Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 09 July 2021

DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy
- Ruixue Huang 1 &
- Ping-Kun Zhou ORCID: orcid.org/0000-0001-7666-1209 2
Signal Transduction and Targeted Therapy volume 6 , Article number: 254 ( 2021 ) Cite this article
44k Accesses
229 Citations
18 Altmetric
Metrics details
- Cancer genomics
- Molecular medicine
Genomic instability is the hallmark of various cancers with the increasing accumulation of DNA damage. The application of radiotherapy and chemotherapy in cancer treatment is typically based on this property of cancers. However, the adverse effects including normal tissues injury are also accompanied by the radiotherapy and chemotherapy. Targeted cancer therapy has the potential to suppress cancer cells’ DNA damage response through tailoring therapy to cancer patients lacking specific DNA damage response functions. Obviously, understanding the broader role of DNA damage repair in cancers has became a basic and attractive strategy for targeted cancer therapy, in particular, raising novel hypothesis or theory in this field on the basis of previous scientists’ findings would be important for future promising druggable emerging targets. In this review, we first illustrate the timeline steps for the understanding the roles of DNA damage repair in the promotion of cancer and cancer therapy developed, then we summarize the mechanisms regarding DNA damage repair associated with targeted cancer therapy, highlighting the specific proteins behind targeting DNA damage repair that initiate functioning abnormally duo to extrinsic harm by environmental DNA damage factors, also, the DNA damage baseline drift leads to the harmful intrinsic targeted cancer therapy. In addition, clinical therapeutic drugs for DNA damage and repair including therapeutic effects, as well as the strategy and scheme of relative clinical trials were intensive discussed. Based on this background, we suggest two hypotheses, namely “environmental gear selection” to describe DNA damage repair pathway evolution, and “DNA damage baseline drift”, which may play a magnified role in mediating repair during cancer treatment. This two new hypothesis would shed new light on targeted cancer therapy, provide a much better or more comprehensive holistic view and also promote the development of new research direction and new overcoming strategies for patients.
Similar content being viewed by others
Targeting DNA damage response pathways in cancer
Florian J. Groelly, Matthew Fawkes, … Madalena Tarsounas
A new wave of innovations within the DNA damage response
Qi Li, Wenyuan Qian, … Yuanfeng Xia
Leveraging the replication stress response to optimize cancer therapy
Emily Cybulla & Alessandro Vindigni
Introduction
The journey of dna repair machinery system discovery.
In recent years, the availability of high-quality data on DNA from in vivo and in vitro research reported in the literature, as well as international conferences, funding, and collaboration among scientific communities has increased. In addition, new technologies related to translational research, targets for clinical therapy, and expertize among scientists have developed rapidly, indicating that DNA damage repair and genomic stability research has entered a new era after a century of steady progress. This field continuous to offer unprecedented opportunities for exploring further the secret of our genomic DNA structure integrity and function harmonization while also promoting clinical disease prevention and therapeutic options, especially boosting the precise cancer therapy. The secret veil of DNA was uncovered 70 years ago, since the famous “Photo 51”was published 1 along with the ground breaking report entitled “Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid” by James Watson and Francis Crick 2 in 1953. In this landmark study, DNA was illustrated as a double helix, resembling a ladder twisted along its length.. Over the following decades, many distinct biological topics such as DNA damage repair, genomic instability, cancer therapy and control of genetic diseases have been explored and found to be associated with DNA sequences and genomic profiles. 3 , 4 , 5 , 6 In Fig. 1 , we illustrate the brief history of the DNA and DNA damage repair discovery journey. Some important moments and scientists should be noted for their groundbreaking contributions to this journey. In 1927, the landmark discovery of gene mutation induced by X-ray was claimed by Gager and Blakeslee, Miller. 7 One year later, the genetic transformation of bacteria was reported by Frederick Griffith. 8 In 1946, Hermann J. Muller was awarded the Nobel Prize in Physiology or Medicine for his contribution in discovery of genetic mutations in fruit flies and revealed that higher the dose of X-ray and other ionizing radiation exposed, the greater the number mutations that occurred. 9 , 10 In 1944, Oswald Avery, Colin MacLeod and Maclyn McCarty provided robust evidence demonstrating that DNA was our genetic material. 11 Shortly thereafter, Watson and Crick published the structure of DNA and announced that “we have discovered the secret of life” in 1953 2 . In 1964, the keyword “DNA repair” was formally introduced with the discovery of “Dark Repair” and photo reactivating “repair-replication” of UV light-induced E. Coli DNA injury by excision of damaged areas containing thymine dimers. 12 , 13 , 14 , 15 Since then, the DNA damage repair research rapidly spreads into the area of photobiology, radiobiology and cancer biology, etc. We understand presently that the term of “DNA repair” defines the biochemical and molecular biological processes of DNA damage removing and genomic integrity restoring, which including DNA damage sensing and signaling, repair machinery proteins recruited onto the damage sites, functioning and released step by step to restore the genomic integrity. The first evidence of the direct association between DNA repair deficiency and human disease and cancer predisposition was demonstrated in Xeroderma pigmentosum. 16 , 17 In 2015, the Nobel Prize in Chemistry has been awarded to Tomas Lindahl, Paul Modrich and Aziz Sancar for their pioneering and fundamental contributions of mapping, at a molecular level, how cells repair damaged DNA and safeguard the genomic stability. Their work has provided fundamental knowledge of how a living cell functions and is, especially, used for the development of new cancer treatments.
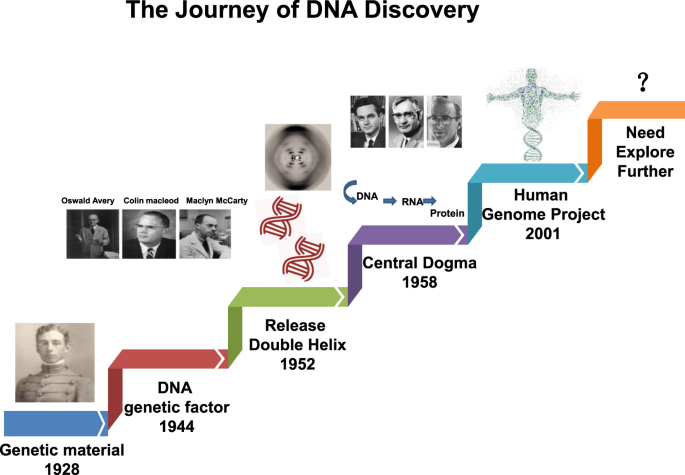
The journey of DNA discovery
Now, it is well known that mammalian cells have evolved multiple and diverse machineries for repairing every type of spontaneously occurring as well as exogenous factors-induced DNA damage.
Following these critical discoveries and foundational works, the Human Genome Project was initiated in 2001, which provided a deep understanding of the evolution of the human population as well as relationships between human health and diseases. 18 , 19 Other projects exploring the topics of genome organization and function, such as the Encyclopedia of DNA Elements(ENCODE),have provided significant conclusions based on extensive sequencing results. 20 , 21 Over the past century, understanding of the DNA double helixintegrityled to extremely significant advances in our appreciation of biological processes including gene transcription, replication and protein expression. Notably, clinical medicine has become more broadly based on genetics and DNA function, 22 , 23 the increasing knowledge of DNA repair opened up a new irreplaceable path of precisely targeted cancer therapy. Over the next century, many more promising advancements will undoubtedly occur in DNA-related research. Despite these significant past discoveries, the underlying molecular mechanisms and functions of DNA have remained unclear in recent decades. Clinical applications for disease therapy necessarily lag behind research into DNA damage repair-based medical diagnosis and therapeutic targets. Nevertheless, DNA damage repair research is leading to better appreciation of the complexity and diversity of diseases. Future efforts are needed to elucidate further the secrets of life using DNA and to introduce novel concepts and hypotheses as powerful and compelling as the discovery of the DNA double helix and DNA repair-based genomic integrity maintaining mechanisms. 22 , 23 , 24 , 25
A historical perspective of linking the genomic distortion with cancer
The origin of cancer medicine is associated with a clinical discovery based on medical record analysis and an epidemiological survey. Percival Pott, known as the father of epidemiology, observed a high prevalence of scrotal cancer among the boys who were employed as chimney sweeps, and attributed the cancer to soot exposure. 26 This was the first evidence of occupational exposure to hazard factors associated with cancer development. Prior to the discovery of the structure of DNA, Dr. Theodor Boveri proposed in 1914 the remarkable theory that the origin of malignant tumors was from cancer cells, and that cancer cells formed through alteration of normal cells. 27 He expounded on this theory by suggesting that tiny microscopic bodies called chromosomes might be abnormally distributed in tumor cells. 28 , 29 In the late 1920s, Hermann Muller, the principal discoverer of gene mutations mentioned above, reported that exposing Drosophila melanogaster to ionizing radiation from X-rays could result in the “transmutation” of a gene, contributing to aberration of the chromosome. 9 , 10 In the 1930s, it was observed that, compared to the normal human cells with 46 chromosomes, the number of chromosomes in cancer cells typically varies and frequently exceeds 46. 30 , 31 Meanwhile, scientists noted that cancer cells have more rapid and stronger growth ability than normal cells. 32 , 33 , 34 By the 1950s, shortly after the DNA structure was described, it was shown that exposure to chemical mutagens such as the chemical benzene could produce chromosome breakage and increase DNA mutation rates. 35 , 36 In the 1980s, the process of carcinogenesis was described, with necessary conditions of DNA mutations generated due to environmental mutagen insult and the occurrence of DNA damage without effective repair. 37 , 38 Thus, the critical role of DNA damage response(DDR) was determined. In the following years, extensive evidence obtained using many new methods developed from the study of DDR processes indicated that DNA repair, 39 DNA damage signaling and repair pathways, 40 , 41 cell cycle checkpoints, 42 , 43 apoptosis, 44 , 45 , 46 fidelity of replication, 47 , 48 DNA re-replication 49 and telomeres 50 , 51 are all closely associated with cancer. 51 Based on these studies of the DNA molecule integrity and the process of genetic mutation, the linkage between DNA mutation and cell carcinogenesis became increasingly clear. Phil Lawley, a pioneering researcher of DNA damage and carcinogenesis, found that some alkylating agents, such as butadiene dioxide, 52 , 53 , 54 could interact with DNA, forming harmful adducts and eventually disrupting the normal role of DNA as a molecule template. 55 The hypothesis that certain cancer genes are susceptible to such agents was proposed and extensively studied over the past few decades. Since then, chemotherapeutic agents and radiotherapy have been found to treat various cancers effectively through DNA damage induction. In the war against cancer, numerous agents have been developed and novel technical strategies have also been explored. However, many challenges and unsolved issues remain that require further study, such as: (i) the detailed molecular mechanisms underlying the cancer cell DNA response to chemotherapeutic agents and radiotherapy; (ii) how cancer cells become resistant to chemotherapeutic agents and radiotherapy; (iii) possible new and promising biomarkers for investigation as novel inhibitors or therapy agents; and (iv) most importantly, the basic biological mechanisms underlying the DDR. With such information, effective cancer therapies could be developed to target DDR and ultimately prevent or cure cancer.
DNA damage, cellular response, repair and cancer
Genome stability.
To support survival and reproduction, maintaining genome stability is a critical priority of all cells. 56 Any abnormal alterations of the genetic base sequence can disrupt cellular biological processes, hampering cellular functions and possibly inducing carcinogenesis or even cell death. 57 Specifically, strong evidence has indicated that genomic instability promotes cancer pathogenesis through a cascade response involving a series of proto-oncogenes that are continuously triggered or anti-oncogenes that are suppressed. 58 , 59 , 60 In this context, the EGFR (epidermal growth factor receptor), MYC and RAS families have been commonly recognized as proto-oncogenes, 61 whereas TP 53 is a well known tumor suppressor gene. 62 , 63 Accordingly, to reduce the possibility of genetic dysregulation of genome stability, cells have evolved a range of genome stability-related signal pathways and post-translational modifications, 64 which assess the accuracy of DNA metabolism and prevent accumulation of DNA damage. 65 For example, multiple families including ATM (ATM serine/protein kinase), ATR (ATR serine/threonine kinase), and DNA-PKcs (DNA-dependent protein kinase catalytic subunit) can initiate the signaling cascade in mammalian cells. 66 A recent review by Monique PCM et al. summarized the advances of ubiquitination research and noted that ubiquitination performs vital roles in regulating cellular homeostasis through numerous enzymes 67 and proteins. The complex functions of this compound have become known as the “ubiquitin code” in the scientific community. 56 Genomic instability is a common characteristic of most cancer cells. 68 For example, a high ratio of chromosomal instability is associated with mitotic spindle checkpoint deficiency in most breast cancer cell lines. 69 The molecular mechanisms through which cells maintain genome stability and the repercussions of genomic instability are essential emerging issues relevant to clinical cancer avoidance.
Accumulating evidence has shown that a DNA double-strand break(DSB) is typically the most harmful type of DNA damage, and that it compromises genome stability. 70 In mammalian cells, a number of vital DNA repair functions and processes against various DNA damage have evolved. For example, the mismatch repair pathway, base excision repair pathway and nucleotide excision pathways have been well characterized. 71 However, cancer cells have frequently evolved in relation to abnormal DNA damage repair functions and processes. For example, in many cancer cell lines, such as mantle cell lymphoma(MCL), ATM is recurrently mutated in around thirty to almost fifty percent of cases. 72 These mutations may be linked with cancer chemotherapy resistance. 73 Furthermore, cell cycle machinery-related genes play critical roles in driving avoidance of chemotherapy and radiotherapy treatment effects by cancer cells. 74 Most measures developed to kill cancer cells involve: (i) stimulating G1 phase aberrant homologous recombination in cancer cells; (ii) inducing mitotic catastrophe in cancer cells; or (iii) deleting the cell cycle checkpoint. 70 , 75 , 76 Despite data showing that genomic instability may be associated with ROS(reactive oxygen species), 77 , 78 in this review, we focus on DNA damage repair, as it is a major clinical target of cancer chemotherapy and radiotherapy.
DNA damage and cancer
It is critical for maintaining genomic DNA stability due to its role as the template for replication and transcription. 79 As described above, damage to DNA from environmental hazards insult as well as endogenous toxic agents such as free radicals can compromise genome stability and cause or promote many diseases, particularly cancer. 37 , 80 , 81 As the DNA molecule is the basic genetic material, it is vital for ensuring the integrity of DNA structure and function to support normal life activities and stable species characteristics. 82 , 83 Indeed, when experiencing either endogenous or exogenous stresses, cells can generate various types of DNA damage, including base pair alterations, DNA replication errors 84 and distortion and breakage of the DNA double helix strands. 85 Common exogenous factors, especially certain environmental hazards such as toxic heavy metals and ionizing radiation, have been intensively studied and found to cause serious DNA damage. 86 , 87 , 88 , 89 , 90 Endogenous materials are often released during the metabolism of exogenous materials in the body or after cell damage and the loss of cell membrane integrity. 91 DNA damage can occur through two pathways, namely direct effects and indirect effects. In the direct pathway, endogenous or exogenous materials directly contact DNA, leading to the breakage of chemical bonds in DNA molecules, and thereby changing the structure and activity of DNA. 92 , 93 In the review by Anthony T et al., endogenous stresses including gene transcription and replication in cancer cells are noted to cause genomic instability. 79 In the indirect pathway, endogenous or exogenous materials activate products such as free radicals 94 , 95 that can damage DNA. 96
Several types of DNA damage have been reported previously, as follows: (i) single-strand breaks; (ii) double-strand breaks (DSBs); 97 (iii) base damage; (iv) sugar damage; (iv) DNA cross-linking and (v) clustered damaged sites, 98 of which the most deleterious lesion and the most severe threat to cells is the DSB. DSBs that occur without effective repair or error-prone repair can cause carcinogenesis or cell death. 99 Lindahl et al. reported that, each day, our cells may be subject to around 70,000 instances of DNA damage. 100 Most of these lesions are single-strand breaks, and only a few are DSBs, which are less frequent. Numerous studies have illustrated that DNA subject to oxidative stress exhibits a large number of base and sugar lesions, 101 such as guanine modification or 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-OH-dG). Base lesions are usually caused indirectly by ROS generated due to oxidative stresses such as radiolysis of water molecule induced by ionizing radiation. 102 Sugar damage, such as 8,5′-cyclopurine-2′-deoxynucleosides, can be caused by free radical insult to the sugar moiety. 103 , 104 DNA cross-linking is often attributed to exposure to chemical cross-linking agents, e.g. cis-platinum, or free radical-generating ionizing radiation. 105 With this type of damage, DNA repair-related proteins are trapped with DNA, causing the proteins to adhere to the 5′ or 3′ end of the DNA strand break. 106 Furthermore, DNA cross-linking can hinder the activities of some vital enzymes such as DNA helicases and polymerases. 107 , 108 A review described the formation of DNA cross-links due to exposure to various endogenous, environmental and chemotherapeutic agents. 106 However, elucidation of how this process is regulated and its full biological functions in mammalian cells and cancer cells require further research. Clustered DNA damage, sometimes described as multiple local damage sites, refers to damage in which at least 20 base pairs are separated. 109 Clustered DNA damage usually consists of multiple lesions such as base damage, a basic site damage and single-strand breakage. 110 However, in contrast to DSBs, the multiple lesions of clustered DNA damage may be present on the same DNA strand or on opposing strands within a tiny range. Figure 2 illustrates the main types of DNA damage along with differential definitions of double-strand breakage-based and non-double-strand breakage-based clustered DNA damage. In general, clustered DNA damage results in enhanced mutation frequency, 111 cancer, and cell death. The mechanism of clustered damage has been described as a base obtaining a single electron, after which multiple electron pathways are activated. 111 However, whether a beneficial result (friend) or non-beneficial result (foe) is obtained from clustered DNA damage in cancer cells or normal cells requires further study. 112 As cells face a tremendous amount of DNA damage arising from various exogenous and endogenous stressors, such as ionizing radiation or ROS, 112 , 113 recognition of how DNA damage occurs requires deeper investigation. Many scientific issues remain to be addressed in future research, such as: (i) excluding the currently known DNA damage types, other novel DNA damage styles may exist that have not yet been discovered; (ii) methods to evaluate and measure DNA damage types and degrees, or visualization techniques for DNA damage; (iii) monitoring processes for DNA damage and identification of effective biomarkers for early detection of DNA damage; and (iv) obtaining reference values for the exogenous and endogenous stressors that drive DNA damage. Investigating these issues may help to standardize DNA damage caused by various insults. Importantly, innovative technologies and unique theoretical models would be developed while exploring these interesting issues.
