in pancreatic ductal adenocarcinoma (PDAC), but success is limited by pathway reactivation, which drives resistance. Here, the authors investigate the mechanism underlying HER2-reactivation post KRAS-MAPK inhibition, identifying combination of MAPK and HER2 inhibition as a therapeutic strategy.
- Ashenafi Bulle
- Kian-Huat Lim
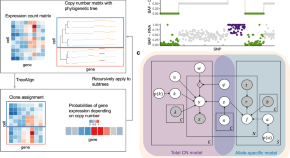
Allele-specific transcriptional effects of subclonal copy number alterations enable genotype-phenotype mapping in cancer cells
Quantifying the impact of copy-number alterations (CNAs) on gene expression at the subclone level in cancer remains a challenge. Here, the authors develop TreeAlign, a method that integrates sample-matched single-cell DNA and RNA sequencing data to infer the impact of CNAs on subclonal gene expression.
- Marc J. Williams
- Sohrab P. Shah
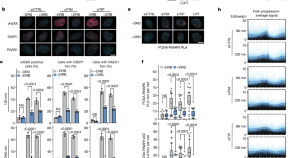
Transcription–replication conflicts underlie sensitivity to PARP inhibitors
Poly(ADP-ribose) polymerase 1 (PARP1) functions together with TIMELESS and TIPIN to protect the replisome in early S phase from transcription–replication conflicts, and inhibiting PARP1 enzymatic activity may suffice for treatment efficacy in homologous recombination-deficient settings.
- Michalis Petropoulos
- Angeliki Karamichali
- Thanos D. Halazonetis
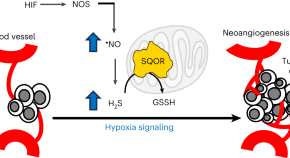
Sulfide oxidation promotes hypoxic angiogenesis and neovascularization
Hypoxia induces ·NO-dependent hydrogen sulfide (H 2 S) biogenesis by inhibiting the transsulfuration pathway. H 2 S oxidation promotes endothelial cell proliferation to support neovascularization in tissue injury and tumor xenograft models.
- Roshan Kumar
- Victor Vitvitsky
- Ruma Banerjee
A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche
A study reveals that Fusobacterium nucleatum subspecies animalis is bifurcated into two distinct clades, and shows that only one of these dominates the colorectal cancer niche, probably through increased colonization of the human gastrointestinal tract.
- Martha Zepeda-Rivera
- Samuel S. Minot
- Christopher D. Johnston
The pleiotropic functions of reactive oxygen species in cancer
Papagiannakopoulos and colleagues discuss the roles of reactive oxygen species in cancer and the ways in which redox mechanisms may be exploited for cancer therapy.
- Katherine Wu
- Ahmed Ezat El Zowalaty
- Thales Papagiannakopoulos
Bone marrow inflammation in haematological malignancies
Haematological malignancies are associated with inflammation in the bone marrow. In this Review, the authors discuss how tumour-associated inflammation affects the normal functions of the bone marrow and supports the outgrowth and survival of malignant cells. Moreover, they describe how the inflammatory changes in the bone marrow differ in myeloid and lymphoid malignancies.
- Madelon M. E. de Jong
- Lanpeng Chen
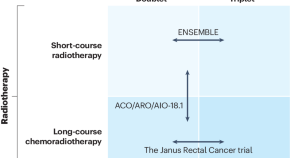
Future direction of total neoadjuvant therapy for locally advanced rectal cancer
In this article, the authors discuss the use of total neoadjuvant therapy for locally advanced rectal cancer. They highlight ongoing trials and discuss future treatment options, including the potential use of multi-omics and artificial intelligence to facilitate treatment selection and prediction of response.
- Yoshinori Kagawa
- J. Joshua Smith
- Takayuki Yoshino
Targeting cuproplasia and cuproptosis in cancer
Copper is an essential trace element with inherent redox properties and fundamental roles in a diverse range of biological processes; therefore, maintaining copper homeostasis is crucial. In this Review, the authors discuss new insights into the mechanisms by which disrupted copper homeostasis contributes to tumour initiation and development, including the recently defined concepts of cuproplasia (copper-dependent cell growth and proliferation) and cuproptosis (a mitochondrial pathway of cell death triggered by excessive copper exposure). They also discuss potential strategies to exploit cuproplasia and cuproptosis for the treatment of cancer.
- Daolin Tang
- Guido Kroemer
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
In this Review, Meier et al. discuss the molecular mechanisms of necroptosis, delineate how this form of immunogenic cell death activates antitumour immune responses and explore the opportunities and limitations of targeting necroptosis for anticancer therapy.