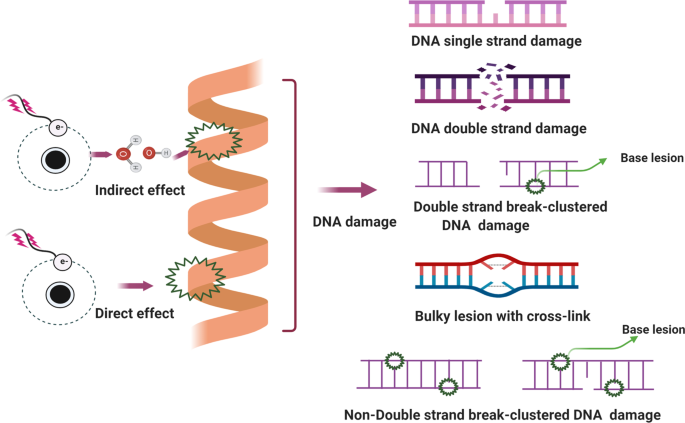
Main types of DNA damage along with differential definitions of double-strand breakage-based and non-double-strand breakage-based clustered DNA damage
DNA damage response
Sensors in the dna damage response.
To avoid DNA damage, cells have evolved numerous interacting mechanisms for ensuring genomic stability or have even used DNA damage to produce new opportunities for natural selection. 114 , 115 These mechanisms have been identified as the DNA damage response (DDR). Generally, DDR mechanisms involve feedback signals from damage sites and movement of repair factors to cluster at damage sites. In our previous published review, we used ionizing radiation to explore how the cell’s DNA damage sensors and signaling transducers interact in the DDR. We focused on the critical issue of recognizing and identifying DNA damage signals to activate the subsequent biological response cascade. 6 Therefore, in this review, we focus on the association between DDR and cancer. Due to the characteristic genomic instability of cancer cells, mutations and tumor heterogeneity are common and widespread. 116 These features suggest that cancer cells are prone to enhanced proliferation, growth and tumorigenes is due to dysregulation of DDR-related mechanisms. 117 The acquisition of specific mutations in cancer cells might, in turn, increase the dependence on other DDR factors for survival. 118 The development of cancer requires both mutagenic and non-mutagenic events. Cells exposed to endogenous and exogenous factors that act as mutagenic agents show impacts throughout the process of cell oncogenesis, but these effects are stronger in cancer cells with mutated or deficient DDR genes. 119 , 120 Alteration of DDR genes has been demonstrated in various cancers, including breast cancer and prostate cancer. 40 , 118 For example, BRCA1 or BRCA2 inactivating mutations were found in ninety out of almost six hundred breast cancer patients. 121 Moreover, DNA mismatch repair-dependent DDR pathways, such as loss of non-canonical mismatch repair gene functions, contribute to improved treatment outcomes of colorectal cancer. 122
Here, as a few DNA damage sensors such as γH2AX, Mre11-RAD50-NBS1 complex, Ku70/Ku80, MDC1 and 53BP1 can initiate the damage signaling thus trigger the DDR, 123 In a study,γH2AX may be expressed not only to detect genetic effects caused by various toxic substances but also to monitor the clinical efficacy of chemotherapy and radiotherapy and the sensitivity alternations of cancer cells to anticancer agents. 124 Another study assessed DDR processes after hepatocellular cancer therapy and found that γH2AX expression increased. 125 Screening for H2AX variant functions and targeting of H2AX have been proposed as cancer treatments. 126 Ku70/Ku80 expression also exhibited a significant increase in rectal cancer patients after chemotherapy and radiotherapy treatment, and further study showed that the increase in Ku70/Ku80 expression was correlated with chemo- and radio-resistance in various cancers. 127 Ku70/Ku80 expression can be used as a molecular cluster for predicting the susceptibility of rectal cancer to chemoradiotherapy. 128 In contrast to oxygen sensors, which have been extensively investigated, these DDR sensors are still in the early stages of molecular characterization, and their roles in sensing DNA damage and signaling, cancer progression and therapy require further study.
DNA damage response and cancer
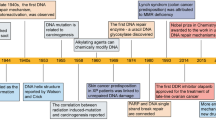
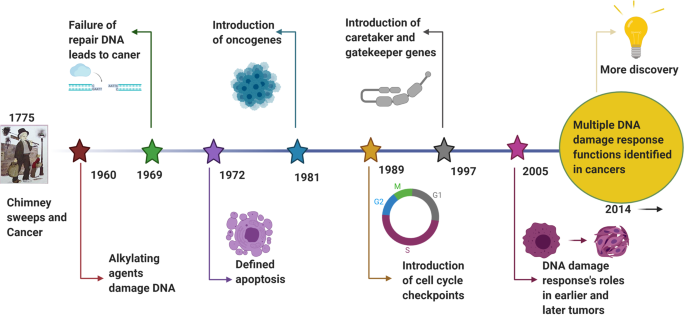
A review literature highlighted the various concepts behind targeting of DDR in cancer, 129 which were summarized that (i) DDR can be used as a target of anticancer drug treatments; (ii) as most cancer cells have a deficiency of some DDR pathways’ ability, inhibition targets can be explored in the remaining pathways; (iii) DDR inhibition can be used to investigate cancer replication stress; and finally (iv) the author considered using DDR inhibitors in specific DDR-lacking backgrounds initially to promote exploration of DDR-based agents for cancer treatment in the future. The first description on the association of cancer with occupational exposures was presented in 1775 by the British surgeon Percivall Pott, 130 who first showed the link between the occupational exposure of chimney sweeps and scrotal cancer. In 1946, the X-ray induced recessive lethal of Drosophila was first reported to be related to the chromosomal breakage. 131 , 132 Later, the discovery of the helical structure of DNA led to the introduction of the concept of DNA as the hereditary material. 133 , 134 Soon thereafter, repair of X-ray damage to DNA was reported in bacteria in 1966 135 and in eucaryotic cell in 1967. 136 Significant research showed that defective DNA repair resulted in many diseases and, in particular, cancer propensity, during the period from 1969 to 2015. 137 , 138 In 1972, apoptosis was defined, which is a programmed cell death pathway occurring in cells during the normal tissue development or encountering exogenous stresses, especially DNA damage. 139 , 140 In 1981, the concept of oncogenes was introduced, 141 followed by the concept of tumor suppressors three years later. 142 In 1989, cell cycle checkpoints were proposed and in 1990, p53 was reported to be mutated in various cancers. 143 Then, in 1997, caretaker and gatekeeper genes were proposed based on the research discoveries on DNA repair genes BRCA 1/2, RAD 51. 144 , 145 , 146 In 2002, ROS production and DNA damage attributable to deregulated metabolism induced by oncogene expression were reported. 147 , 148 In 2005, DDR was described as an anticancer barrier in early-stage tumorigenesis, but the genes showing DDR mutations were absent from later-stage tumors. 149 To date, a large number of DDR genes have been identified in various cancers. 150 , 151 Figure 3 illustrates the timeline of DDR-related findings and concepts related to cancer, highlighting the scientists who worked to provide a deeper understanding of the roles of DDR in cancer.
Timeline of DDR-related findings and concepts related to cancer, highlighting the scientists who worked to provide a deeper understanding of the roles of DDR in cancer
Alongside the DDR processes described above, including cell cycle checkpoints and apoptosis, we present DDR signaling by way of a brief introduction to how DDR pathways can affect cancer development. First, a healthy cell affected by environmental hazards, viral or bacterial infection, or ROS may initiate DNA damage and mutations, increasing oncogene activation, tumor suppressor inactivation and replicative and oxidative stresses. The oncogene activation might occur directly or indirectly. As a result, DNA replication fork fidelity and replication recovery are compromised. 152 , 153 , 154 Hence, increased DNA damage and mutations in normal cells would hamper genomic stability. At this stage, the damaged cells still exhibit a range of responses, including activation of checkpoint arrest and triggering of increased p53 expression to protect cells against further damage. However, downregulation of DDR processes should disturb the proliferation of pre-cancerous cells. 155 , 156 , 157 General DDR pathway research in relation to cancer development provides important information that may be useful for the design of targeted cancer therapies. 158 , 159 More importantly, understanding DDR can also help elucidate why targeted clinical therapy strategies often fail. 160 , 161 Debjani P et al. assessed the performance of cancer cells escaping targeted lung cancer therapy, found that the key event was activation of the TGF-β signaling pathway in some cancer cells after targeted therapy. 162 TGF-β activation can inhibit the expression of DDR-related genes, resulting in decreased DNA repair ability and, thus, accumulation of mutations. Other advances have shown that tumor heterogeneity may influence the outcomes of targeted cancer therapy. 163 , 164 For example, some scientists have reported that ALK-targeted therapy differs among cancer patients, with many patients treated with ALK-targeted therapy developing therapy resistance, which results in cancer progression. 165 Targeted therapies against other cancers, such as non-small cell lung cancer (NSCLS), also face challenges related to tumor heterogeneity, which impact acquired and inherent drug resistance. 166
New technologies, such as cancer genome profiling using deep sequencing and microarrays and single-cell sequencing offer more information about which DDR-related genes are mutated or mis-regulated. 62 , 167 , 168 , 169 , 170 However, a better understanding of DDR pathways and discovery of new and valuable ideas for improving cancer treatment are still urgently needed.
DNA damage repair
A historical perspective of dna damage repair.
Many human pathologies such as tumors and chronic metabolic diseases can be clearly attributed to DNA damage induction. 171 Indeed, although DNA damage is common and its occurrence is very frequent, such damage must be repaired immediately and correctly to ensure the exact transfer of genetic information during cell division. 172 , 173 Without appropriate DDR capacity after insult from environmental or endogenous stressors, negative effects may occur in normal cells, as follows: (i) increasing opportunities for genomic defects; (ii) possible genomic instability and malignant transformation; (iii) enhanced development of cancer; and (iv) further injury to cellular DNA repair ability, as DNA damage signaling and inappropriate repair processing in cancer cells would benefit cancer cell growth and proliferation while disrupting the outcomes of cancer chemo-and radiotherapy. Over the long period of around 4 billion years of evolution, it is unsurprising that cells have prioritized minimizing mutagenesis and protecting genomic replication through effective and quick repair of DNA damage. 174 In recent years, numerous studies have reported evidence of the importance of DNA damage repair: (i) a few types of DNA damage have been illustrated over recent decades, of which DNA double-strand breaks (DSBs) represent the greatest risk for causing genomic instability; 173 (ii) some components of DNA repair pathways are so important that life would not be sustained without them, such as ATR, which is critical for early embryonic development and its deficiency results in chromosomal fragmentation and early embryonic lethality; 175 (iii) many hereditary disorders have been attributed to DNA repair deficiencies, such as the observation by Jim Cleaver that patients with the rare autosomal-recessive cancer predisposition syndrome, xeroderma pigmentosum, lacked the ability to perform DNA repair after damage due to UV exposure; 138 and (iv) defective DNA repair pathways are associated with cancer initiation, as shown by a study in the 1990s, which found Lynch syndrome was related to mutation of the DNA repair proteins MutS and MutL. 176
From a historical perspective, early research into DDR focused on observations, as described above. 177 In 1940, American biologist Albert Kelner identified photo reactivation, which is an enzyme-catalyzed reaction, as the enlightenment DNA repair mechanism. 178 Then, in 1964, Setlow RB and Carrier WL reported an error-correcting mechanism in which intrastrand thymine dimers formed after UV radiation of DNA accounted for a large fraction of the observed biological damage to DNA. 15 , 179 In the same year, the term “DNA repair” was formally founded with the discovery of “Dark repair” and “Repair-replication” or “unscheduled DNA synthesis” of ultraviolet injury to the DNA in Escherichia coli . 12 , 13 , 180 These studies showed that one strand of damaged DNA could be excised, and the resulting gap could be repaired, using the intact complementary strand as a template. 12 , 181 This repair pathway is known as nucleotide excision repair. In 1968, J R Cleaver et al. validated it as a repair replication mechanism through observation of UV-induced lesions to HeLa cell DNA. 182 Soon thereafter, polynucleotide ligase activity was discovered in the cell-free extracts from E. Coli by Gellert M in a study showing that E. coli extracts could convert lambda DNA to covalent circles. 183 In other words, polynucleotide ligase can combine a newly synthesized patch with the contiguous parental DNA strand.
In the mid-1970s, the excision repair processes of base excision repair and mismatch repair were described. Lindahl revealed that an N-glycosidase was active in DNA repair based on its ability to deaminate dCMP residues into an easily repairable form. 184 Meanwhile, Wagner Jr. and Meselson used E.coli to identify repair tracts originating at mismatches. The repair process propagatesin the direction of 5′ to 3′ and can cover approximately three thousand nucleotides. 185 In terms of excision repair, the optimal outcome is that the DNA can be replicated normally after excision and repair, but it is also possible for an advancing replication fork to encounter the damage site after excision and prior to the completion of repair, which is known as synthetic death. 174 Compared to the former repair type, the latter is more complicated and more likely to be lethal. In 1975, an interesting hypothesis was raised by Radman, who suggested that E. coli possesses an inducible DNA repair system, called “SOS repair”. 186 The main components of this hypothesis were: (i) DNA damage initiated the “SOS repair” process; (ii) de novo protein synthesis is involved in the repair process; and (iii) physiological and genetic status requirements must be met for further prophage induction. 186 This hypothesis was confirmed through many later experiments, and some new concepts have been incorporated into this model. For example, a study showed that cells treated with rifampin to eliminate their ROS repair ability exhibited reduced repair efficacy of global cyclobutane pyrimidine dimer (CPD) formation due to UV radiation exposure. 187 These findings provided the insights into the enzymes responsible for DNA damage detection, and showed that they may attack undamaged DNA, with deleterious consequences. In other words, these sensitive DNA repair enzymes perform dual roles depending on their concentrations. At low concentration, these enzymes are kept in check until needed for repairing specific DNA damage. Since these studies, the concepts of transcription-coupled repair sub-pathway and global genome repair sub-pathway of nucleotide excision repair (NER) have been supported by numerous studies. 188 , 189 The DNA damage in the active transcription gene or the transcribing strand is preferentially repair through the transcription-coupled repair sub-pathway. 188 , 190 Global genome repair is another sub-pathway of nucleotide excision repair, through which the DNA damage in whole genome is repaired with equal efficiency. The mechanistic difference between transcription-coupled repair and global genome repair is mainly that, in the former process, the stalling of RNA polymerase at transcriptionally active genes favors the recruitment of Cockayne syndrome proteins A and B, whereas in the latter process, helix-distorting damage is recognized by XPC and its partners RAD23B (Rad 23 homolog B) and CETN2 (centrin 2). 191 However, compared with global genome repair, transcription-coupled repair is more constitutive and is evidently not inducible as a DDR response. Excluding excision repair, several other repair pathways that support improved replication to overcome the obstruction of replication caused by lesions without their removal have been reported; they are known as tolerance pathways. These pathways require the function of specialized DNA polymerases. 93 , 192 , 193 At this point, a “collapsed replication fork” had been defined and its role in the loss of DNA synthesis capacity was known. 194 In this process, the fork collapse contributes to genomic instability or even death. 194 , 195 In general, after reviewing DNA damage repair from a historical perspective, 196 concerns remain, which can be summarized as follows: (i) Is the previous DNA damage repair definition sufficient to represent fully the process and its significance? Based on the rapid development of DNA damage research and a deeper appreciation of DNA damage repair, the definition should be expanded to include exogenous and endogenous insults, genomic early and later responses, DNA repair-related enzymes, and early events associated with later outcomes. (ii) Most of previous researches aimed to uncover new targeted proteins and enzymes rather than considering the interactions among multiple DNA repair pathways. Sometimes, various DNA repair pathways can handle the same damage sites in competing ways, but how this interaction occurs remains unclear. Moreover, the processes that occur at each step of multiple repair routines require further investigation. (iii) The threshold level is an essential concern for initiation of DNA damage repair, but leads to low-level lesions often being overlooked. However, multiple long-term low-level lesions may lead to DNA repair via some novel pathway or mechanism, which requires further validation and testing. (iv) Finally, basic information about DNA repair is lacking, including how damage to bases and other structures of DNA is sensed in cells, what roles the sensing machinery plays in the cellular response to DNA damage, and how the cells perform cell cycle arrest in response to DNA damage in normal cells and cancer cells. Furthermore, in the context of cell mutagenesis or lethality, more information is needed about how DNA damage repair-related enzymes and proteins regulate downstream events in combination with other factors after the recognition of an aberration. These questions are very basic, but have yet to be answered fully and clearly. In addition, it is important to learn from previous research and apply these discoveries in the clinic setting in the future. The greatest value of DDR research is that a deeper understanding of the secrets of life will allow us to face the challenges that arise from environmental, social and technological issues more effectively.
DNA damage repair pathways
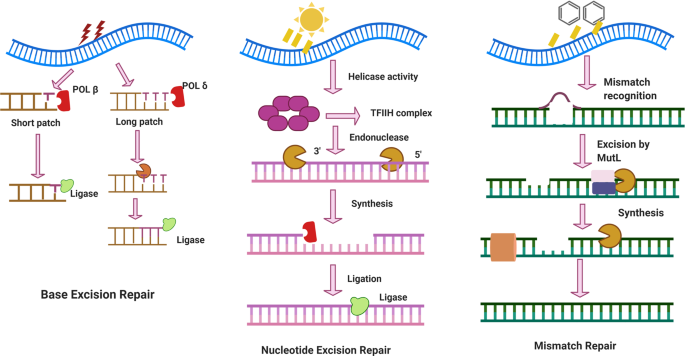
Several repair pathways exist, including direct reversal, base excision repair, nucleotide excision repair, mismatch repair, single-strand break repair and DSB repair. 197 , 198 , 199 Direct repair generally refers to the repair of pyrimidine dimers formed due to UV exposure or other factors or the repair of alkylated bases. Nucleotide excision repair refers to repair of DNA replication lesions or bulky adducts arising from distortions of the DNA structure. 3 , 200 Mismatch repair refers to adjustment of mismatched base pairs in double-stranded DNA, as well as repair of some insertions or deletions of less than 4 nt. 201 , 202 Double-strand break repair refers to repair of DSB lesions. 203 , 204 , 205
Direct reversal repair
As described above, induced DNA damage often refers to damage caused by alkylation, oxidation, UV and cross-linking agents. 206 Direct reversal of the base lesion rather than excision is the one simplest step error-free and most economical DNA repair mechanism to have evolved. 206 , 207 , 208 Cells have also developed direct reversal mechanisms for several types of DNA damage, such as alkylation, inter/intra-strand cross-link. In E. coli , the mechanism of the direct DNA damage reversal reaction was described as a “flip-out”process. 209 Briefly, enzymes first form a long loop, then DNA photolyase binds to duplex DNA, goes through a series of energy transfer, single electron transfer and enzymatic catalysis steps, and forms a flip-out helix structure to skip the break site for direct reversal. 209 , 210 , 211 This form of direct DNA damage reversal is considered to be beneficial to cells, as it is a highly effective and simple method to address an important and necessary issue.
Unlike other molecules, which can be replaced, DNA cannot be replaced after being damaged, and must instead be repaired. Three classical DNA damage direct reversal mechanisms have been described, namely, repair of O-alkylated DNA damage by alkyltransferases and dioxygenases, repair of photolesions caused by ultraviolet (UV) radiation through the work of spore photoproduct lyases and photolyases, 212 , 213 and reversal of N-alkylated base adducts by AlkB family dioxygenases. 214 DNA is constantly subjected to numerous environmental insults. Among such hazards, alkylating chemicals, which are often applied as cancer chemotherapy agents, can cause DNA damage in the form of alkylation. 215 , 216 Endogenous products, including metabolites such as adenosylmethionine arising from many biological processes, may also damage DNA. 217 , 218 After damage from these agents, some typical damage response molecules such as methylguanine and methyladenine are formed on double-stranded DNA. 219 , 220 These materials can increase the cell’s mutagenic and carcinogenic potentials, for example, by increasing the chance of base misincorporation. 221 Alkyltransferases are associated with direct removal of DNA alkylation damage. Studies have shown that this enzyme is responsible for the removal of mutagenic alkyl adducts on the bases of the O6 atom of 2′-deoxyguanosine and the O4 atom of thymidine. 222 , 223 In cells, the importance of O6-alkylguanine DNA alkyltransferase-based direct reversal is greater than that of nucleotide excision repair or base excision repair, suggesting the critical role of alkyltransferases. 224 The potential role of this protein in DNA repair was reported 40 years ago. Its main roles include prevention of mutations, cytotoxicity, and cancer development. 225 Alkyltransferases have been identified in many living things. 226 However, whether other co-factors or energy resources interact with alkyltransferases to perform direct DNA damage reversal remains unknown. 225 Meanwhile, although multitudinous studies have been conducted to investigate the activities of alkyltransferases, how their polymorphisms relate to health, and specifically cancer therapy, remain unclear at present.
Photolyases, which are 50–55kD single-chain flavoproteins, are damage-specific binding proteins active in the response to the formation of UV-induced cyclobutane pyrimidine dimers (CPDs) 227 , 228 and 6–4 photoproducts. 229 From the perspective of LO Essen et al., photolyases are highly effective light-driven DNA repair enzymes, which function specifically in the reversal of genomic lesions induced by UV radiation. 230 The mechanism of photolyase-related reversal is generally similar to that following induction of DNA lesions by UV insult; specifically, an electron is injected at the lesion site, activating cleavage of cyclobutane-pyrimidine dimers or 6–4 photoproducts inside the duplex DNA structure. 230 This reversal method is highly effective and simple, as an electron is shuttled to the lesion site for direct destabilization. 231 The energy to drive this reversal reaction comes from chromophores excited through intake of a photon. 232 , 233 , 234 With the advancement of this research field, other mechanisms, including an exclusive bifurcating-electron-transfer method with a cyclic radical mechanism, have been continuously reported. For dimer repair, six steps have been identified, typically including three electron transferences and two bindings to lesions. 229 Through dynamic analysis, new discoveries such as a unique electron-tunneling pathway and essential residues at repair trigger sites have been revealed. 229 , 235 , 236 Importantly, recent crystal structure determination of photolyases has provided new direct insights into the relationship between photolyase structure and its roles in DNA damage repair, 237 supporting further comparison between DNA photolyases and spore photoproduct lyases. 237 , 238 , 239
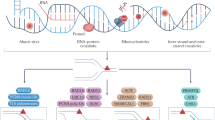
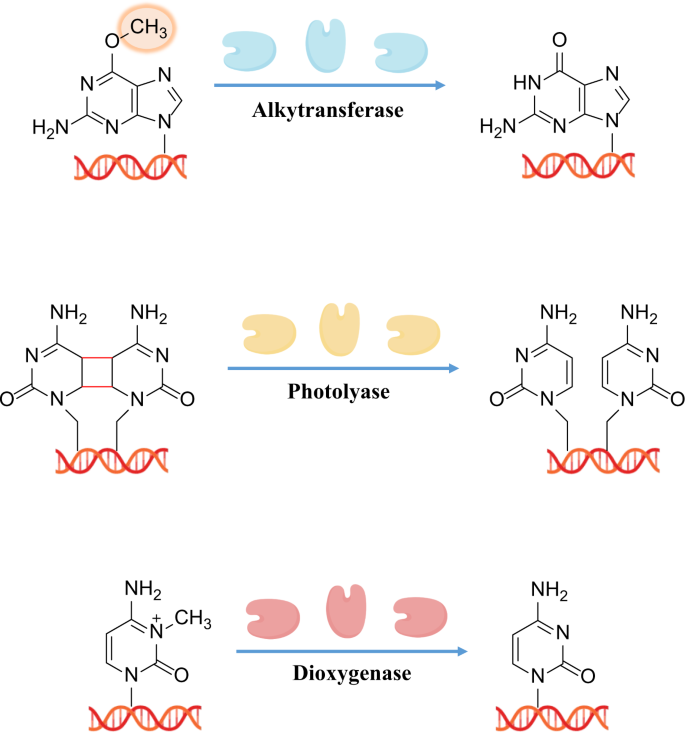
In recent years, the AlkB family of demethylases has attracted increasing attention for its regulatory role in oxidative DNA repair. In 2002, studies by Pal F et al. 240 and Sarah CT et al. 241 revealed a third type of direct reversal mechanism for DNA damage, reporting that AlkB protein in E. coli can repair cytotoxic damage due to 1-methyladenine and 3-methylcytosine in DNA, and that this reaction by AlkB is dependent on oxygen and α-ketoglutarate. Figure 4 lists the three types of direct DNA damage reversal described above, including representative substrates, repair proteins, cofactors, and the corresponding repair products.
Three types of direct DNA damage reversal including representative substrates, repair proteins, cofactors, and the corresponding repair products
Base excision repair
Exposure to ionizing radiation produces radicals. 242 , 243 Radicals may cause base lesions and thus, base excision repair may be initiated to address these lesions. 244 , 245 Key enzymes such as OGG1 can sense damaged bases and are responsible for the recognition and removal of 8-oxoguanine. 246 , 247 The result of base excision repair is that 1 to 10 nucleotides, but no more, can be replaced by short to long patches. 248 The cell’s status and background, lesion style, and levels of exogenous and endogenous materials affect the selection between short patch and long patch base excision repair. 249 , 250 , 251 The importance of base excision repair is not only for ensuring genomic stability but also that its dysregulation would lead to increased risks of cancer, aging-related diseases and other serious disorders. 252 However, base excision repair does not simply serve as an isolated repair pathway, it is one component of the larger DNA damage repair machinery. It forms a network in combination with other pathways and may in turn be regulated by other pathways via a feedback mechanism. 253
Nucleotide excision repair
Compared to base excision repair, nucleotide excision repair is more complex, as it is responsible for addressing complicated lesions including bulky adducts and cross-linking lesions, 254 , 255 and there are two sub-pathways of TCR-NER and global genome repair (GGR)-NER as mentioned above. The source of this type of DNA damage is alkylating or cross-linking agents. Deficiency of nucleotide excision repair is typically associated with several human diseases, including xeroderma pigmentosum and other neurological diseases. 200 The repair process has been elucidated through research over previous decades. In TCR-NER, the hampered RNA polymerases by the damage constitutes the initial step for recognizing DNA lesions, then recruits the CSB/ERCC6, which in turn recruits CSA/ERCC8 complex. In GGR-NER, the protein complex XPC/RAD23B/CETN2 can sense and recognize DNA distortion and recruit helicase TFIIH to form a XPC-RAD23B-TFIIH complex to unwind the DNA helix. Once the pre-incision complex is ready, the endonuclease XPG and XPF/ERCC1 are recruited, which can cut the strand at the 3′ and 5′ flanks of damage site, respectively, to ensure that of a piece of damage-containing nucleotides are removed. Then, the proteins responsible for synthesizing the missing nucleotides are recruited and, finally, DNA ligase fills the gap to complete the repair process. 256 , 257 , 258
Mismatch repair
The primary purpose of mismatch repair is to counteract replication errors and thus improve the fidelity of replication. 259 This repair pathway is mainly used to resolve single nucleotide mismatches and small insertion loops generated by DNA polymerase. 119 , 260 The mismatch repair pathway consists of three steps. First, protein complexes such as the MSH2-MSH6 heteroduplex sense and recognize the mismatch and identify the site of the insertion-deletion loop. 261 , 262 These protein complexes will quickly move to the mismatch lesion site and bind to the DNA molecule to form a sliding clamp. At this point, many proteins gather to perform various functions. For example, exonuclease 1 (EXO1) has been reported to carry out excision of nucleotides in the 5′- > 3′ direction. 263 Another protein, replication protein A (RPA) serves as a binding function of single-strand DNA produced by EXO1 to prevent further DNA degradation. 264 MLH1, a subunit of MutLα, whose defect is responsible for ~50% of MMR defected cancers, may restrained DNA excessive excision by EXO1. 265 Immediately following the recognition step, the removal step occurs. The mismatched bases are removed, and then the replacement DNA is synthesized by DNA polymerase δ while DNA ligase ligates the remaining nick. 259 , 266 , 267 Certain proteins are also necessary for this synthesis step. Proliferating cell nuclear antigen (PCNA) functions not only in the mismatch recognition step but also in the processing of DNA polymerase during the final synthesis step. 268 , 269 As reported by Kira CB et al., PCNA can trigger other proteins to cut the error-containing strand, leading to more rapid and effective excision and synthesis. 270 Due to their critical roles in DNA damage repair and, in particular, mismatch repair, loss of expression of essential proteins such as MSH, EXO and PCNA is closely and significantly linked to increased predisposition to a number of diseases, including various cancers 202 and other metabolic pathologies. 201 , 202 , 271 , 272 , 273 Recent reports indicate a broader spectrum of non-canonical roles of mismatch repair. These roles include the responses to oxidative DNA lesions, helix-distorting nucleotide lesions and environmental chemical toxicants such as benzo(α)pyrene-induced cellular senescence, as well as regulation of the cell cycle. 269 , 274 , 275 , 276 Undoubtedly due to its importance in maintaining genomic stability, mismatch repair deficiency leads to increased DNA mutations. The opportunity for secondary mutations increases by 100 or even 1000 times in mismatch repair-deficient cells. 201 , 277 With the development of high-throughput sequencing technology and compound screening strategies, many novel functions and mechanisms of mismatch repair have been identified, and further research should aim to clarify the genes and proteins of the mismatch repair pathway in depth and elucidate how each gene or protein may differentially function in each step. Moreover, non-canonical roles of the mismatch repair pathway should be further investigated to provide new insights into DNA damage repair and identify potential new fields for targeted cancer therapy or improvement of chemo-and radio-therapy outcomes in the future.
Figure 5 illustrates the main characteristics of base excision repair, nucleotide excision repair and mismatch pathways and the main differences in their lesion sensors, mediator proteins and effector proteins. 278 Generally, in base excision repair, APE1 and DNA glycosylases are the main DNA damage sensors. Meanwhile, the damage sensors in nucleotide excision repair are XPC and CSA; the mediator proteins are XPA, XPF and RPA; and the effect or proteins are XPG, ERCC1, and POLD1. In mismatch repair, the roles of synthesis proteins and protein complexes differ.
Main characteristics of base excision repair, nucleotide excision repair and mismatch pathways and the main differences in their lesion sensors, mediator proteins and effector proteins
Single-strand break repair can be conducted through the base excision repair, nucleotide excision repair and mismatch repair pathways. 279 In this section, we focus on the DSB repair pathway, as this damage type is most deleterious to genomic stability.
Double-strand break repair
Two main types of DSB repair pathways have been reported, namely, homologous recombination (HR) 280 and non-homologous end joining (NHEJ). 281 Compared to the NHEJ pathway, HR is more conservative and error-free due to its dependence on the existence of sister chromatids. 282 , 283 However, this property limits the HR pathway repair to the cell cycle S/G2 phase when sister chromatids exist, whereas the NHEJ pathway can repair DSBs throughout the cell cycle. 284 , 285 , 286
Homologous recombination (HR)
The HR pathway is comprised of three processes: (i) double-strand break recognizing, DSBR; (ii) synthesis-dependent strand annealing, SDSA; and iii) break-induced replication, BIR. After a DSB occurs, a single-strand is cut from the DSB’s end to form two single-strand ends, of which the 3-terminal ends pair with the homologous templates to form a D-loop structure. The single-stranded DNA (ssDNA) that breaks after this step is synthesized using homologous DNA as a template. After D-loop formation, the repair modes differ among the three pathways. The resected 3′ ssDNA end(s) of the DSB sense, bind with, and insert a homologous DNA sequence to prime the synthesis of repaired DNA. Somatic cells use the sister chromatid rather than the homologous chromosome as the repair template. In the DSBR pathway, the D-loop structure becomes stable through capture of another 3′ terminus, forming a double Holliday junction (dHJ) structure. In the SDSA pathway, the 3′ end is extended and released from the template, and then matches with another 3′ end to continue the DNA repair process. In the BIR pathway, the D-loop forms a replication fork to ensure synthesis of the following chain and leading chain. Within the HR pathway, many proteins must combine with each other to function. After the formation of DSBs, under the action of specific nucleases (e.g., the Mre11-Rad50-Nbs1, or MRN, complex), the 5′ ends of DSBs are excised to form a 3-terminal single-stranded DNA (ssDNA). Subsequently, the ssDNA is encapsulated by RPA, which is replaced by Rad51 to form the nuclear fiber structure of Rad51. Mediators such as RAD52 and BRCA2 participate in this process. Subsequently, with the help of PALB2 and rad51ap1, the Rad51 nuclear fiber combines with the homologous double-stranded DNA to form a D-loop structure. After this association, the D-loop dissociates under the action of FANCM to form a product without cross exchange. The dHJ structure formed in the DSBR repair pathway can also be dissolved by a helicase topoisomerase complex (BLM-TopoIII) to form a product without cross exchange.