- Pascal Meier
- Arnaud J. Legrand
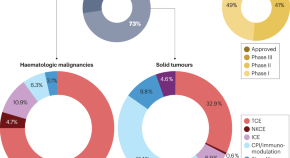
The present and future of bispecific antibodies for cancer therapy
Bispecific antibodies (bsAbs) can mediate therapeutic effects beyond those of natural monospecific antibodies. This Review provides an overview of recent developments in the field of bsAbs for cancer therapy and an outlook into next-generation bsAbs in earlier stages of development.
- Christian Klein
- Ulrich Brinkmann
- Roland E. Kontermann
Fungi in cancer
In this Viewpoint article, we asked three scientists working on the cancer mycobiome to provide their opinions on advancements and challenges and what the future holds for this exciting field of cancer research.
- Jessica Galloway-Peña
- Iliyan D. Iliev
- Florencia McAllister
Cancer burden in low-income and middle-income countries
In this Viewpoint, we asked four experts to discuss the increasing burden of cancer in low- and middle-income countries; they explore the changes that are necessary to improve cancer diagnosis, prevention and treatment within these nations.
- Sharmila Anandasabapathy
- Chite Asirwa
- Chemtai Mungo
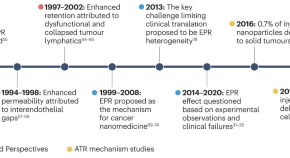
The mechanisms of nanoparticle delivery to solid tumours
The mechanisms of nanoparticle delivery to solid tumours guide the engineering of nanoparticles for cancer applications. This Review discusses two contrasting nanoparticle delivery mechanisms, the enhanced permeability and retention effect and the active transport and retention principle, and their implications for the design of cancer nanomedicines.
- Luan N. M. Nguyen
- Warren C. W. Chan
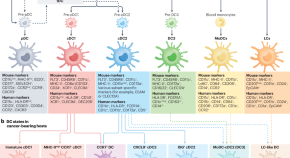
Dendritic cells as orchestrators of anticancer immunity and immunotherapy
Dendritic cells (DCs) are antigen-presenting cells that function at the interface between innate and adaptive immunity, thereby acting as key mediators of antitumour immune responses and immunotherapy efficacy. In this Review, the authors outline the emerging complexity of intratumoural DC states that is being revealed through single-cell analyses as well as the contributions of different DC subsets to anticancer immunity and the activity of immune-checkpoint inhibitors. The authors also discuss advances in the development of DC-based cancer therapies and considerations for their potential combination with other anticancer therapies.
- Ignacio Heras-Murillo
- Irene Adán-Barrientos
- David Sancho
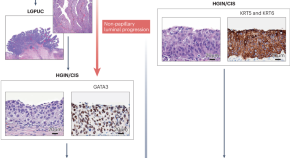
Molecular profile of bladder cancer progression to clinically aggressive subtypes
In this Review, the authors describe the molecular profile of bladder cancer progression associated with the subtypes of this disease and comment on their potential diagnostic, prognostic and therapeutic importance.
- Charles C. Guo
- Sangkyou Lee
- Bogdan Czerniak
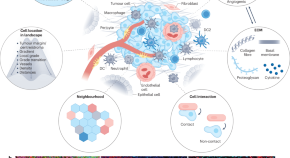
Multiplex protein imaging in tumour biology
In this Review, de Souza et al. discuss how advances in the ability to image protein markers at high-plex, at single-cell and even subcellular resolution, are expanding our understanding of tumour biology and clinical outcomes, and outline the future promise of combining such multiplex protein imaging methods with other forms of spatial omics.
- Natalie de Souza
- Bernd Bodenmiller
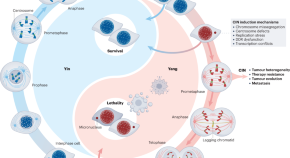
The yin and yang of chromosomal instability in prostate cancer
Chromosomal instability is a hallmark of advanced prostate cancer. In this Review, the authors discuss the biological causes and paradoxical consequences of chromosomal instability, its potential clinical role in the stratification of prostate cancer aggressiveness and the development of novel treatment strategies.
- Marc Carceles-Cordon
- Jacob J. Orme
- Veronica Rodriguez-Bravo
Management of patients with muscle-invasive bladder cancer with clinical evidence of pelvic lymph node metastases
Muscle-invasive bladder cancer with clinically positive pelvic lymph nodes is a particular situation at the interface between localized and metastatic disease. In this Review, the authors discuss the advances and challenges of currently available strategies for the diagnosis and treatment of patients with muscle-invasive bladder cancer with clinically positive pelvic lymph nodes.
- Elisabeth Grobet-Jeandin
- Louis Lenfant
- Thomas Seisen
The complex interplay of modifiable risk factors affecting prostate cancer disparities in African American men
African American men are disproportionately affected by prostate cancer in the USA. In this Review, the authors discuss the complex interplay of modifiable risk factors that might underlie the glaring prostate cancer disparities observed.