Rad51 plays an important role as the core molecule in the HR pathway. 287 Rad51 in mammalian cells is similar to Rad51 in yeast cells and RecA in bacteria, with specific functions before, during, and after HR association. 288 First, Rad51 interacts with DNA to form the Rad51 nuclear fiber structure, which effectively elongatesss DNA and is therefore conducive to ssDNA encountering its homologous DNA template. 289 Rad51 can promote the combination of ssDNA with homologous DNA templates and, thereby, promote formation of the D-loop. After association, Rad51 breaks away from the leading strand of DNA during DNA synthesis, exposing its 3-terminal sequence, which is used as the primer for DNA synthesis. 290
RPA is a single-stranded DNA-binding protein comprised of a trimer of RPA1, RPA2 and RPA3. 291 RPA1 has four domains in the trimer, which play roles in DNA synthesis. 292 The N-terminus of RPA1 has a protein-binding domain, DBD-F, along with three domains that bind to ssDNA, DBD-A, DBD-B and DBD-C. The second large component of the trimer, RPA2, has a central structure domain, DBD-D. RPA3 has only one domain, DBD-E. 293
CtIP (CtBP-interacting protein) plays important roles in cell cycle regulation and DNA damage repair. 294 , 295 It contains a dimerization domain (40–165 amino acids) at the N-terminus, which has the same amino acid sequence and binding site as the RB family. CtIP possesses a central domain, which interacts with CtBP. The C-terminal sae2/ctp1-like domain of CtIP is conserved between human and yeast. The phosphorylation of the CtIP S327 site promotes its binding to BRCA1, which then binds to itself, and then is ubiquitinated by BRCA1 and recruited to the damage site. 296 The DNA-binding domain of CtIP is located between amino acids 515–557, which is conducive to the recruitment of CtIP to DSB sites. The two lysine sites in this domain, K513 and K515, are crucial to the interaction between CtIP and DNA. The N-terminus and C-terminus of CtIP contain structural domain segments that interact with MRN. The T847 site of CtIP can be phosphorylated by CDK, which helps CtIP to activate the nuclease activity of MRN and, thus, to promote the single-strand excision of DNA DSB ends. 297
Non-homologous end joining (NHEJ)
In the classical NHEJ pathway, the heterodimer of Ku70 and Ku80 first binds to the broken DNA ends and then recruits DNA-PKcs (DNA-dependent protein kinase catalytic subunit). DNA-PKcs is a member of the phosphatidylinositol 3-kinase (PIKK)kinase family that can pull two broken DNA ends together and recruit processing-related enzymes, such as Artemis, PNKP (polynucleotide kinase/ phosphatase), APE1 (AP endonuclease 1) and Tdp1 (tyrosyl DNA phosphatase 1), and then recruit the XRCC4-XLF-LIG4 complex. 298 Ku70 and Ku80 are subunits of the first protein complex to be recruited to the damage site, both of which have a central domain (Ku core) that binds to DNA. An acid domain, serine 6, is present in the N-terminus of Ku70 that can be phosphorylated by DNA-PKcs. SAP (SAF-A/B, Acinus and PIAS) possesses a C-terminal domain. 299 There is a linking region between SAP and the Ku core of about 536–560 amino acids. Both SAP and this linking region can bind to DNA, so SAP may anchor the Ku dimer to chromatin. The C-terminal region of Ku80 interacts with the Ku core through a highly flexible linking region. 300 At the end is a 12-amino-acid region that can directly interact with DNA-PKcs. The Ku dimer can recruit DNA-PKcs, XRCC4 and XLF to a damage site. When the Ku dimer binds to DNA, Ku70 is directed toward DSBs, while Ku80 is directed away from DSBs. 301
DNA-PKcs, as a member of the PIKK family of serine/threonine protein kinases, 302 contains a leucine-rich domain (LRR) at the N-terminus, which may play an important role in DNA binding, and a series of heat repeat sequences (huntingtin, elongation factor 3, a subunit of protein phosphate 2a and tor1, heat). The C-terminus contains a FAT (FRAP, ATM, TRRAP) domain. The PIKK regulation domain (PRD) may be located between the kinase domain and the FAT domain. 303 Cells lacking DNA-PKcs showed high radio sensitivity. Moreover, mouse experiments showed that mice lacking DNA-PKcs might suffer from severe comprehensive immunodeficiency (SCID). 304 The binding region between DNA-PKcs and Ku is located in the C-terminal region of DNA-PKcs. When the Ku dimer is on DNA, Ku recruits DNA-PKcs to the DNA break terminus, and two DNA-PKcs molecules interact with the DSB site to form a synaptic complex. The DNA-PKcs/Ku/DSB complex can fix the ends of DSBs, thereby protecting the DSB site from nuclease digestion.
After the end of DNA is processed by Artemis and other end-processing molecules, the subsequent repair process must connect the disconnected DNA, and LIG4 executes the DNA connection. XRCC4 has no known enzymatic activity, but can function as a scaffold protein, aiding the recruitment of other NHEJ pathway molecules. In structure, the best binding ligand of XRCC4 is LIG4. 305 The C-terminus of LIG4 contains two BRCT domains, with a connecting region between the two domains. The highly stable complex XRCC4-LIG4 can be formed through interaction with the helical region at the C-terminus of XRCC4. XRCC4 can stabilize LIG4 and promote its activity. The XRCC4-LIG4 complex can interact with Ku, PNK, APLF, XLF and DNA. 306 XRCC4 can be highly phosphorylated, and DNA damage can promote its self-phosphorylation. DNA-dependent protein kinase (DNA-PK)is necessary for the phosphorylation of XRCC4 induced by DNA damage, and promotes the binding of XRCC4-LIG4 to DSBs. 307 The SUMO modification of XRCC4 is essential to its nuclear localization and DSB repair function.
53BP1 (p53-binding protein 1) is a very important molecule in the DSB repair pathway, functioning as an intermediary molecule or effector. 308 It can promote the terminal junction of DNA after DSB occurrence. To be recruited to DNA, 53BP1 must directly recognize the specific histone structure produced by the DSB. Moreover, 53BP1 can promote the NHEJ pathway and inhibit the HR repair pathway. The N-terminus of 53BP1 contains 28 serine/threonine glutamine sites (s/t-q), which are the target sites of ATM. When the N-terminus of 53BP1 is phosphorylated by ATM, the interaction of 53BP1 with Rif1 (Rap1-interacting factor 1) and PTIP(Pax activation domain-interacting protein) is promoted. 309 , 310 The C-terminus of 53BP1 contains a BRCT domain, which interacts with p53 and EXPAND1. The minimal focal region of 53BP1 contains an OD (oligomerization domain), a glycine- and arginine-rich (GAR) motif, and a ubiquitination-dependent recruitment (UDR) domain. It can be dimethylated atlysine 20 within its GAR motif, and the UDR domain can interact with ubiquitinated H2AK15. 311
Alternative end joining
While the c-NHEJ and HR pathways are primarily responsible for repairing DSBs of DNA, alternative end joining (alt-EJ) was considered responsible for residual DSBs that c-NHEJ and HR are unable to repair. 312 , 313 However, it is unsure whether alt-EJ represents a standing pathway or only the end-joining components of the pathway usually serving in dsDNA processing of other functions, such as in replication, recombination or repair. Alt-EJ is also called microhomology-mediated end joining(MMEJ). 314 Alt-EJ refers to repair of DSB damage independently of classical NHEJ factors such as Ku70, DNA-PKcs and lIG4. 315 Although this process appears similar to c-NHEJ, alt-EJ is Ku-independent, depending instead on regions of microhomology on each side of the breakage site. 315 Specific proteins including PARP-1[Poly(ADP-ribose) polymerase] are critical for facilitating the alt-EJ pathway. 316 As reported by Huang YJ et al., PARP-1 is vital to DSB repair in breast cancer cells, and the alt-EJ pathway is triggered by radiomimetic agents. 317 Other studies have shown that PARP-1 and DNA ligases are required for chromosomal translocation followed by alt-EJ activation due to ionizing radiation. 318 In addition, alt-EJ is mainly mediated by the CtIP/MRN complex. 319 Furthermore, Polθ can indicate the microhomologous DNA ends to support the joining of DNA ends. 320 Moreover, the maximum activity of the alt-EJ pathway was observed in the G2 phase of the cell cycle. 321 In mammalian cells, PARP-1 binds completely to the DNA ends with the Ku heterodimer, and resection is then triggered by the MRN complex, followed by DNA ligase III mediating DNA end ligation. 322 , 323 , 324 DNA polymerase theta (pol teta) is an evolutionarily conserved protein encoded by the POLQ gene in mammalian genomes 325 with the ability to mediate joining of single-stranded 3′ ends. Without pol theta, end joining is damaged and residual repair would create large deletions flanking the break site. 326
Figure 6 illustrates DNA DSB repair pathways with the roles of the relevant proteins. As shown in Fig. 6 , DSB repair depends on whether end resection occurs. If the end resection process is blocked, the only repair pathway available is NHEJ, whereas if end resection occurs, three repair pathways, namely HR, NHEJ and alt-EJ, can be initiated to repair lesions in a competitive model. The competitive model includes two layers, as NHEJ competes with the resection-dependent pathways, while HR and alt-EJ compete for lesion repair. The consequences of these three pathways differ. The outcome of NHEJ is accurate deletions of 1–4 nt, while the outcome of HR is loss of heterozygosity, and that of alt-EJ is mutagenesis rearrangement (insertions/deletions). In the review by Anabelle D, this pathway was described as relying on a subset of HR enzymes, and alt-EJ is a highly mutagenic pathway in vivo, driving telomere fusion events and tumor-related chromosomal translocations in various mouse models. 327 These findings raise vital questions about the rules for selecting a repair pathway and the consequences of each option for DSB repair.
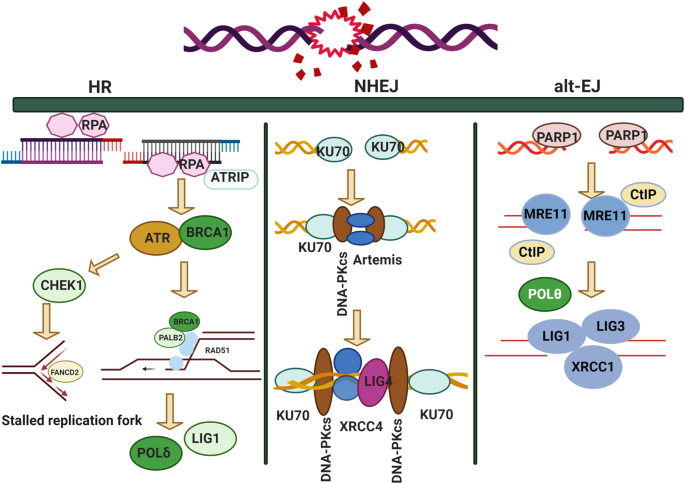
DNA DSB repair pathways with the roles of the relevant proteins
According to the review by Raphael et al., 97 certain factors may influence DSB damage repair pathway selection. First, the role of end resection affects the DDR choice. 328 Nicole et al. indicated that resection is commonly used by cells for selection of DSB repair pathways, 329 especially in the G1 phase of the cell cycle or with complex damage. End resection includes two phases, of which the first is “end clipping”, where in a small number of base repairs occur, making the DNA ends available for alt-EJ. 314 In the second phase, known as “extensive resection”, proteins including CtIP and EXO1 produce long stretches of ssDNA, which are then subject to HR. 330 , 331 Thus, the factors that affect the end resection process also influence DNA DSB repair pathway selection. For example, CDKs phosphorylate the long-range resection nuclease EXO1 in the S to G2 phases of the cell cycle, regulating the DNA end resection and repair pathway choice. 332 However, in non-cycling cells, DSB end resection is significantly reduced, facilitating the c-NHEJ repair pathway. 97 Post-translational modification was also found to regulate end resection. For example, acetylation plays a dual role, inhibiting end resection in budding yeast while promoting end resection via sumoylation. 333 , 334 Sonia et al. demonstrated that protein deneddylation is another major controller of DSB repair pathway choice. RNF111/UBE2M-mediated neddylation can inhibit end resection mediated by CtIP through regulation of ssDNA length. 335 Additional factors, such as maintenance of the balance between BRCA1- and 53BP1-mediated end resection, also modulate pathway selection. In BRCA1-knockout cells, 53BP1 can end resection by blocking CtIP from DNA ends, leading to c-NHEJ pathway selection. 336 Furthermore, hyperactivity of 53BP1 drives genomic instability in BRCA1-/- mice through inhibition of HR pathway selection. 337 In addition to these factors associated with end resection, the repair pathway-related protein complex can affect complex formation or dissociation, and may thus also influence end resection, as the linkage among the protein complexes is likely to be an elaborate interaction network. Alteration of either protein in this network may affect end resection, leading to resection-dependent repair pathway selection. 338 A recent study showed that ATM mediated the interaction of the UBQLN4-MRE11 complex to repress HR further, indicating that ATM not only initiates the HR pathway but also suppresses excessive end resection through various protein interactions. 339 Importantly, competitive relationships among various DSB repair pathways have also been reported. For example, an enzyme in the alt-EJ pathway, Polυ, can inhibit HR pathway activity through binding with RAD51, indicating that Polυis vital for alt-EJ to compete with the HR pathway. 340 In contrast, HR factors such as FA (Fanconi anemia) proteins also promote alt-EJ activity, 341 suggesting that these factors, which interact with ATM and RAD51, can influence repair pathway selection in a background-dependent manner. 97 , 342 In general, these studies confirm that multiple connections exist between alt-EJ and HR. These connections may be competitive and one protein may function in multiple pathways under multiple avenues of regulation, increasing the complexity of DSB repair pathway selection.
Over the past decade, many hypotheses as to how DSB repair pathways are selected have been proposed. From the perspective of George et al., alt-EJ may operate as a backup to the c-NHEJ and homologous recombination repair pathways in the G2 phase. However, as the fidelity of alt-EJ is lower than that of c-NHEJ, chromosome translocations become more likely. 343 Previous reports have noted that the cell cycle and chromatin context also affect the selection of the double-strand repair pathway, and some studies have shown that euchromatin and heterochromatin both affect this choice. 344 Shuren et al. hypothesized that the DNA end structure is another major determinant of the DSB repair pathway. 345 However, these hypotheses were published considering single factors rather than from a global perspective of the DSB repair process. In this review, we raise a novel hypothesis based on a global view of DSB causes, repair selection and consequences. We designated this hypothesis “environmental gear selection”. As shown in Fig. 7 , environmental hazards such as radiation, ROS, alkylating agents, cross-linking agents, topoisomerase inhibitors, and UV light can affect DSB repair pathway selection. For example, alkylating agents may lead to the NER and BER [Please define these abbreviations] repair pathways, whereas radiation damage may be repaired through the BER, HR, c-NHEJ or alt-EJ pathways. UV light leads to NER selection over c-NHEJ and alt-EJ. After insults from different DNA damage sources, different sensors, proteins and protein complexes are activated to initiate various repair pathways. Some proteins function in specific pathways, such as ATM and DNA-PKcs in the HR and c-NHEJ pathways, respectively. The activities of such specific proteins contribute to repair ability and subsequent repair outcomes. This process is similar to the principle of gears’ function in a hydrodynamic force model as illustrated in Fig. 7 . Comparing a watch to genomic stability, the rollinggears in the watch represent the working proteins or protein complexes in a repair pathway to genetic damage. When the watch is disturbed by radiation, gear A may be chosen to trigger the BER repair pathway, whereas when the watch is lesioned by antimetabolites, causing more serious damage, the HR, c-NHEJ and alt-EJ pathway choice may be activated by gear B. At this stage, these three pathways compete depending on the cell cycle phase and end resection status. When exposed to UV light, gear C is activated to select the NER pathway. This “environmental gear selection” hypothesis may provide new insights into the environment-dependent selection among DNA damage repair pathways. In certain significance, this selection hypothesis is generated from the natural selection and can be considered as a result of evolution. Notably, this hypothesis will help clarify the roles of DNA damage, response, and repair, along with providing targets for cancer therapy.
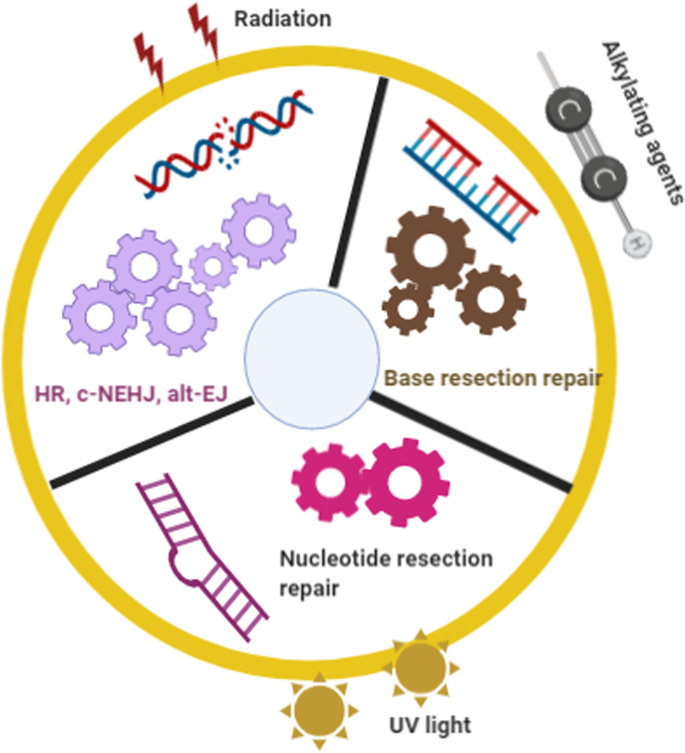
Hypothesis of “environmental gear selection” to describe DNA damage repair pathway evolution. Environmental hazards such as radiation, ROS, alkylating agents, cross-linking agents, topoisomerase inhibitors, and UV light can affect DSB repair pathway selection
Targets for cancer therapy
Cancer, one of the main causes of death around the world, is a threat to human health that requires urgent attention. Currently, chemotherapy, radiotherapy and immunotherapy are the main treatment measures for cancer. Some of these therapy strategies have been found to inhibit cancer through disruption of the DDR process, interfering with DNA replication and inducing DNA lesions in cancer cells, and signaling cell death. Thus, it has been suggested that improved cancer treatment outcomes may be obtained through targeting the DDR and DNA replication along with promotion of mitotic catastrophe in cancer cells. 346 To achieve stronger oncogene triggering ability, cancer cells generally exhibit genomic instability, cancer suppressor gene inactivation and tolerance of attenuated DNA damage repair. 347 DDR enables cells to respond to a variety of exogenous and endogenous insults that threaten the cell’s genomic stability. 348 A number of essential components of this response are enzymes and proteins, which are encoded by various genes. 349 , 350 The specific activation or inactivation of these factors in various cancers and the development of corresponding inhibitors or activators represent a recent hot spot of cancer therapy research. 351 However, some studies have found that inducing DNA damage in cancer cells during cancer therapy may have unfavorable side effects. 347 For example, the cancer suppressor proteins related to DDR, DNA-PK and CHK1, exhibit oncogenic functions in the later period of cancer development. 352 Moreover, much serious obstacle to cancer prevention and control is cancer cell resistance to therapy. 353 , 354 , 355 Numerous studies have discussed this problem of resistance, some in the cancer microenvironment and others using cancer stem cells 356 or cancer heterogeneity. 357 In this review, we focus on the targeting of DDR and repair-related proteins, kinases and pathways to explore possible cancer therapies.
Targeting the DNA damage response for cancer immunotherapy
Pivotal milestones in cancer immunotherapy.