- Jabril R. Johnson
- Nicole Mavingire
- Rick A. Kittles
Extrachromosomal DNA in cancer
Extrachromosomal DNA (ecDNA) is now accepted as a major contributor to cancer pathogenesis. In this Review, Yan, Mischel and Chang highlight the recent advancements in ecDNA research, providing new insights into the biogenesis and maintenance of ecDNA, as well as its role in altering gene expression and promoting tumour heterogeneity.
- Xiaowei Yan
- Paul Mischel
- Howard Chang
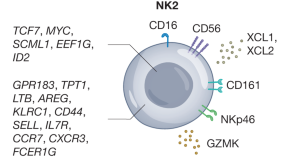
Natural killer cell therapies
This Review explores in detail the complexity of NK cell biology in humans and highlights the role of these cells in cancer immunity.
- Eric Vivier
- Lucas Rebuffet
- Valeria R. Fantin
Clinical and translational attributes of immune-related adverse events
Suijkerbuijk et al. summarize the clinical manifestation and classification of immune-related adverse events, discuss the immunopathogenesis of immune-related adverse events and provide key insights into their management and future therapeutic directions.
- Karijn P. M. Suijkerbuijk
- Mick J. M. van Eijs
- Alexander M. M. Eggermont
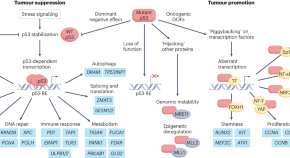
Translating p53-based therapies for cancer into the clinic
Although p53 was once considered undruggable, in this Review, Peuget et al. discuss the progress made in targeting p53 as a form of cancer therapy with approaches ranging from restoration of mutant p53 function to inhibition of the negative regulator of p53, MDM2, as well as newer strategies, including p53-based mRNA vaccines and antibodies.
- Sylvain Peuget
- Xiaolei Zhou
- Galina Selivanova
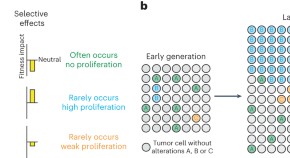
Aneuploidy and complex genomic rearrangements in cancer evolution
Van Loo and colleagues review the mechanistic underpinnings of large genomic alterations and their roles in tumor development and evolution.
- Toby M. Baker
- Peter Van Loo
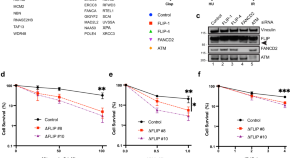
FLIP(C1orf112)-FIGNL1 complex regulates RAD51 chromatin association to promote viability after replication stress
Recombination is essential for life. Here, the authors characterize FLIP as a novel regulator of the key recombination protein RAD51’s functions. FLIP loss caused marked sensitivity to DNA damage, increased DNA breakage and defective replication.
- Jessica D. Tischler
- Hiroshi Tsuchida
- Richard O. Adeyemi
BRAF — a tumour-agnostic drug target with lineage-specific dependencies
Various BRAF alterations are found and function as oncogenic drivers across diverse cancer types. BRAF inhibitor-based therapy has improved outcomes for patients with cancers harbouring BRAF V600 mutations, although resistance develops in most, and the current inhibitors are not effective against other types of BRAF alterations. In this Review, the authors describe the mechanisms underlying oncogenic BRAF signalling, as well as pan-cancer and lineage-specific mechanisms of intrinsic, adaptive and acquired resistance to BRAF inhibitors. They also discuss novel RAF inhibitors and drug combinations designed to overcome these resistance mechanisms and/or expand the applicability of molecularly targeted therapy to a broader range of BRAF -mutant cancers.
- Aphrothiti J. Hanrahan
- David B. Solit
Lipids as mediators of cancer progression and metastasis
Schulze and colleagues discuss the latest advances in understanding the role of lipids in cancer progression and metastasis and reflect on opportunities to target lipid metabolism in tumors.
- Felix C. E. Vogel
- Adriano B. Chaves-Filho
- Almut Schulze
New immune cell engagers for cancer immunotherapy
Immune cell engagers — antibody-based molecules engineered to direct immune effector cells to recognize and kill cancer cells — represent a rapidly expanding approach in cancer therapy. Here, the authors bring us up to date with the targets, challenges and opportunities for harnessing the anticancer activities of T cells, natural killer cells and myeloid cells with immune cell engagers.
- Aurore Fenis
- Olivier Demaria
- Emilie Narni-Mancinelli
Metabolic heterogeneity in cancer
Demicco, Liu et al. discuss how metabolic adaptations in cancer contribute to tumour progression. These adaptations entail high spatial and temporal metabolic heterogeneity, based on local adaptations in different regions of the tumour microenvironment, as well as metabolic evolution over time as the tumour progresses and metastasizes.
- Margherita Demicco
- Xiao-Zheng Liu
- Sarah-Maria Fendt
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Renal cell carcinoma is a metabolic disease linked to a variety of alterations in genes that regulate cellular metabolism. Here, the authors examine cell-intrinsic metabolic alterations in hereditary and sporadic renal cell carcinoma, and how they can be exploited to develop novel therapeutic interventions.