Immune evasion, a hallmark of cancer cells, causes difficulty and frequent failure of cancer therapies aimed at activating the immune system against malignancy. A recent review described the immune escape mechanisms in lung cancer. The authors noted that specific mechanisms, including impaired antigen presentation and activation of immune checkpoints, lead to immunotherapyresistance. 358 Despite this difficulty, the application of immunotherapy has changed the treatment of clinical cancer patients in recent decades. 359 In particular, the advancement of immune checkpoint blockade therapy provides hope for improving therapy outcomes and life quality in future cancer sufferers. 360 , 361
From a historical perspective, the earliest application of immunotherapy for cancer treatment occurred more than a century ago. The first therapeutics applied, Coley’s toxins, were used by Dr. William Coley et al. to stimulate the immune systems of cancer patients using a complex of heat-killed bacteria. 362 Based on this treatment, Dr. William Coley came be called the “Father of Immunotherapy”. 363 Dr. William Coley reported that he was able to use these toxins successfully to induce cancer regression. 364 At the time, his reports were highly controversial and received strong criticism from other researchers, who criticized the efficacy and safety of toxins used for cancer treatment. 365 In 1909, Paul Ehrlich proposed the hypothesis that cancer cells can protect themselves through immune recognition. 366 Almost 50 years later, Mithison et al. observed passive transfer of anticancer immunity in transplanted mice. A milestone in cancer immunotherapy development was the discovery of CTLA-4 in 1985. 367 Walunas et al. found that CTLA-4 is associated with glycoproteins found in T cells. Its expression increases with the activation of T cells. 368 In 1992, the United States FDA (Food and Drug Administration) approved high-dose IL-2 therapy for clinical use, which enables expansion of cultured lymphocytes. 369 Soon thereafter, PD-1 (programmed death ligand 1) was discovered, also in 1992. 370 Then, in 1994, Allison et al. reported cancer rejection following anti-CTLA-4 blockade. 371 , 372 Since then, numerous advances have been achieved. In 2002, a very important hypothesis was proposed by Schreiber et al., known as the immunoediting hypothesis, which states that cancer cells play dual roles of promoting host protection against cancer and also escaping immune destruction. 373 Then, CAR-T cells were found to elicit clinical responses in patients with B-cell lymphoma. 374 The first vaccine to treat cancer was developed from dendritic cells by Dendreon Pharmaceuticals, called sipuleucel-T, and was approved by the FDA in 2010. 375 In 2011, the FDA approved the CTLA-4 blockade agent, ipilimumab, a human IgG1l anto-CTLA-4 monoclonal antibody, 376 which is the first FDA-approved immune checkpoint inhibitor. 377 Following this approval, the PD-1/PD-L1 blockade agent nivolumab was approved by the FDA in 2014. 378 Immunotherapy with nivolumab and ipilimumab is now the first-line therapy for patients with unresectable malignant pleural mesothelioma. 378 , 379 In 2015, the first, and to date only, oncolytic virus, talimogene laherparepvec, was approved by the FDA for cancer immunotherapy. 380 , 381 This virus has the ability of inducing oncolysis directly and activating adaptive anti-cancer immunity. 382 , 383 Interestingly, in 2018, a study reported that the gut microbiome influences the efficacy of checkpoint blockade. 384 In a study by Gopalakrishnan V et al., the gut microbiome modulated the tumor response to checkpoint blockade immunotherapy in mouse models. 385 Meanwhile, a human clinical test showed that primary resistance to immune checkpoint inhibitor therapy could be attributed to dysregulation of gut microbiota composition and abundance. 386 These are the major milestones in the history of cancer immunotherapy. Now, as we begin a new century, the main priority is exploitation of the mechanisms underlying immunotherapy, while further research should also be conducted to identify new checkpoint inhibitors.
DNA damage response deficiency is associated with activation of anticancer immunity
Compelling evidence has shown that DNA damage signals and endogenous DDR can activate the innate immune response. 387 Some reports have noted an association between DDR deficiency and the activation of anticancer immunity. The cGAS-STING pathway is a canonical defense mechanism against viral infections, in which the cGAS (cyclic GMP-AMP synthase) can detect and sense the exogenous viral DNA entered into the cytosol, stimulates the adapter protein STING (Stimulator of interferon genes) to trigger interferon (IFN) signaling. Two research groups SM Harding, et al. and KJ Mackenzie, et al. simultaneously reported that cGAS can also sense the cytosolic micronuclei DNA originated from the nuclear chromosomal fragments following DNA double-strand breaks induced by ionizing radiation. Upon binding the cytosolic DNA, cGAS produces the second messenger cGAMP, which in turn activates STING- inflammatory signaling, and leading to regression of abscopal tumors. 388 , 389 Moreover, a non-canonical cGAS-independent STING activation pathway has also been revealed to be triggered by the DNA damage signaling in nucleus. 390 It was found that upon genomic DNA damage, the STING was activated by the DNA repair proteins ATM and PARP1,together with the DNA sensor IFI16 (interferon-inducible protein16). 391 On the basis of summarizing the literatures regarding the activation of cGAS-STING pathway by radiation-induced DNA damage signaling, Storozynsky and Hitt concluded that the cGAS-STING signaling pathway is a bridge between the DNA damaging abilities of exogenous toxicants and the activation of CD8+ cytotoxic T cells, showing that this pathway can induce anticancer immune responses. 392 Moreover, the DNA damage-induced alt-EJ pathway can also induce an innate immune response. 393 As reported by Dunphyet al., ATM and PARP-1, which are DDR factors, can combine with the DNA-binding protein IFI16(TNF receptor associated factor 6) to activate STING through the alt-EJ pathway, leading to the assembly of an alternative STING signaling complex that contributes to the recruitment of the tumor suppressorp53 and the E3 ubiquitin ligase TRAF6 to STING. 391 TRAF6catalyzes the K63-linkedubiquitination of STING, which promotes the activation of nuclear factor kappa B (NF-κB).These data indicate that DNA damage-mediated signaling pathway activation can promote anticancer immune responses.
Because of their intrinsic characteristic of genomic instability, cancer cells may trigger innate immunity through activation of a series of inflammatory pathways aimed at facilitating the targets of cytotoxic lymphocytes, 394 such as the report showing that IFN-gamma is critical for cytotoxic T cell-dependent genome immunoediting of cancer cells. 395 Insufficient genome integrity due to DNA repair and replication defects may also activate the innate immune response. Recently, concern has been raised about how cellular DNA is driven out of the cytoplasm, which causes a cascade effect. Some studies have indicated that EXO1, BLM and SAMHD1 can promote double-strand DNA damage and stalling of the replication fork, thereby releasing genomic fragments into cytoplasm. 396 , 397 These fragments produced in response to exogenous insult such as radiation exposure can also activate the cGAS-STING signaling pathway, indicating that cGAS is linked to a subset of the induced micronuclei and downstream cascade events of IRF3 phosphorylation and increased IFN-α expression. 388 A famous discovery is that the abrogation of BRCA1 or BRCA2, which are cancer suppressors, causes DSB accumulation and induces IFN signaling and anti-cancer immunity. 398 These findings suggest that an effective therapeutic strategy can be obtained through combined usage of genotoxic agents and immune checkpoint blockade. 389 Furthermore, these studies provide further evidence that cancer-causing genomic instability is typically associated with the induction of innate immunity.
Cancer cells have the capacity for immune escape
Multiple studies have described the ability of cancer cells to escape from immune system detection and attack, which depends in part on expression of cell surface proteins that perform immunosuppressive functions. 358 , 399 Vital proteins such as PD-L1 400 and cytotoxic T-lymphocyte-associated antigen 4(CTLA-4) are expressed on the surface of T cells. Their receptors may interact with B7 to ensure the inactivation of T cells. 401 As a result, immune checkpoint blockade would promote adaptive immunity and inhibit cancer proliferation and growth. 402 From the perspective of DDR, a hallmark of cancer, the immune system response can be initiated through induction of cell surface ligands to trigger disruption of cancer surveillance by a series of immune receptors, such as NKD2D. 403 This finding indicates that DDR can act as a bridge between alteration of cancer cell surface immune-related receptor expression and the cancer cell immune response. 404 Indeed, prior to elucidating how cancer cells develop the capacity for immune escape, it is essential to determine how immune sensors detect nuclear DNA damage and how nuclear DDR sensors sense immune signaling molecules in the cytoplasm. 405 Nakad et al. noted that a series of sensors, including protein complex of replication protein A (RPA) and MRN, can detect DNA damage and activate the TKR9 signaling pathway, inducing further NF-κB expression in the nucleus, while in the cytosol, the Ku70 complex can sense DNA to activate the IFN-regulatory factor (IRF) response and the innate immune adapter CARD9 can trigger NF-κB through activation of the STING signaling pathway. 405 DDR is not only able to induce inflammatory signaling factors as described above but also induces ligands to bind to the immune receptors NKG2D and DNAX Accessory Molecule-1 (DNAM-1). 406 However, the upregulation of these ligands requires activation of DNA damage sensors such as ATM and ATR protein kinases, as well as checkpoint pathway-related proteins such as Chk1. 407 , 408 In cancer cells, these mechanisms have been demonstrated to have significant associations with immune escape. In a study by Masahisa J et al., a DNA damage signal mediated by ATM facilitates cancer immune escape and increases cancer cell resistance to phagocytosis 409 in an integrin αvβ3-dependent manner. A recent study found that DNA-PKcs, a well-known protein in the non-HEJR pathway that plays a pivotal role in genome stability, also has functions in immune escape. 410 In addition, p53, a vital cancer suppressor, activates the innate immune response against cancer cells and can directly control immunosurveillance to achieve successful cancer therapy. 411 Thus, DDR-related sensors and protein complexes as well as the immune escape mechanism are very important to cancer immune therapy. In Fig. 8 ,we illustrate how immune checkpoint blockade promotes the T cell response. As shown in Fig. 8 , after T cells are activated, the expression of immune checkpoints including PD-1 and CTLA-4 increases, and then T cells are able to enter the cancer tissues, causing the cancer tissues to release large amounts of cytokines such as IFN-γ and ultimately resulting in increased expression of immune checkpoints. Anti-immune checkpoints can block immune checkpoint expression and in turn promote the T cell response.
Immune checkpoint blockade promotes the T-cell response
Clinical application of immune checkpoint inhibitors based on the DNA damage response
The PD-1/PD-L1 axis is well recognized as a promising therapeutic target. 412 The agents that regulate PD-1/PD-L1 axis expression can thus be considered possible clinical cancer immune therapies. To date, several immunomodulatory agents targeting the PD-1/PD-L1 axis have been approved by the US FDA. These agents include avelumab, durvalumab, pembrolizumab and ipilimumab. Table 1 lists the clinical trial outcomes of typical agents targeting the PD-1/PD-L1 axis and CLA-4. The anticancer activity, safety and tolerance of these targeting agents have been supported in a series of clinical trials (Table 1 ), but many challenges and limitations to their application remain. (i) Although the selection of predictive biomarkers from genomic targets for clinical cancer therapy is essential for successful treatment, for immunotherapy, the checkpoint blockade of immune cell responses to the treatment agents is more important. 413 Unlike the genes that are mutated in cancer cells, such as p53, the immune system is significantly affected by stressors including complex environmental toxicants and endogenous materials. Based on this consideration, future research may shift its focus to immune response effectiveness rather than the identification of additional biomarkers for immune checkpoint-related mechanistic studies. The effectiveness of an evolving immune response indicates the major clinical benefits provided by immune therapy to cancer patients. These benefits should be further evaluated in the clinical setting. (ii) Among cancer cells, immune cells do not exist alone; instead, many cell types including fibroblasts and endothelial cells make the microenvironment around cancer cells highly complex, providing opportunities to escape immune detection and monitoring. Thus, the targeting of immunotherapy agents should focus on uncovering the underlying mechanism of immune escape and the relationship between the cancer cell microenvironment and immune cell responses in terms of therapy efficacy. (iii) The mechanism through which immune T cells provide the therapeutic response remains unclear. Because this response process is generally complex, involving numerous signaling pathways, its regulation may be either stimulatory or inhibitory. This complexity causes great difficulty in the transfer and application of clinical agents. Thus, an in-depth understanding of the regulation pathway of immune T cells in response to environmental insults and therapeutic agents is urgently needed. (iv) It has been suggested that the combination of multiple therapeutic agents may be more effective than a single application. However, which agents are best used in combination and their safety and efficacy in clinical settings remain unknown. More importantly, the molecular mechanisms underlying the effects of combination therapy on the immune T cell response require further investigation. Nevertheless, immune checkpoint therapies and combinations of multiple agents have provided robust evidence of their benefits to clinical patients and have the potential to elicit durable control, and even curing, of cancer. 413 , 414 , 415 (v) Based on the current understanding of immune therapy causing checkpoint blockade, factors that can activate T cells and increase immune checkpoints, such as PD-1/PD-L1 and CLTA-4,are promising agents for future application. For example, new evidence has shown that human microbiota play roles in immunotherapy. According to Vanessa et al., Bifidobacterium spp. probiotics can improve the effects of PD-1 and CTLA-4. 416 The authors suggested that in the era of immunotherapy, immunotherapies that are more selective and effective while being less toxic should be investigated. Thus, action should be taken immediately to reveal the mechanisms of the T cell activation and response processes under various environmental stresses and endogenous insults, which would identify potential effective agents for future clinical use.
Targeting DNA damage repair for cancer chemotherapy
Chemotherapy is currently the most common clinical option for cancer treatment. 417 As discussed above, BER, NHEJ, alt-NHEJ and HR are the four major repair pathways of DNA damage repair. The key proteins in BER are PARP-1 (poly(ADP-ribose) polymerase), APE1(abasic endonuclease), XRCC1(X-ray cross-complementing protein 1) and DNA ligase III; those in the NHEJ pathway are KU70/Ku80, DNA-PK, Artemis, XRCC4, DNA ligase IV and XLF; the alt-NHEJ pathway includes PARP-1, XRCC1 and DNA ligase III; and HR involves RPA, BRCA1, PALB2, BRCA2 and RAD51. 418 , 419 According to numerous studies, DNA repair pathway defects facilitate genomic instability, leading to increased cancer cell proliferation and survival time. 420 Nonetheless, cancer cells depend on the residual DNA repair capacity to protect them against damage. In cancer chemotherapy, therapeutic agents applied in the clinical setting rely on mechanisms of inducing DNA lesions, resulting in cell death. Therefore, in this section, we focus on targeting these key proteins as potential cancer chemotherapy agents.
Targeting PARP-1
Numerous studies have shown that PARP-1 performs critical functions in many biological processes, including regulation of transcription, apoptosis, and DDR. 421 In particular, once a single-strand break or DSB occurs, the expression of PARP-1 is activated, enhancing poly(ADP-ribose) (PAR) activity, which adds branched PAR chains to enhance recruitment of repair proteins to the damage location. 422 , 423 This function was first identified in 2007 by Fisher et al. 424 in an investigation of the DNA single-strand breakage mechanism. Since then, further evidence has shown that PARP-1 also has a repair function in the alt-EJ pathway. 425 Inhibitors targeting PARP-1 have been used as anticancer therapies, with the characteristic mechanism of catalyzing the PARP active site through competitively binding with NAD + . 426 PARP-1 inhibitors have demonstrated effectiveness for the treatment of cancers characterized by BER and alt-EJ defects. The first report of specific PARP activity inhibition was in 1971, based on the treatment of HeLa cells with thymidine and nicotinamide. 427 Subsequent studies reported the identification of numerous compounds that inhibit PARP activity. In general, the efficacy of PARP inhibitors for treatment of cancers with BER and alt-EJ repair defects is due to such lethal synergistic interactions. 428 , 429 In 2005, Helen EB et al. first indicated that knockout cells of BRCA2 showed defects in HE [Please define this abbreviation] pathway repair, which made the cells sensitive to PARP inhibitors and showed that PARP-1 activity is vital in HE-deficient BRCA2 mutant cells. 430 Thus, in BRCA2 mutant cells, the replication fork lesion cannot be repaired and the cancer cells die. Over the past decade, the US FDA and European Medicines Agency (EMA) have approved several PARP inhibitors. These inhibitors include olaparib, which was approved in 2014 for advanced ovarian cancer therapy 431 and for primary peritoneal cancer therapy in 2017, 432 in 2018 for HER-2 negative breast cancer treatment, 433 and in 2020 for metastatic castration-resistant prostate cancer; 434 rucaparib, which was approved in 2016 for ovarian cancer therapy, 435 in 2018 for treatment of recurring ovarian and primary peritoneal cancers, and in 2020 for treatment of metastatic castration-resistant prostate cancer; niraparib, which was approved in 2017 and 2020 for primary peritoneal cancer and advanced ovarian cancer, respectively; 436 , 437 and talazoparib, which was approved in 2018 for treatment of advanced or metastatic HER2-negative breast cancer. 438 In addition, veliparib (ABT-888) is currently in the clinical trial process, 439 while another new inhibitor, fluzoparib, which was initially identified in 2017, is also in clinical trials for treatment of ovarian, breast and lung cancers. 440
Targeting APE1
APE1, is a vital BER pathway protein that is known to be a necessary intermediate in the processing of potentially cytotoxic abasic DNA damage sites. 441 Previous research has indicated that the biological functions of APE1 are complex. APE1 expression is ubiquitous in various normal and cancer tissues, but shows differences among tissues. Moreover, APE1 in the nucleus has been suggested to carry out a DNA repair function, whereas its presence in the cytoplasm is assumed to support regulation of mitochondrial DNA repair or regulation of transcription factors. 442 CJ Herring et al. showed that APE1 expression was associated with intrinsic radiotherapy sensitivity in cervical cancer tissues. 443 In lung cancer tissues, APE1 shows high expression levels, particularly in cisplatin-resistant cancers. 444 Other studies have shown that the expression of APE1 is significantly associated with DNA repair capacity in cancer tissues such as seminomas and malignant teratomas. 445 , 446 , 447 These data not only indicate that increased APE1 levels are associated with DNA damage but also that they show enhanced resistance to chemotherapy and radiotherapy. To support its use as an anticancer drug target, efforts have been made to develop drugs that inhibit APE1 activity or show strongly enhanced sensitivity to DNA base lesions. Over the past decade, a number of APE1 inhibitors have been developed through cell, animal and clinical trials. These inhibitors can be classified into the following types: APE nuclease activity inhibitors, including methoxyamine, CRT0044876, lucanthone and hycanthone; APE1 redox activity inhibitors, including resveratrol and E3330; and inhibitors of the APE1/nucleophosmin interaction, including gossypol. 448 Michael SG et al. indicated that cancer patients treated with methoxyamine tolerated a maximum oral dose of 60mg/m 2 /d. 449 CRT0044876, which plays a role in inhibition of apurinic/apyrimidinic (AP) endonuclease, as well as 3′-phosphodiesterase and 3′-phosphatase activities, can potentiate the cytotoxicity of DNA base-targeting compounds 450 and is considered a potent and selective APE1 inhibitor. 451 Lucanthone acts as an ionizing radiation enhancer as well as an APE1 endonuclease inhibitor, 452 andis considered a novel inhibitor of autophagy that can induce cathepsin D-mediated apoptosis. 453 Hycanthone is a derivative of lucanthone, which also shows promise as a cancer therapy. 454 Lucanthone is a well-known topoisomerase II inhibitor. 455 HeLa cells treated with lucanthone can exhibit AP site accumulation. 456 Resveratrol is a natural phenol that enhances the cytotoxicity of chemotherapy in human melanoma cells. 457 E3330 caused significant inhibition of cancer cell proliferation through upregulation of endogenous ROS levels. 458 , 459
Targeting XRCC1
Almost 30 years ago, the gene encoding XRCC1 was cloned out and its known biological functions expanded greatly. 460 Typically, XRCC1 is known as a molecular scaffold protein that can provide a platform for recruiting multiple enzymatic components, such as DNA kinase and DNA phosphatase, to improve the repair of DNA single-strand breaks. 461 , 462 Among the functions of XRCC1, its interaction with its protein partner, PARP1, is particularly interesting. Such interactions are important for XRCC1 functions, especially for cancer cell resistance to chemotherapy. 460 , 463 Accumulating evidence shows that XRCC1 mutations are strongly associated with various diseases, including neurological diseases 464 and cancers. 465 , 466 For example, the XRCC rs25487 AA genotype is linked to increased risk of severe radiation-induced lymphopenia during cancer treatment. 467 Another study showed that XRCC-1 arg194trp polymorphism led to increased acute radiation-induced toxicity in patients with advanced laryngeal squamous cell cancer undergoing cisplatin-related chemotherapy. 468 In a prospective cohort study, patients with head and neck squamous cell cancers with polymorphism variants had significantly increased acute radiation-related morbidities. 469 A phase II study evaluated the effects of XRCC1 polymorphisms on the chemotherapy agents satraplatin and prednisone, monitoring therapeutic outcomes in patients with metastatic castration-resistant prostate cancer, and showed that, compared to XRCC1 polymorphisms, patients with the wild-type allele had longer progression-free survival. 470 Currently, most XRCC1-related cancer therapy studies are focused on the effects of XRCC1 polymorphisms on chemotherapy and radiotherapy outcomes 471 or the use of XRCC1 as a biomarker for cancer clinical outcome prediction. 472 , 473 In addition, studies on the regulation of XRCC1 protein expression and its effect on chemotherapy outcomes are underway. Decreased XRCC1 expression due to gefitinib, a selective epidermal growth factor receptor-tyrosine kinase inhibitor and Hsp90 inhibitor led to synergistic cytotoxic effects on non-small cell lung cancer cells. 474 We screened the clinical trials related to XRCC1 using the keyword “XRCC1” ( https://clinicaltrials.gov/ ). A total of 15 trials were listed, of which most are associated with the effects of XRCC1 polymorphisms on chemotherapy outcomes.
Targeting DNA-PKcs
DNA-PKcs, a vital component of the NHEJ machinery, belongs to the phosphatidylinositol-3 kinase-like kinase family (PIKK). One of the important roles of DNA-PKcs is inducing ATM (ataxia-telangiectasia mutated) and ATR (Rad3-related protein) in response to DNA lesions. During NHEJ initiation, after the DNA DSB is recognized by Ku70/80, DNA-PKcs binds to Ku70/Ku80 to form a complex, leading to a conformational change in the structure of DNA-PKcs that allows the N-terminus region of DNA-PKcs to modulate the activation of enzymatic activity. 475 However, the mechanisms underlying the interaction of DNA-PKcs with Ku70/Ku80 remain unclear. Furthermore, the reason why multiple sites of DNA-PKcs is frequently phosphorylated by a number of kinases including PIKK members ATM and ATR, but few phosphorylation events other than S2056 and T2609-T2647 cluster were clearly confirmed in the NHEJ process, has not been fully elucidated. 476 , 477 In the final stage of the NHEJ pathway, break gaps are filled by a series of DNA synthases and DNA ligase IV. DNA ligase can be stabilized and triggered by XRCC1, forming XRCC4-DNA ligase IV complexes that further ligate non-cohesive ends of DNA in the final step of NHEJ. In addition to the DNA repair-related function of DNA-PKcs, it has other functions including roles in mitosis regulation, protection of telomeres, and the inflammatory and immune responses. 478 , 479 These data indicate that the biological functions of DNA-PKcs are critical. Our lab team has extensively investigated the functions of DNA-PKcs over the past three decades. We have identified expression level changes in hepato- and cholangio-carcinomas, 480 and observed that downregulated expression of DNA-PKcs alters the transcriptional levels of IL8, IL10RA and DAPK3. 481 We also found that DNA-PKcs activity is necessary for c-myc protein expression, as DNA-PKcs deficiency suppressed c-myc expression. 482 Recently, we reported that knockdown of HUWE1-dependent DNA-PKcs neddylation decreased the repair efficacy of the NHEJ pathway. 483
In clinical cancer therapy, the use of inhibitors of retained DNA repair machinery components is a promising method for eradicating cancer cells. Inhibitors targeting DNA-PKcs include NU7441, nedisertib, AZD7648, VX-984, berzosertib, ceralasertib, VX-803, and BAY1895344. 40 Among these inhibitors, some have reached the clinical setting. Nedisertib has been approved for phase II trials for various indications. 484 NU7441, a very strong inhibitor of cancer cell proliferation and growth, shows synergy with enzalutamide against advanced prostate cancer in the clinical setting. 485 Nedisertib, also known as M3814, can modulate ATP-binding cassette transporter family-mediated multiple resistance in lung cancer chemotherapy. 486 AZD7648, which is now in phase I trial, has been described as a potent and selective DNA-PKcs inhibitor and is associated with good chemotherapy outcomes and enhanced olaparib activity. 487 VX-984, also in phase I trial, showed potential for selective inhibition of NHEJ in transformed cells, 488 and enhanced the sensitivity of glioblastoma cells to radiation therapy. 489 Berzosertib, which is an ATR inhibitor currently in phase I trial, is considered a safe and effective inhibitor of advanced solid cancers. 490 In some studies, berzosertib has synergized with gemcitabine to enhance therapeutic outcomes in some serous ovarian cancers including platinum-resistant high-grade serous ovarian cancer. Ceralasertib, also known as AZD6738, showed synergistic activity in combination with olaparib in ATM-deficient cancer cells, and was found to be tolerable and effective for prolonging progression-free survival of patients with platinum-sensitive relapsed epithelial ovarian cancer. 491 VX-803 and BAY1895344 are also selective ATR inhibitors currently in phase I trials. 492 Although numerous studies have indicated that inhibition of DNA-PKcs is deleterious to cancer cells, with very promising results, further pre-clinical and clinical trials should be conducted to obtain more substantial data. Meanwhile, the upstream and downstream targets of DNA-PKcs should be explored to support development of additional targeted therapeutic agents for cancer control.
Targeting ligases
In 2008, three types of ligases were cloned and their structures, comprised of a catalytic region, nucleotidyltransferase domain and oligonucleotide domain, were identified. 493 Subsequent functional investigation showed that DNA ligases I and IV have regulatory functions in the nucleus, whereas DNA ligase III is active in both the nucleus and mitochondrion. Ligase I can interact with proliferating cell nuclear antigen to function in the NER, BER and alt-EJ pathways. Ligase III can interact with other proteins including UNG2, APE1, APTX, PCNA, PNKP and Pol β, providing a specific role for ligase II in DNA replication and repair. 494 , 495 Notably, DNA ligase III is the sole enzyme active in the mitochondrion. DNA ligase IV can form a complex with XRCC4 during the early stage of DNA DSB repair via NHEJ through binding with Ku70/Ku80 at a DNA end.
In 2019, a new DNA ligase I inhibitor designated S012-1332 was developed that showed in vivo activity against breast cancer. 496 Previously, the small-molecule inhibitors L82, a ligase I selective inhibitor, and L67, a selective inhibitor of ligases I and III, were reported to function in cancer cells. 497 Another ligase I inhibitor, benzocoumarin-stilbene hybrid, can reduce cancer cell proliferation and growth, suggesting that it may be a promising chemotherapy agent for cancer through regulation of DNA damage repair pathways. 498 L189 is an inhibitor of three DNA ligases characterized in 2008, which targets DNA replication and repair. 499 SCR7, targeting NHEJ, blocks ligase IV-mediated joining rather than that by DNA ligases I and III. 500 Recently, Ujjayinee R et al. developed a new ligase IV-specific inhibitor, SCR130, which could inhibit NHEJ and induce cancer regression in mice while having minimal or no effect on joining mediated by ligases I and III. 501 Unexpectedly, the effects of these inhibitors on cancer cell therapy provide evidence to support further development of effective therapeutic strategies.
The rapid progress in understanding the cellular machinery associated with DDR pathways and its roles in various cancers has led to the discovery of new potential targets, including selective and specific DNA repair-related kinase inhibitors. Among these inhibitors, some have been approved for clinical application, while others are currently in the clinical trial stage or being investigated in cell or animal models. In addition, targets on other DNA damage repair kinases, which are not discussed in detail in this section but described in our previously published review, such as ATR, CHK1 and WEE1, are being investigated for clinical application in cancers, making them potential new cancer chemotherapy agents in the future. In addition to the cytotoxicity to cancer cells of these inhibitors, their safety and tolerance should be evaluated carefully for dose-response effects, which may limit their therapeutic application. Notably, combination therapy methods with two or more inhibitors applied together should also be evaluated in terms of potential efficacy, safety and tolerance. Chemotherapy patients often experience off-target toxicity and detrimental side effects due to the impacts of chemotherapy on healthy tissue. Importantly, chemotherapy programs should focus on precision therapy, meaning that therapy with inhibitors associated with DNA damage repair defects should be employed with consideration of the cancer patient’s traits, such as genetic background, environment, lifestyle, diet, culture and race, which are important factors that can affect therapy results. 502 Finally, chemotherapy resistance is a serious threat to the fight against cancer, and the mechanisms underlying resistance, such as that reported for PARP-1 inhibitor resistance, require further elucidation.
Targeting PI-3 kinase (Phosphatidylinositol 3-kinase)
The PI3K family of kinase consists of I, II and III classes. Each class has its specific substrates and potential roles. 503 Previous studies found that activation of PI3K pathway would initiate a cascade of downstream signals to regulate biological roles including cell proliferation or survival. In brief, PI3K class I can catalyze the conversion of phosphatidylinositol 4,5-bisphosphate(PIP2) to PIP3, leading to the activation of AKT. If the PI3K was activated in an aberrant way, either amplifications or mutations would occur in this signaling pathways-related genes, 504 resulting in many serious diseases. Approximately 50% breast cancer patients have PI3K pathway-related genes’ mutations. 505
As of the frequent involvement of the PI3K pathway in various cancers, inhibitors have been developed and are being under surveyed in clinical trials over the past a few decades. These inhibitors include PI3K inhibitors, isoform-specific PI3K inhibitors and dual PI3K/mTOR inhibitors. In terms of the PI3K inhibitors’ anticancer effects, for instance, in breast cancer therapy, most of PI3K inhibitors were reported the modest therapeutic index such as buparlisib. Additional, these inhibitors have been found with mood adverse effects, raising the concerns about the safety and need further investigative efforts in breast cancer therapy. 506 Till now, several PI3K pathway inhibitors are reported in the clinical trials for various cancers. Copanlisib, an intravenous pan-class I PI3K inhibitor, illustrated improved progression-free survival in patients with relapsed or refractory indolent non-Hodgkin lymphoma compared with placebo plus rituximab in a Phase III trial. 507 Alpelisib, the first PI3K inhibitor approved by FDA showed had effect on treatment of PIK3CA-mutated, hormone receptor-positive breast cancer. 508 Buparlisib, a pan-class I PI3K inhibitor had a clinical benefit rate of 12% in a Phase II clinical trial. 509 Some trials evaluated the combination anticancer effects of multiple drugs, such as combination of buparlisib, taselisib and alpelisib or combination of AZD8186(PI3Kβ/δinhibitor) in treatment of breast cancer 510 or serabelisib(PI3Kα inhibitor) and copanlisib(PI3Kα and PI3Kδinhibitor) in treatment of relapsed follicular lymphoma. 511 Furthermore, the safety data from PI3K inhibitor studies should be assessed to reduce potential adverse effects in cancer patients and therefore help maximize cancer therapy outcomes.
Targeting DNA damage repair pathways to improve cancer radiotherapy responses
Radiotherapy, in which certain doses of ionizing radiation are applied to induce cancer cell death or control growth and proliferation through triggering of DNA DSBs, is a major treatment strategy used for patients with various cancers. Currently, approximately half of cancers require radiotherapy for clinical treatment. 512 In addition, radiotherapy is often used to shrink the cancer prior to surgery or used following surgery to kill residual cancer cells. Ionizing radiation penetrates into cancer cells and tissues, with direct and indirect destructive functions. Direct function refers to the induction of single-strand breaks and DSBs of DNA, whereas indirect functions include the activation and formation of ROS or free radicals that subsequently damage DNA or alter cellular DNA damage repair pathways. Radiation often destroys DDR pathways in cancer cells, but cancer cells have evolved rescue pathways that allow cancer cells to survive radiotherapy. Therefore, targeting these rescue pathways is expected to be an effective therapeutic approach for reducing cancer recurrence or improving radiotherapy sensitivity. 513 , 514 However, radio therapy still faces many challenges. One challenge is that damage to normal tissues and cells and adverse effects due to radiotherapy often occur during radiotherapy treatment. Another challenge is radiotherapy resistance. At present, no radio sensitizers for use in combination neo-chemo- and radiotherapy have been approved for clinical application. Typically, most chemotherapeutic drugs are used in combination with radiotherapy to enhance the cancer’s sensitivity to treatment. In a recently reported phase III trial, avelumab and cetuximab in combination with radiation were tolerated by patients with advanced squamous-cell cancer. 515 A clinical trial conducted by Charles AK et al. showed that the combination of cisplatin and radiotherapy improved progression-free survival time by three years. 516 As currently available radiotherapies cause toxicity in normal tissues with adverse effects, it is essential to improve radiotherapy sensitivity through the discovery of new targeted agents, especially these based on the mechanism of DDR pathway regulation. In other words, there is urgent need for improvement of radio-sensitivity to develop and exploit highly effective targeted inhibitors with low toxicity. The review by Amy MB et al. summarized a few key hallmarks of cancer that can be targeted for further improvement of radiotherapy outcomes. These hallmarks of cancer cells include enhanced DDR, inflammation, altered mitochondrial and energy metabolism, apoptosis evasion, repopulation by cancer stem cells, hypoxia, expanded cancer subclones, immune evasion, and alteration of the cell cycle. 512 Regarding these cancer hallmarks, many issues require elucidation, such as (i) why cancer cells possess so many mechanisms against radiation insult; (ii) which hallmarks have the predominant position or most important functions; (iii) whether the activation of these pathways in cancer cells after irradiation occurs simultaneously or sequentially; (iv) the relationships among these hallmarks; and (v) methods for targeting two or more hallmarks at the same time, In this section, we focus on the targeting of DNA repair pathways as a viable therapeutic method to improve the efficacy of cancer radiotherapy.