- Nathan J. Coffey
- M. Celeste Simon
News & Commentary
The death gaze of MEDUSA
Chemogenetic profiling can reveal genetic determinants that coordinate phenotypic responses to therapeutics, along with predicting potential pathways of resistance. A new analytical method for evaluating chemogenetic profiles reveals contributions from death-regulatory genes.
- Jesse D. Gelles
- Jerry Edward Chipuk
Mirvetuximab soravtansine has activity in platinum-sensitive epithelial ovarian cancer
- Diana Romero
Enhancing diagnostic precision in liver lesion analysis using a deep learning-based system: opportunities and challenges
A recent study reported the development and validation of the Liver Artificial Intelligence Diagnosis System (LiAIDS), a fully automated system that integrates deep learning for the diagnosis of liver lesions on the basis of contrast-enhanced CT scans and clinical information. This tool improved diagnostic precision, surpassed the accuracy of junior radiologists (and equalled that of senior radiologists) and streamlined patient triage. These advances underscore the potential of artificial intelligence to enhance hepatology care, although challenges to widespread clinical implementation remain.
- Jeong Min Lee
- Jae Seok Bae
IL-13Rα2-targeted CAR T cells show promise in patients with recurrent high-grade gliomas
- David Killock
Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost
The treatment, called NexCAR19, raises hopes that this transformative class of medicine will become more readily available in low- and middle-income countries.
- Smriti Mallapaty
A new standard of care for advanced-stage urothelial carcinoma
- Peter Sidaway
Identification of dynamic microbiota signatures in patients with melanoma receiving ICIs: opportunities and challenges
The composition of the gut microbiota has emerged as a tumour-extrinsic factor that modulates response to immune-checkpoint inhibitors (ICIs), although the lack of consistency in microbiota signatures across studies has limited their value as reliable biomarkers. Herein, we discuss a recent study in which longitudinal microbiome profiling identified several taxa that are persistently enriched in patients with melanoma and a favourable response to ICIs.
- Saman Maleki Vareki
- Diwakar Davar

‘Woah, this is affecting me’: why I’m fighting racial inequality in prostate-cancer research
Olugbenga Samuel Oyeniyi sought a career with a stronger public-health focus after learning that Black men are twice as likely as white men to get prostate cancer.
- Jacqui Thornton
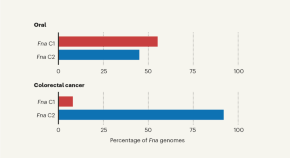
Whittling down the bacterial subspecies that might drive colon cancer
Understanding the factors that drive formation of particular types of cancer can aid efforts to develop better diagnostics or treatments. The identification of a bacterial subspecies with a connection to colon cancer has clinical relevance.
- Cynthia L. Sears
- Jessica Queen
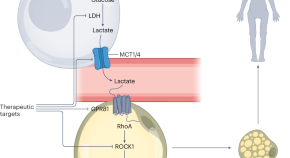
A waste product’s unexpected role in wasting
The mechanisms that drive cancer cachexia are unclear. Adipocyte activation of GPR81 by high levels of lactate is now shown to drive adipose tissue browning, thermogenesis and a loss of body weight in mouse models of cancer.
- Jack D. Sanford
- Marcus D. Goncalves

Why are so many young people getting cancer? What the data say
Clues to a modern mystery could be lurking in information collected generations ago.
- Heidi Ledford
Deadly brain cancer shrinks after CAR-T therapy — but for how long is unclear
Early studies with engineered immune cells show drastic but often short-lived results in glioblastoma, the most aggressive brain cancer.
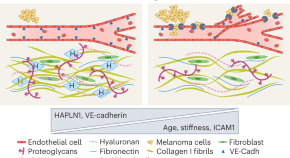
Mechanisms of melanoma aggressiveness with age
The extracellular matrix is an essential component of the tumor microenvironment and affects cancer progression. Weeraratna and colleagues have now uncovered that age-related reductions in the level of hyaluronan and proteoglycan link protein 1 (HAPLN1) stimulate neoangiogenesis and compromise the vascular integrity of intratumoral blood vessels. These biological modifications converge to fuel distant melanoma metastasis.
- Corine Bertolotto

Engineered T cells call for back-up
- M. Teresa Villanueva
New pathogen on the block
Fu et al. provide data indicating a pathogenic role for Streptococcus anginosus in gastric cancer.
MEGA CRISPR rejuvenates exhausted CAR T cells
MEGA is a new CRISPR-based RNA-editing platform with the ability to enhance the fitness of CAR T cells; it may also overcome certain limitations of conventional DNA-targeting CRISPR–Cas9 systems.