Targeting non-homologous end joining
Previous studies have been conducted to determine the effects on cancer radiotherapy outcomes of employing NHEJ-related proteins as efficacy targets. DNA-PKcs, a key factor in the NHEJ pathway, was found to improve radiotherapy sensitivity in numerous cell and animal studies investigating the effects of its defect. Using NU7441 to knockdown DNA-PKcs expression enhanced the sensitivity of various cancers to radiotherapy. 517 For example, in post-irradiation colon cancer cells, an inhibitory peptide BTW3, targeting DNA-PKcs T2647 phosphorylation, enhanced sensitivity to radiation treatment. 518 In breast cancer, inhibition of DNA-PK with a histone deacetylase inhibitor, TMU-35435, which can degrade DNA-PKcs with proteasomes, enhances radiotherapy sensitivity. 519 M3814, a pharmacological inhibitor of DNA-PKcs, can potentiate radiotherapy in ovarian cancer animal models and non-small cell lung cancer models. 520 Thus, among the scientific community, the leading perspective is that DNA-PKcs is a promising therapeutic target for human cancer treatment. However, challenges remain for the treatment of some cancers, such as esophageal cancer, for which potential targeted DNA-PKcs inhibitors have not yet been discovered. In addition to DNA-PKcs inhibitors, recent research has indicated that the expression of APLF, a key protein regulating DNA end excision in NHEJ, increased in radiation-resistant glioblastoma cells, suggesting that it may be a useful novel target for glioblastoma radiotherapy. 521 Another study found that thymine DNA glycosylase (TDG) can regulate the NHEJ pathway through demethylation of cytosine-guanine (CpG) islands in the TAZ gene, thereby enhancing esophageal cancer cell proliferation and radiation resistance. 522
Targeting homologous recombination
To improve radiotherapy efficacy, it is essential to understand radiotherapy resistance in HR-defect cancers. Compared to the NHEJ repair pathway, cancer cells with rapid replication capacity tend to employ the HR-mediated DSB repair pathway. Thus, understanding how cancer cells respond to radiation through the HR pathway would benefit the development of novel approaches to exploring radiotherapy resistance. Notably, HR repair pathway deficiency has been observed in some cancers. 523 , 524 HR-deficiency caused cancer cells to become sensitized to chemo-and radio-therapeutic agents, suggesting that HR deficiency is a valuable pathway to improve radiotherapy resistance. 525 Some factors influence the formation of HR deficiency including promoter methylation, BRCA1/2 mutations and somatic HR mutations. 526 Telomeric allelic imbalance (TAI) with breakage sites can result in HR deficiency, and is part of the “genomic scar” induced by HR deficiency. 527 Mutations of BRCA1/2 are the most common source of HR deficiency. When hyperthermia is used to inhibit the HR pathway, BRCA2-deficient cancer cells become more sensitive to radiation treatment. Combining a PARP inhibitor with hyperthermia showed good treatment efficacy for cancers with HR deficiency. 528 Gregory et al. showed that YU238259, an inhibitor of DNA DSB repair, has synergistic effects with ionizing radiation and PARP inhibition, and that this synergism is enhanced with BRCA2 deficiency. Thus, this inhibitor has strong potential as either a monotherapy or an adjuvant for radiotherapy. Treatment with the combination of AZD6738, an inhibitor of ATR, and olaparib significantly improved ionizing radiation-induced resistance, suggesting that combination therapy with two or more agents might be an effective approach for treating cancers intrinsically resistant to radiation. 529
Double targeting of HR and NHEJ
A number of inhibitors have been developed to improve radiotherapy resistance, which target both the HR and NHEJ pathways. The MEK1/2 inhibitor, GSK212, also known as trametinib, has shown promising anticancer efficacy and confers radio sensitization to pancreatic adenocarcinoma cells through double suppression of HR and NHEJ. 530 Additional studies have reported the use of this double targeting approach in other cancers. Cells of squamous cell cancer of the head and neck that were treated with valproic acid, a histone deacetylase inhibitor, enhanced the radiosensitizing effect by increasing radiation-induced DNA DSBs through double targeting of HR and NHEJ. 531 , 532 A clinical trial conducted by Mary et al. showed that the combined use of the MEK1/2 inhibitor, selumetinib, with 5-fluorouracil (5-FU) significantly reduced the clonogenic survival time of colorectal cancer cells and increased their radiation sensitivity. 533 In a phase I study, Christina W et al. reported that the combination of trametinib, 5-FU and chemoradiation treatment was safe and well tolerated in patients with stage II or III rectal cancer. 534 Meanwhile, Chang et al. found that BEZ235, a dual inhibitor of PI3K and mTOR, greatly improved radiation sensitivity through inactivation of HR and NHEJ proteins in radioresistant prostate cancer cells. 535 Using a high-throughput cell-based small molecule screening method, Alexander et al. identified a collection of novel compounds that can selectively modulate both the HR and NHEJ repair pathways. Among these compounds, some have been approved by the FDA, such as the calcium channel blocker mibefradil dihydrochloride, and have predicted activities as HR and NHEJ repair inhibitors and radiosensitizers. Moreover, they found that helenine, an antiviral drug, can reduce NHEJ and HR repair activity to 20% and 6%, respectively, suggesting that it could be of great benefit for future clinical use. 536 With the development of high-throughput technologies, numerous studies have aimed to discover potential inhibitors with activities against both HR and NHEJ, through which many novel compounds have been identified, some of which have reached phase I trials, such as the radiosensitizer mibefradil (Trial# NCT02202993). However, a few major questions remain about combination treatment with two or more therapies or the discovery of targets with dual functions in both the HR and NHEJ pathways. (i) Off-target effects are possible, and their underlying mechanisms are highly complex. Such effects may be associated with mitochondrial alteration or immune responses. 537 As off-target effects are a major obstacle to the clinical application of combinations of inhibitors, it is essential to reveal the molecular mechanisms of off-target effects. (ii) To effectively enhance radiosensitivity, more attention should be paid to investigations of the safety and tolerance of combinations of inhibitors. (iii) The inherent DNA repair ability of cancer cells is a key factor affecting the efficacy of combination treatment with multiple inhibitors. Thus, identification of potential biomarkers associated with various cancer types or cancer stages, as well as with gender or environmental factors, would aid in the development of optimal radio sensitizers and personalized cancer therapies.
Clinical therapeutic drugs for DNA damage and repair
Most of cancer therapeutic drugs have been applied in clinic for a few decades with the enough evidence of efficacy in killing proliferating cancer cells(Table 2 ). Typically, these agents comprise of antimetabolites such as 5-fluorouracil, radiotherapy and radiomimetics such as Bleomycin, Monofunctional alkylators such as Temozolomide, Bifunctional alkylators such as Cisplatin, Topoisomerase inhibitors such as VP16 and Replication inhibitor such as Aphidicolin based on the induced cancer cells’ toxic DNA lesions18256616. In general, the effects of these agents on cancer therapy are affected by a few following factors. (i) DNA replication is one of the targeted period by some of cancer chemotherapy agents. These agents may produce excessive DNA damage resulting in cell death following DNA replication; (ii) cell repair ability can influence the efficacy of cancer therapy agents. For instance, some DNA repair inhibitors have been identified the efficacy in preclinical models (Table 2 ); (iii) tumor survival microenvironment and development are linked with perturbed DNA damage response and DNA damage repair pathways. Due to this perturbation effect, the DNA repair ability is reduced, leading to the cancer cells’ genomic instability. However, one DNA repair pathway destroyed by agents may be compensated by other alternative pathways. These alternative other compensated pathways can be identified to treat the cancer with DNA repair-defective. Currently, in clinic, the advantages and disadvantages by using single DNA repair agents have been reported. The advantages consist of that single agents could exploit cancer-specific defects in checkpoint signaling and DNA repair. This advantage can convert endogenous DNA lesions into fatal replication lesions in cancer cells resulting in death. Another advantage is the side effects of single DNA repair inhibitors would be minimized through cross-talk among normal cells. However, the most limitation of single agents in treatment of cancer is the acquisition of resistance in cancers. This limitation may be caused by the mechanism of cross-talk among DNA repair pathways.
These influence factors make the strategies using DNA repair inhibitors in cancer treatment had some specific properties. First, in clinic, these inhibitors can be combined with DNA damage anticancer drugs to increase the cancer therapy outcomes because combination usage can inhibit DNA-repair-associated removal of toxic DNA lesions. Second, DNA repair inhibitors could be adopted to kill tumor cells in a way of monotherapy or selectively either in DNA damage-defective response or DNArepair. Furthermore, the synthetic lethal interactions within the defective cancer and DNA repair pathway could be served as identification of new therapy strategies. Third, we can further consider that the DNA repair inhibitors would be served to promote cancer-associated replication lesions to kill them in a selective way.
Theoretically, DNA repair inhibitors are used to kill the cancer cells with replication lesions and convert them into fatal replication lesions, thus the cancer cells present to be killed specifically. Thus, in the future, it can be proposed that DNArepair inhibitors should be developed to make replication lesions more toxic, leading to more fatal replication lesions selectively killing oncogene-expressing cancer cells. Actually, our understanding regarding a few replication repair pathways remains limited, in particular, their complicated interplay reaction. Thus, more extensive basic experiments and study are needed to explore more and more new anticancer targets in this field.
Hypothesis: “DNA damage baseline drift”-mediated repair may be magnified in cancer therapy
Based on the interpretation of DNA damage, response and repair, and their associations with cancer therapies described above, we formulated a new hypothesis to provide new insights into the functions of DDR in cancer therapy. This hypothesis states that “DNA damage baseline drift”-mediated repair may be magnified in cancer therapy.
First, we must define “DNA damage baseline drift”. Compared to normal cells, cancer cells undergo a process known as carcinogenesis, in which DNA damage leads to a series of genetic mutations and finally to formation of a mass of cells, finally grows into tumors known as tumorigenesis. 538 This process, in which normal cells become cancer cells, includes two critical periods: the benign accumulation period and malignant period. 539 , 540 Because DNA can be repaired after damage, during the initial period of environmental hazards or endogenous insults, the DNA repair system maintains genomic stability; however, as exposure to insults continues, long-term exposure may cause cancer, a state of abnormal and uncontrollably rapid growth. During these two vital periods, the cells experience the following stages of cancer cell transformation: exposure to a carcinogen, initiation of DNA damage, enhancement of the mutated cell, growth, and replication. This process of DNA damage occurs in normal cells after insult from carcinogens, and has shown properties of dose-response and time-response in numerous studies. 541 , 542 In accordance with this background, we formulated the new concept of a baseline level of DNA damage repair ability in normal cells that become tumor cells. Below this baseline level, normal cells can repair their DNA damage and maintain genomic stability, whereas beyond this baseline level, DNA damage repair becomes unable to reverse the carcinogenetic effects effectively, or DNA damage repair shifts to supporting the proliferation and growth of tumor cells. As shown in Fig. 8 , under the effects of continuous exogenous and endogenous carcinogen insults, one or a few normal cells cannot tolerate the stress, leading to DNA damage baseline drift, which results in the hallmarks of cancer cells, such as genomic instability, genetic mutations, enhanced growth, altered cellular energy metabolism, inflammation, and promotion of invasion and metastasis. There could be an exceptional situation, i.e., the repair pathway is chosen inappropriately, leading to error-repair or “non-fidelity repair”, which may also have the potential of carcinogenesis even at the baseline level.
The second significant aspect of this hypothesis is that “DNA damage baseline drift”-mediated repair could be magnified in cancer therapy. Here, the term magnification refers to the multiple possible roles of DNA damage repair. During the early stage of cancer immuno-, chemo-, and radio-therapy, DNA damage repair-related proteins or complexes could be targets for improving therapy efficacy; however, as time goes on, tumor cells may initiate specific DNA damage repair machinery and thus develop resistance to therapy. For this reason, therapy-resistant cancer cells can carry out many new functions, as discussed in the previous section. These therapy-resistant functions and phenotypes include evasion of immune monitoring, alteration of the cell cycle, activation of inflammation-related cytokines, dysregulation of mitochondrial and energy metabolism and evasion of apoptosis. This magnification effect acts like a lever mechanism (Fig. 8 ), meaning that DNA damage repair can follow specific repair pathways to shift the phenotype of cancer cells toward treatment resistance. Evidence of this magnification effect has been provided in some published studies. Matthew JS et al. demonstrated that PARP-1, a key protein for DNA damage repair, plays dual roles in the regulation of cancer growth and progression. 543 The review by Carol B et al. summarized a number of DNA damage repair-related proteins with dual roles, including BRCA1, ATM, ATR and p53. p53 is a classical example of a gene with dual roles in cancer development. p53 is the most frequently mutated gene in human cancers. 544 , 545 Furthermore, in some cell types, p53 can enhance apoptosis, whereas in other cell types, p53 intensifies cell cycle progression. 546 p53 inactivation leads to intratumoral heterogeneity and, compared to wild-typep53, mutant p53 may exhibit gain-of-function mutations related to apoptosis, anti-oxidant activity or the epithelial-to-mesenchymal transition (EMT) in addition to its DNA damage repair functions. According to Qing S et al., the effects of combination therapy with a DNA-PK inhibitor, M3814, and radiation were more dependent on p53 regulation of cell-cycle blockade and senescence, whereas knockout of p53 expression led cancer cells to initiate hallmarks events such as mitotic catastrophe and apoptotic cell death. 547 In cells of human head and neck squamous cell cancer exhibiting downregulation of the MRN complex, the accumulation of lethal DNA damage in the form of DSBs and telomere length shortening contributed to the death of BRCA-proficient cancer cells. 548 Theoretically, DNA damage baseline drift may suggest the reason why cancer cells gain so many functions, as doing so would avoid further damage from cancer treatment. Cancer therapy-induced resistance may also be subject to magnification effects caused by the continuous accumulation of DNA damage repair sites.
The purpose and significance of raising this hypothesis relates to the following aspects of cancer biology and treatment: i) Addressing DNA damage caused by environmental hazards and endogenous toxicant insults, which induce accumulation of errors in a time- and dose-dependent manner. DNA damage repair capacity depends on numerous factors including cell background, exposure time and dose, as well as genetic and epigenetic factors. These factors indicate that DNA damage in excess of the exposure repair threshold, or in other words, DNA damage accumulation at a level that cannot be repaired, leads to a cascade of changes in cancer- and cancer therapy-related phenotypes. ii) Providing a novel perspective for clarifying further DNA damage repair functions during cancer development and their impacts on cancer therapy outcomes. This novel perspective may pave the way for establishment of new study fields or new technologies to explore DNA damage repair in a systematic manner. iii) Providing new prospective cancer therapeutics for future clinical application, as a small investment in understanding DNA damage repair could yield enormous returns. Better understanding of this process would improve the future prospects of personalized precise cancer therapy.
Current challenges and future perspectives
DNA damage, response and repair have garnered the attention of cancer researchers, physicians and surgeons, while intensive research has produced new fundamental insights into the mechanisms underlying cancer development and cancer therapy-induced resistance. In addition, the development of new potential inhibitors to improve cancer therapy would benefit from research on DNA damage, response and repair. This review highlights the roles of DNA damage, response and repair in cancer development and cancer therapy from perspectives of the historical research timeline and clinical applications. Based on this background, we suggest two hypotheses, namely “environmental gear selection” to describe DNA damage repair pathway evolution, and “DNA damage baseline drift”, which may play a magnified role in mediating repair during cancer treatment (Fig. 9 ). This “DNA damage baseline drift” hypothesis may provide a novel holistic concept at the genetic level in the area of DNA damage repair and cancer therapy and care for serious conditions such as chemotherapy resistance and radiotherapy resistance, etc.
Hypothesis of “DNA damage baseline drift”. Compared to normal cells, cancer cells undergo a process known as carcinogenesis, in which DNA damage leads to a series of genetic mutations and finally to formation of a mass of cells, finally grows into tumors known as tumorigenesis
Genomic stability is important for cellular survival and evolution, and cells respond to environmental hazards and endogenous stresses through complex interactions between DNA damage-related sensors, activators, repair pathways, and protein complexes, with additional effects from cellular context and status. While this complex network underlies the molecular mechanisms of DDR and repair-mediated cancer therapy, it also provides a resource for identifying potential inhibitors in a systematic manner. However, the plethora of DNA damage, response and repair processes, along with their profound and complex interactions, have not yet been fully elucidated. Similarly, the advent of personalized cancer therapy and high-throughput sequencing technologies provide hope for future prediction of many gene alterations associated with specific conditions. Thus, despite decades of extensive research and countless discoveries, much more work is needed to appreciate fully the roles of DNA damage, response, repair and their regulation in cancer and cancer treatment.
Challenge I: Considering that DNA damage, response, and repair are critical to cancer therapy, it is reasonable to recategorize cancers from the perspective of their DNA repair deficiency status. This new categorization could provide a new perspective for the development of personalized cancer therapies. For example, cancer patients with DNA repair defect in the NHEJ pathway could be treated with therapy inhibitors specific to the HR pathway. This process is achievable due to the rapid development of whole-genome cancer sequence detection, and supports exploitation of all relevant alterations and mutations for cancer therapy.
Challenge II: Early biomarkers for identifying DNA damage, response, and repair defects should be identified and used for cancer therapy selection. Although many relevant biomarkers have been reported in recent decades, most require improvement. For example, deficiency of RAD51 foci has been used as a biomarker for the detection of DNA repair ability via immunohistochemistry. However, as the test method is complex and the results may be affected by many factors, this test has not been widely used in clinics. Moreover, in the era of personalized cancer therapy, a greater number of potential functional markers and those that reflect early changes should be identified for the DNA damage, response and repair process, along with the development of more precise experimental methods. In addition, an ethical issue remains to be addressed. In the real world, to test patient responses to cancer therapy, patients should be tested for potential DNA damage, response and repair defects using these early changing biomarkers, but these biomarkers must be activated using activators such as radiation, chemotherapy or immunotherapy, which would be very difficult for ethical reasons. Therefore, prior to the clinical application of biomarkers, ethical issues should be addressed.
Challenge III: The mechanism underlying the activity of DNA damage repair inhibitors in cancer therapy remains unclear, although a number of potential inhibitors have been approved for clinical trials. Because their mechanisms have not been fully revealed, targeted therapies for cancer may have off-target effects. If we can solve this problem, targeted cancer therapy based on exploitation of DNA damage repair can be expected to improve therapeutic outcomes in the future. Recently, the combined usage of two or more inhibitors or therapy methods has increased in popularity. Combined treatment may increase the efficacy of a therapy, but can also enhance toxicity or adverse effects, as its molecular mechanisms are much more complex and difficult to elucidate than those of single treatments. Therefore, the creation of usage criteria and principles for combination treatment to ensure that such therapies are more effective against cancer and less harmful to health is urgently needed.
Challenge IV: Cancer resistance and normal tissue severe side-effects are the major obstacles to cancer therapy, the goal of personalized therapy strategy is to overcome these obstacles. Many resistance mechanisms have been reported to chemo-, radio-, and immuno-therapy. For example, cancer cells with mutated BRCA1 and BRCA2 may be sensitive to chemotherapy using a PARP inhibitor, while the normal tissue cells if without the germling mutation of BRCA1/2 are relatively resistance as compared to the mutated cancer cells. Obviously, in the clinical setting, testing for gene mutation seven the secondary mutations in the recurrence tumors should be popularized and, most importantly, resistance mechanisms should be explored through cell, animal and clinical experiments in the near future.
Challenge V: Distilling the convergent findings obtained from the enormous amount of complicated research conducted on the relationships of DNA damage, response, and repair processes with cancer therapy remains a challenge, as does translating these basic research outcomes into clinical applications. At present, a plethora of inhibitorsare in clinical trials or approved for clinical use that originated from basic cell and animal experiments. Additional biomarkers and agents show promise at the preclinical level, but their translation to the clinical setting has failed for many reasons, such as not providing superior therapeutic outcomes and serious adverse effects. To achieve the purpose of translation study in the clinic in the future, more basic molecular mechanism of DNA damage and repair in cancer therapy should be extensive studied.