- Karen O’Leary
First cell therapy for solid tumours heads to the clinic: what it means for cancer treatment
Therapy built on tumour-infiltrating lymphocytes is now being prepared for at least 20 people in the United States with advanced melanoma.
- Sara Reardon
FIRSTMAPPP prospectively charts the efficacy of sunitinib for phaeochromocytoma and paraganglioma
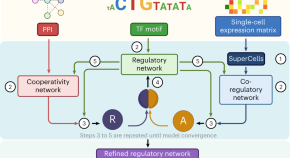
Enabling comparative gene regulatory network analysis on single-cell data with SCORPION
We present SCORPION, a computational tool to model gene regulatory networks based on single-cell transcriptomic data and prior knowledge of gene regulation. SCORPION networks can be modeled for specific cell types in individual samples, and are therefore suitable for conducting comparisons between experimental groups.
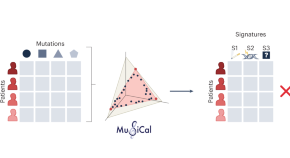
Improved identification of cancer mutational processes
Mutational signatures help to deconvolve the different processes that shape cancer genomes. A new tool now alleviates some of the persistent challenges in the field.
- Tom L. Kaufmann
- Roland F. Schwarz
Non-inferiority of simple versus radical hysterectomy in low-risk cervical cancer
Personalized cancer care can’t rely on molecular testing alone.
- James Larkin
- Chloe Beland
- Alexander R. Lyon

The future of precision cancer therapy might be to try everything
Researchers are blasting patients’ cancer cells with dozens of drugs in the hope of finding the right treatment.
- Elie Dolgin
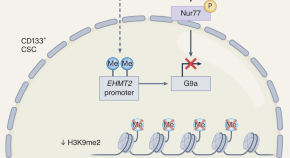
Repurposing the dopamine transporter antagonist vanoxerine to treat colorectal cancer
Self-renewing cancer stem cells drive tumor initiation and progression and represent a major target for therapeutic development. A study now shows that vanoxerine, a dopamine transporter antagonist, precisely inhibits this cell population in colorectal cancer, which leads to attenuation of tumor initiation and increased infiltration by immune cells.
- Winnie Chen
Low risk of CAR T cells going rogue
- Maria Papatriantafyllou
Rare CTR9 variants and myeloid malignancies
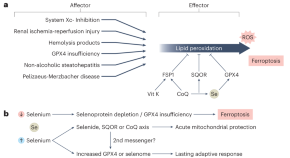
Selenium abandons selenoproteins to inhibit ferroptosis rapidly
Selenium is usually incorporated into selenoproteins, with important functions in redox regulation. A new study in Nature Metabolism reveals a previously unappreciated role for selenium-based chemical species as direct electron donors to reduce ubiquinone, thus contributing to redox homeostasis by preventing lipid peroxidation.
- Ian G. Chambers
- Rajiv R. Ratan
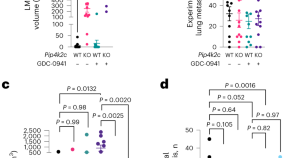
Melanoma leverages local cues to promote liver-specific metastasis
Using CRISPR–Cas9 screens, we found that cancer-cell-intrinsic loss of Pip4k2c conferred liver-metastatic organotropism in melanoma through hypersensitization to insulin-mediated PI3K–AKT signaling via co-optation of distinct hepatic metabolic cues. Additionally, we showed that combinatorial therapies that abolished physiological and drug-induced changes in glucose and insulin levels specifically reduced liver metastasis.
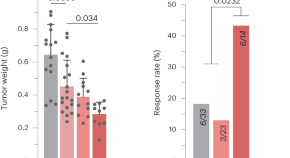
Mitochondrial DNA mutation enhances sensitivity to immunotherapy in melanoma
Mitochondrial DNA mutations are present in over 50% of all cancers, and truncating mutations in several genes encoding components of complex I of the respiratory chain are most recurrent. We found that these mutations are a source of Warburg-like metabolic shifts that promote a pro-inflammatory immunological state, enhancing sensitivity to checkpoint blockade.

Why we need to rethink how we talk about cancer
Naming metastatic cancers after parts of the body could be holding up research and preventing people from accessing the best treatment
- Lucy Odling-Smee
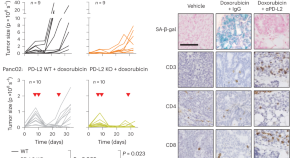
PD-L2 expression in senescent cancer cells limits tumor clearance after chemotherapy
Senescent cancer cells, which are characteristically present in tumors after genotoxic therapies, upregulate the immune checkpoint ligand programmed cell death 1 ligand 2 (PD-L2). We show that genetic or pharmacological ablation of PD-L2 prevents the accumulation of intratumoral senescent cells, reducing the recruitment of immunosuppressive myeloid cells and facilitating tumor clearance by T cells.
Methods & Protocols
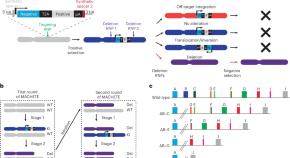
Engineering megabase-sized genomic deletions with MACHETE (Molecular Alteration of Chromosomes with Engineered Tandem Elements)
The authors introduce MACHETE (molecular alteration of chromosomes with engineered tandem elements), a clustered regularly interspaced short palindromic repeats directed Cas9-based system for the efficient deletion of megabase-sized genomic regions.
- Francisco M. Barriga
- Scott W. Lowe
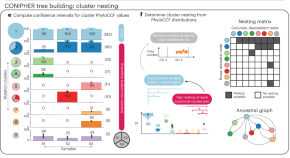
CONIPHER: a computational framework for scalable phylogenetic reconstruction with error correction
CONIPHER is a computational framework for accurately inferring subclonal structure and the phylogenetic tree from multisample tumor sequencing, accounting for both copy number alterations and mutation errors.
- Kristiana Grigoriadis
- Ariana Huebner
- Nicholas McGranahan

Robust scoring of selective drug responses for patient-tailored therapy selection
This protocol presents a computational approach to scoring drug sensitivity that integrates multiple dose–response parameters into a single response metric and identifies differences in drug-response patterns between cancer cells and healthy control cells.
- Yingjia Chen
- Tero Aittokallio
INVADEseq to identify cell-adherent or invasive bacteria and the associated host transcriptome at single-cell-level resolution
Invasion–adhesion-directed expression sequencing adapts the 10x Genomics 5′ single-cell RNA sequencing protocol to enable generation of bacterial and eukaryotic DNA libraries to identify adherent or invasive bacteria and the associated host transcriptome at a single-cell level.
- Jorge Luis Galeano Niño
- Susan Bullman
Genome-wide pooled CRISPR screening in neurospheres
The authors present a protocol for genome-wide clustered regularly interspaced short palindromic repeat screening of three-dimensional neurospheres.
- Amy B. Goodale
- David E. Root
Newly diagnosed AML: quizartinib improves OS

Promising results for antisense RNA therapy in mouse models of diffuse midline glioma
- Caroline Barranco
Serum tumour markers for testicular cancer recurrence
The current serum tumour markers α-fetoprotein, human chorionic gonadotrophin, and lactate dehydrogenase show limited value for testicular cancer relapse detection. A recent study highlights that false-positive elevations in follow-up monitoring are common and, conversely, many patients do not have elevations despite proven relapse. These findings highlight the potential for circulating microRNAs to be used as improved biomarkers for relapse detection.
- Matthew J. Murray
- Cinzia G. Scarpini
- Nicholas Coleman
Stress response in tumor-infiltrating T cells is linked to immunotherapy resistance
A newly composed single-cell transcriptomic atlas of tumor-infiltrating T cells across 16 cancer types revealed previously undescribed T cell states and heterogeneity. A unique T cell stress response state, T STR , was linked to immunotherapy resistance. Our high-resolution T cell reference maps, web portal, and annotation tool can assist efforts to develop T cell therapies.
CD4 + CAR T cells — more than helpers
Therapeutic products containing CD8 + and CD4 + T cells expressing CARs are effective at inducing remission in patients with cancer. How CD4 + CAR T cells contribute to the anti-tumor response has not been well established. A study uses syngeneic models and in vivo imaging to glean mechanistic insights into how CD4 + T cells target tumors.
- M. Eric Kohler
- Terry J. Fry
Venetoclax–obinutuzumab combinations are effective in fit patients with CLL
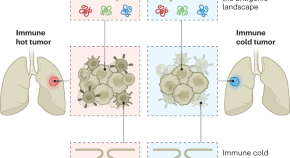
Taking the temperature of lung cancer antigens
Antigen presentation is fundamental to anti-tumor immunity, but our understanding of the physiological and molecular inputs to the process in different contexts remains limited. Two new studies explore the contribution of cell-intrinsic proteolytic mechanisms and cell-extrinsic hot and cold tumor microenvironments in shaping the antigenic landscape in lung cancer.
- Paul A. Stewart
- Alex M. Jaeger
A skull bone marrow niche for antitumour neutrophils in glioblastoma
A preprint by Lad et al. shows that tumour-associated neutrophils in glioblastoma originate from skull bone marrow and acquire an antigen-presenting cell phenotype intratumorally in the presence of local T cells.
- Austeja Baleviciute
Impaired RNA clearance
In this study, Insco et al. find patient-specific CDK13 mutations to impede RNA surveillance, leading to the accumulation and translation of prematurely terminated RNAs that drive malignancy in melanoma models.
- Gabrielle Brewer
Peptide-mediated CRISPR engineering of cells
An article in Nature Biomedical Engineering reports a simple and hardware-independent peptide-mediated delivery method for the CRISPR-mediated engineering of T cells.
- Nesma El-Sayed Ibrahim
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
New discovery gives hope to fight metastatic cancer
Cancer that splits and develops in new organs around the body becomes significantly more difficult to fight. Now, researchers at Chalmers University of Technology, Sweden, have shown that these metastatic cancers, that spread from the original, adapt their metabolism to the tissue in which they grow. The discovery represents a breakthrough for the understanding of metastatic cancer and is an important piece of the puzzle in the search for more effective treatments.
Metabolism in the human body can be likened to its internal engine. It is a prerequisite for our cells to grow and receive energy. Therefore, it is also an important target for cancer treatments, where the focus is on stopping the progress of cancer cells.
In a new study, which was recently presented in the scientific journal PNAS , researchers in Systems and Synthetic Biology at Chalmers have examined how metabolism works in cancer cells that have spread via metastases -- also called secondary tumors -- to new organs. The study gave the researchers new insights into how the metastases adapt to their new environment.
"Obviously, the local environment affects the cancer cells more than previously known. The metastatic tumours should show the same metabolic properties no matter where in the body they are located, but we discovered that the cancer cells largely adapted their metabolism to the new tissue in order to continue to develop and grow. This is important knowledge, which shows that we cannot consider the metastases as their original tumors," says Fariba Roshanzamir, PhD in Systems and Synthetic Biology at Chalmers and the study's lead author.
Tools for inhibiting cancer metabolism
Fariba Roshanzamir works in Professor Jens Nielsen's research group at Chalmers and has, together with Swedish and international colleagues, been able to establish the groundbreaking results. The study focused primarily on so-called triple-negative breast cancer -- a severe breast cancer that is difficult to treat with drugs -- but the conclusions can, according to the researchers, be applied to all types of metastatic cancer. This opens new doors to develop more effective treatments.
"If we manage to shut down the metabolism in a tumour, it will stop working and this study provides important keys to better understand what to target. Selecting metabolic inhibitors that specifically target the metastases in the organs to which the tumour has spread, rather than treating them as their original tumours, is of great importance to be able to find good strategies for treatments in the future," she says.
New view of the properties of metastases
Today, the spread of cancer to new organs is one of the leading causes of death in cancer patients. Jens Nielsen, Professor of Systems and Synthetic Biology at Chalmers University of Technology and one of the study's authors, hopes that it will lead to a new view of the properties and behavior of metastases.
"This is a breakthrough in terms of our understanding of metastatic cancer and an important step on the way to more individualised drugs," he says.
- Breast Cancer
- Skin Cancer
- Colon Cancer
- Lung Cancer
- Brain Tumor
- Prostate Cancer
- Stomach cancer
- Brain tumor
- Prostate cancer
- Colorectal cancer
- Breast cancer
- Stem cell treatments
Story Source:
Materials provided by Chalmers University of Technology . Original written by Ulrika Ernström. Note: Content may be edited for style and length.
Journal Reference :
- Fariba Roshanzamir, Jonathan L. Robinson, Daniel Cook, Mohammad Hossein Karimi-Jafari, Jens Nielsen. Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures . Proceedings of the National Academy of Sciences , 2022; 119 (35) DOI: 10.1073/pnas.2205456119
Cite This Page :
Explore More
- Extremely Fast Wound Healing: New Treatment
- Micro-Lisa! Novel Nano-Scale Laser Writing
- Simple Brain-Computer Link: Gaming With Thoughts
- Clinical Reasoning: Chatbot Vs Physicians
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
Trending Topics
Strange & offbeat.

COMMENTS
St. Jude Children's Research Hospital. St. Jude Children's Research Hospital is leading the way the world understands, treats and cures childhood cancer, sickle cell disease, and other life-threatening disorders. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood ...
The researchers later realized why this was so: It wasn't dead tumor cells that were stimulating the immune system; instead, the critical factor was cells that were injured by chemotherapy but still alive. "This describes a new concept of immunogenic cell injury rather than immunogenic cell death for cancer treatment," Yaffe says.
St. Jude Children's Research Hospital scientists reversed an aggressive cancer, reverting malignant cells towards a more normal state. Rhabdoid tumors are an aggressive cancer which is missing a ...
Organoid biology will further develop with a goal of translating the research into personalized therapy. These research areas may result in the creation of new cancer treatments in the future. Keywords: exosomes, immunotherapy, microbiome, organoid. Cancer research has made remarkable progress and new discoveries are beginning to be made.
New research shows how a key immune pathway — the STING pathway — which normally defends the body against viruses and cancer, actually dampens immune responses and helps cancer spread. When STING is persistently activated, it leads to desensitization and a rewiring of downstream signaling that stifles productive, anti-tumor immunity and ...
New research suggests that fungi in the gut may affect how tumors respond to cancer treatments. In mice, when bacteria were eliminated with antibiotics, fungi filled the void and impaired the immune response after radiation therapy, the study found. FDA Approves Belumosudil to Treat Chronic Graft-Versus-Host Disease.
Then, those cancer cells ingest the ADC, where SG3249 is released and kills the cancer cells. ... New CAR T-cell therapy research shows potential in solid tumors. Apr 7, 2023.
Trials of the new form of photoimmunotherapy, led by the Institute of Cancer Research, London, also showed the treatment triggered an immune response that could prime the immune system to target ...
The application of CRISPR tools in eukaryotic cells has greatly facilitated our ability to generate cell-based genetic models and probe gene functions, and has provided new insights into cellular ...
As of April 15, 2022, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape analysis that identified 2,031 such agents, but also a modest deceleration compared to 43% growth in the prior year. CAR-T therapeutics continue to dominate the cell therapy pipeline with growth of ...
Using their new method, the researchers introduced p53 mutations in human lung adenocarcinoma cells, then measured the survival rates of these cells, allowing them to determine each mutation's effect on cell fitness. Among their findings, they showed that some p53 mutations promoted cell growth more than had been previously thought.
December 27, 2023. Estimated reading time: 6 minutes. By Mayo Clinic staff. Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and ...
Writing for the NIH Director's Blog, Dr. Francis Collins highlights how Prof. Tyler Jacks and research scientist Megan Burger's work exploring T cell exhaustion led to the creation of a "strategy for developing cancer vaccines that can 'awaken' T cells and reinvigorate the body's natural cancer-fighting abilities."
(SACRAMENTO) A research team from the UC Davis Comprehensive Cancer Center has identified a crucial epitope (a protein section that can activate the larger protein) on the CD95 receptor that can cause cells to die. This new ability to trigger programmed cell death could open the door for improved cancer treatments. The findings were published Oct. 14 in the Nature journal Cell Death ...
Research led by scientists at Stanford Medicine has shown that long, repetitive DNA sequences may have a role in gene regulation in seven types of cancer — and there may be a way to home in on those sequences to suppress the propagation of cancer cells.. Using a new algorithm to analyze more than 2,000 human cancer genomes, the researchers first identified the repetitive sequences, then ...
T cells recognize and kill cancer cells but quickly lose their effectiveness. This fast dysfunction may help explain why immunotherapy doesn't lead to long-term remission for many patients.
The new cells, which the scientists have dubbed killer innate-like T cells, differ in several notable ways from the conventional target of many immunotherapies. Your source for the latest research ...
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Dong et al. demonstrate that targeting arginine N -methyltransferase 9 (PRMT9) inhibits cancer stem cells in the context of acute myeloid leukemia via type I interferon-associated immunity ...
New research by University of Colorado Cancer Center scientists illuminates the mechanisms at work that prevent p53 activation from triggering effective cancer cell death.
The research should help design radically new ways to treat cancer, they say. The Early Cancer Institute - which has just received £11m from an anonymous donor - is focused on finding ways to ...
ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
The work was supported by funding from the Ludwig Foundation for Cancer Research, the Emerson Collective Cancer Research Fund, the New York Stem Cell Foundation, the Stinehart-Reed Foundation, the Leukemia and Lymphoma Society, the J. Benjamin Eckenhoff Fund, the Blavatnik Family Fellowship, the Deutsche Forschungsgemainshaft, the Knut and ...
Cancer-associated fibroblast phenotypes are associated with patient outcome in non-small cell lung cancer. Cords et al. Published online: January 18, 2024. Cancer Cell. Cancer Cell provides a high-profile forum to promote major translational and clinical advances in cancer research and oncology.
The Nature Portfolio editors who handle cancer primary research, methods, protocols and reviews bring you the latest articles, covering all aspects from disease mechanisms to therapeutic ...
New research advances understanding of cancer risk in gene therapies. Date: November 16, 2023. Source: University of York. Summary: Researchers have discovered that 'cell competition' following ...
Paradigm-shifting work then taught us that our own cells harbor causative oncogenes. Our understanding of cancer—its origins, evolution, and vulnerabilities—exploded from there. Along the way, research outcomes enabled earlier and more accurate cancer diagnosis and drug development, saving millions of lives. On this 50 th anniversary of ...
Your source for the latest research news. Follow ... 2019 — Tumor cells circulating in blood are markers for the early detection and prognosis of cancer. However, detection of these cells is ...
MINNEAPOLIS, April 3, 2024 /PRNewswire/ -- Bio-Techne Corporation (NASDAQ:TECH) today announced it will showcase its portfolio of solutions to advance cancer research from target discovery to cell therapy development at the upcoming annual meeting of the American Association for Cancer Research (AACR), taking place April 5-10, 2024, in San Diego, California.
Oct. 4, 2022 —. Apr. 21, 2022 —. Oct. 6, 2020 —. Cancer that splits and develops in new organs around the body becomes significantly more difficult to fight. Now, researchers have shown that ...