In conclusion, we believe that comprehensive research into the basic biology of DNA damage, response, and repair, accompanied by rapid development of new technologies and further progress in targeted cancer therapy, will drive significant advances in the near future. Hopefully, more robust clinical trial results will also be achieved.
Pederson, T. The double helix: "Photo 51" revisited. FASEB J. 34 , 1923–1927 (2020).
Article PubMed CAS Google Scholar
Watson, J. D. & Crick, F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171 , 737–738 (1953).
da Costa, A. & Baiocchi, G. Genomic profiling of platinum-resistant ovarian cancer: The road into druggable targets. Semin. Cancer Biol . 5 , 30221–30222 (2020).
Sengupta, D., Mukhopadhyay, A. & Sengupta, K. Emerging roles of lamins and DNA damage repair mechanisms in ovarian cancer. Biochem Soc. Trans. 48 , 2317–2333 (2020).
Parekh, V. J. et al. Crucial Role of the C-Terminal Domain of Hfq Protein in Genomic Instability. Microorganisms . 8 , 1598 (2020).
Huang, R. X. & Zhou, P. K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target Ther. 5 , 60 (2020).
Article PubMed PubMed Central CAS Google Scholar
Gager, C. S. & Blakeslee, A. F. Chromosome and gene mutations in datura following exposure to radium rays. Proc. Natl Acad. Sci. USA 13 , 75–79 (1927).
Vasil, I. K. A history of plant biotechnology: from the Cell Theory of Schleiden and Schwann to biotech crops. Plant Cell Rep. 27 , 1423–1440 (2008).
Muller, H. J. The production of mutations by X-Rays. Proc. Natl Acad. Sci. Usa. 14 , 714–726 (1928).
Muller, H. J. Artificial transmutation of the gene. Science 66 , 84–87 (1927).
Portin, P. The birth and development of the DNA theory of inheritance: sixty years since the discovery of the structure of DNA. J. Genet. 93 , 293–302 (2014).
Pettijohn, D. & Hanawalt, P. Evidence for repair-replication of ultraviolet damaged DNA in bacteria. J. Mol. Biol. 9 , 395–410 (1964).
Schuster, R. C. Dark repair of ultraviolet injury in E. Coli during deprivation of thymine. Nature 202 , 614–615 (1964).
Boyce, R. P. & Howard-Flanders, P. Release of ultraviolet light-induced thymine dimers from DNA in E. Coli K-12. Proc. Natl Acad. Sci. USA 51 , 293–300 (1964).
Setlow, R. B. & Carrier, W. L. The disappearance of thymine dimers from DNA: an error-correcting mechanism. Proc. Natl Acad. Sci. USA 51 , 226–231 (1964).
Setlow, R. B., Regan, J. D., German, J. & Carrier, W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc. Natl Acad. Sci. USA 64 , 1035–1041 (1969).
Cleaver, J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 218 , 652–656 (1968).
Chiche, J. D., Cariou, A. & Mira, J. P. Bench-to-bedside review: fulfilling promises of the Human Genome Project. Crit. Care 6 , 212–215 (2002).
Article PubMed PubMed Central Google Scholar
Kelavkar, U. & Shah, K. Advances in the human genome project. A review. Mol. Biol. Rep. 25 , 27–43 (1998).
Harrow, J. et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 22 , 1760–1774 (2012).
Guigo, R. et al. EGASP: the human ENCODE genome annotation assessment project. Genome Biol. 7 , S21–S31 (2006). Suppl 1.
Article Google Scholar
Dolinnaya, N. G., Ogloblina, A. M. & Yakubovskaya, M. G. Structure, properties, and biological relevance of the DNA and RNA G-quadruplexes: overview 50 years after their discovery. Biochem. (Mosc.) 81 , 1602–1649 (2016).
Article CAS Google Scholar
Silverman, M. The discovery of DNA structure: 50 years on. Clin. Invest Med. 26 , 53 (2003).
PubMed Google Scholar
Fu, H., Baris, A. & Aladjem, M. I. Replication timing and nuclear structure. Curr. Opin. Cell Biol. 52 , 43–50 (2018).
Kraus, W. L. PARPs and ADP -Ribosylation: 50 Years and Counting. Mol. Cell. 58 , 902–910 (2015).
Doll, R. Pott and the path to prevention. Arch. Geschwulstforsch. 45 , 521–531 (1975).
PubMed CAS Google Scholar
Harris, H. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. Preface. J. Cell Sci. 121 , v–vi (2008). Suppl 1.
Article PubMed Google Scholar
Wunderlich, V. ["He corrects my view and develops it further." Comments by David von Hansemann on the monograph by Theodor Boveri Concerning the origin of malignant tumors (1914)]. Ber. Wiss. 34 , 263–283 (2011).
Svoboda, J. [Rediscovered Theodor Boveri and his dateless reflections on the development of malignancies]. Cas. Lek. Cesk. 147 , 546 (2008).
Freitas, M. O., Gartner, J., Rangel-Pozzo, A. & Mai, S. Genomic Instability in Circulating Tumor Cells. Cancers (Basel) . 12 , 3001 (2020).
Oster, S. & Aqeilan, R. I. Programmed DNA damage and physiological DSBs: mapping, biological significance and perturbations in disease states. Cells . 9 , 1870 (2020).
Matsuda, S. et al. Role of tumor suppressor molecules in genomic perturbations and damaged DNA repair involved in the pathogenesis of cancer and neurodegeneration (Review). Biomed. Rep. 13 , 10 (2020).
PubMed PubMed Central CAS Google Scholar
Young, R. F. A clinical review of cancer of the breast and antecedent chronic conditions. Edinb. Med J. 52 , 451–459 (1945).
On the Formation and Extension of Cancer-Cells in the Neighbourhood of Cancer, and Their Importance in the Performance of an Operation. Br Foreign Med Chir Rev . 15 , 390–400, (1855).
Bolzan, A. D. Using telomeric chromosomal aberrations to evaluate clastogen-induced genomic instability in mammalian cells. Chromosome Res. 28 , 259–276 (2020).
Luijten, M. et al. Utility of a next generation framework for assessment of genomic damage: A case study using the industrial chemical benzene. Environ. Mol. Mutagen. 61 , 94–113 (2020).
Owiti, N. A., Nagel, Z. D. & Engelward, B. P. Fluorescence Sheds Light on DNA Damage, DNA Repair, and Mutations. Trends Cancer , 7, 240–248 (2020).
Legator, M. S. & Flamm, W. G. Environmental mutagenesis and repair. Annu. Rev. Biochem 42 , 683–708 (1973).
Popescu, N. C. Comprehensive genetic analysis of cancer cells. J. Cell Mol. Med. 4 , 151–163 (2000).
Wengner, A. M., Scholz, A. & Haendler, B. Targeting DNA damage response in prostate and breast cancer. Int. J. Mol. Sci . 21 , 8273 (2020).
Schneeweis, C. et al. The SUMO pathway in pancreatic cancer: insights and inhibition. Br J Cancer , 124, 531–538 (2020).
Dang, F., Nie, L. & Wei, W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ . 28 , 427–438 (2020).
Hartwell, L. H. & Kastan, M. B. Cell cycle control and cancer. Science 266 , 1821–1828 (1994).
Mota, M. B. S., Carvalho, M. A., Monteiro, A. N. A. & Mesquita, R. D. DNA damage response and repair in perspective: Aedes aegypti, Drosophila melanogaster and Homo sapiens. Parasit. Vectors 12 , 533 (2019).
Jing, X. et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 18 , 157 (2019).
Stewart, B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J. Natl Cancer Inst. 86 , 1286–1296 (1994).
Mani, C., Reddy, P. H. & Palle, K. DNA repair fidelity in stem cell maintenance, health, and disease. Biochim Biophys. Acta Mol. Basis Dis. 1866 , 165444 (2020).
O’Connor, P. M. & Kohn, K. W. A fundamental role for cell cycle regulation in the chemosensitivity of cancer cells? Semin Cancer Biol. 3 , 409–416 (1992).
Lee, C. Y. et al. High-throughput screening for genes that prevent excess DNA replication in human cells and for molecules that inhibit them. Methods 57 , 234–248 (2012).
Charcosset, J. Y., Soues, S. & Laval, F. [Poisons of DNA topoisomerases I and II]. Bull. Cancer 80 , 923–954 (1993).
Cummings, J. & Smyth, J. F. DNA topoisomerase I and II as targets for rational design of new anticancer drugs. Ann. Oncol. 4 , 533–543 (1993).
Lawley, P. D. & Brookes, P. Cytotoxicity of alkylating agents towards sensitive and resistant strains of Escherichia coli in relation to extent and mode of alkylation of cellular macromolecules and repair of alkylation lesions in deoxyribonucleic acids. Biochem J. 109 , 433–447 (1968).
Lawley, P. D. & Brookes, P. Interstrand cross-linking of DNA by difunctional alkylating agents. J. Mol. Biol. 25 , 143–160 (1967).
Brookes, P. & Lawley, P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 80 , 496–503 (1961).
Brookes, P. & Lawley, P. D. The reaction of mustard gas with nucleic acids in vitro and in vivo. Biochem J. 77 , 478–484 (1960).
Valles, G. J., Bezsonova, I., Woodgate, R. & Ashton, N. W. USP7 is a master regulator of genome stability. Front Cell Dev. Biol. 8 , 717 (2020).
Burgess, J. T. et al. The therapeutic potential of DNA damage repair pathways and genomic stability in lung cancer. Front Oncol. 10 , 1256 (2020).
Lasolle, H. et al. Chromosomal instability in the prediction of pituitary neuroendocrine tumors prognosis. Acta Neuropathol. Commun. 8 , 190 (2020).
Ma, Q. et al. Increased chromosomal instability characterizes metastatic renal cell carcinoma. Transl. Oncol. 14 , 100929 (2020).
Varella-Garcia, M. Chromosomal and genomic changes in lung cancer. Cell Adh Migr. 4 , 100–106 (2010).
Tosato, V. et al. Warburg effect and translocation-induced genomic instability: two yeast models for cancer cells. Front Oncol. 2 , 212 (2012).
Huang, R. et al. Integrated analysis of transcriptomic and metabolomic profiling reveal the p53 associated pathways underlying the response to ionizing radiation in HBE cells. Cell Biosci. 10 , 56 (2020).
Dai, X. et al. A novel miR-0308-3p revealed by miRNA-seq of HBV-positive hepatocellular carcinoma suppresses cell proliferation and promotes G1/S arrest by targeting double CDK6/Cyclin D1 genes. Cell Biosci. 10 , 24 (2020).
Polo, S. E. & Jackson, S. P. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 25 , 409–433 (2011).
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411 , 366–374 (2001).
Article CAS PubMed Google Scholar
Blackford, A. N. & Jackson, S. P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell 66 , 801–817 (2017).
Mulder, M. P. C., Witting, K. F. & Ovaa, H. Cracking the ubiquitin code: the ubiquitin toolbox. Curr. Issues Mol. Biol. 37 , 1–20 (2020).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability-an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11 , 220–228 (2010).
Yoon, D. S. et al. Variable levels of chromosomal instability and mitotic spindle checkpoint defects in breast cancer. Am. J. Pathol. 161 , 391–397 (2002).
Brockelmann, P. J., de Jong, M. R. W. & Jachimowicz, R. D. Targeting DNA repair, cell cycle, and tumor microenvironment in B cell lymphoma. Cells . 9 , 2287 (2020).
Kottemann, M. C. & Smogorzewska, A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 493 , 356–363 (2013).
Mareckova, A. et al. ATM and TP53 mutations show mutual exclusivity but distinct clinical impact in mantle cell lymphoma patients. Leuk. Lymphoma 60 , 1420–1428 (2019).
Choi, M., Kipps, T. & Kurzrock, R. ATM mutations in cancer: therapeutic implications. Mol. Cancer Ther. 15 , 1781–1791 (2016).
Jiang, H. et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol. Cell. 18 , 699–709 (2005).
Huang, R. et al. BECN1 promotes radiation-induced G2/M arrest through regulation CDK1 activity: a potential role for autophagy in G2/M checkpoint. Cell Death Discov. 6 , 70 (2020).
Zhang, J. et al. Suppression of LINC00460 mediated the sensitization of HCT116 cells to ionizing radiation by inhibiting epithelial-mesenchymal transition. Toxicol. Res (Camb.). 9 , 107–116 (2020).
Basak, D., Uddin, M. N. & Hancock, J. The role of oxidative stress and its counteractive utility in colorectal cancer (CRC). Cancers (Basel) . 12 , 3336 (2020).
Canto-Santos, J., Grau-Junyent, J. M. & Garrabou, G. The impact of mitochondrial deficiencies in neuromuscular diseases. Antioxidants (Basel) . 9 , 964 (2020).
Tubbs, A. & Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 168 , 644–656 (2017).
Lin, L., Cheng, X. & Yin, D. Aberrant DNA methylation in esophageal squamous cell carcinoma: biological and clinical implications. Front Oncol. 10 , 549850 (2020).
Patel, S. M., Dash, R. C. & Hadden, M. K. Translesion synthesis inhibitors as a new class of cancer chemotherapeutics. Expert Opin. Investig. Drugs 30 , 1–12, (2020).
Casati, P. & Gomez, M. S. Chromatin dynamics during DNA damage and repair in plants: new roles for old players. J. Exp. Bot. 72 , 4119–4131 (2020).
Klintman, J. et al. Genomic and transcriptomic correlates of Richter’s transformation in Chronic Lymphocytic Leukemia. Blood . 137 , 2800–2816 (2020).
Ragunathan, K., Upfold, N. L. E. & Oksenych, V. Interaction between fibroblasts and immune cells following DNA damage induced by ionizing radiation. Int. J. Mol. Sci . 21 , (2020).
Marshall, C. J. & Santangelo, T. J. Archaeal DNA repair mechanisms. Biomolecules . 10 , 8635 (2020).
Maremonti, E. et al. Ionizing radiation, genotoxic stress, and mitochondrial DNA copy-number variation in Caenorhabditis elegans: droplet digital PCR analysis. Mutat. Res. 858-860 , 503277 (2020).
Pariset, E., Malkani, S., Cekanaviciute, E. & Costes, S. V. Ionizing radiation-induced risks to the central nervous system and countermeasures in cellular and rodent models. Int. J. Radiat. Biol. 20 , 1–19, (2020).
Wu, R. et al. Crystalline silica particles cause rapid NLRP3-dependent mitochondrial depolarization and DNA damage in airway epithelial cells. Part Fibre Toxicol. 17 , 39 (2020).
Dussert, F. et al. Toxicity to RAW264.7 macrophages of silica nanoparticles and the E551 food additive, in combination with genotoxic agents. Nanomaterials (Basel) . 10 , 1418 (2020).
Huang, R., Yu, T., Li, Y. & Hu, J. Upregulated has-miR-4516 as a potential biomarker for early diagnosis of dust-induced pulmonary fibrosis in patients with pneumoconiosis. Toxicol. Res (Camb.) 7 , 415–422 (2018).
Gupta, N., Khetan, D., Chaudhary, R. & Shukla, J. S. Prospective cohort study to assess the effect of storage duration, leuko-filtration, and gamma irradiation on cell-free DNA in red cell components. Transfus. Med. Hemother 47 , 409–419 (2020).
Fu, J. et al. Epigenetic modification and a role for the E3 ligase RNF40 in cancer development and metastasis. Oncogene 40 , 465–474 (2020).
Tirman, S. et al. PRIMPOL ready, set, reprime! Crit. Rev. Biochem. Mol. Biol. 56 , 1–14 (2020).
Sharma, D. et al. DNA binding and antiradical potential of ethyl pyruvate: Key to the DNA radioprotection. Chem. Biol. Interact. 332 , 109313 (2020).
Verhoven, B. M. et al. Significant improvement in rat kidney cold storage using UW organ preservation solution supplemented with the immediate-acting PrC-210 free radical scavenger. Transpl. Direct 6 , e578 (2020).
Chatterjee, N. & Walker, G. C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen 58 , 235–263 (2017).
Ceccaldi, R., Rondinelli, B. & D’Andrea, A. D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26 , 52–64 (2016).
Frankenberg-Schwager, M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 29 , 273–292 (1990).
Li, J. et al. Pathways and assays for DNA double-strand break repair by homologous recombination. Acta Biochim Biophys. Sin. (Shanghai). 51 , 879–889 (2019).
Lindahl, T. & Barnes, D. E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 65 , 127–133 (2000).
Evans, M. D., Dizdaroglu, M. & Cooke, M. S. Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res . 567 , 1–61 (2004).
Slupphaug, G., Kavli, B. & Krokan, H. E. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. 531 , 231–251 (2003).
Dizdaroglu, M. & Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res 46 , 382–419 (2012).
Rao, K. S. Free radical induced oxidative damage to DNA: relation to brain aging and neurological disorders. Indian J. Biochem Biophys. 46 , 9–15 (2009).
Ide, H., Nakano, T., Salem, A. M. H. & Shoulkamy, M. I. DNA-protein cross-links: formidable challenges to maintaining genome integrity. DNA Repair (Amst.) 71 , 190–197 (2018).
Tretyakova, N. Y., Groehler, A. T. & Ji, S. DNA-protein cross-links: formation, structural identities, and biological outcomes. Acc. Chem. Res. 48 , 1631–1644 (2015).
Stingele, J., Bellelli, R. & Boulton, S. J. Mechanisms of DNA-protein crosslink repair. Nat. Rev. Mol. Cell Biol. 18 , 563–573 (2017).
Nakano, T. et al. Radiation-induced DNA-protein cross-links: Mechanisms and biological significance. Free Radic. Biol. Med. 107 , 136–145 (2017).
Zheng, Y. & Sanche, L. Clustered DNA Damages induced by 0.5 to 30 eV Electrons. Int. J. Mol. Sci . 20 , 3749 (2019).
Eccles, L. J., O’Neill, P. & Lomax, M. E. Delayed repair of radiation induced clustered DNA damage: friend or foe? Mutat. Res. 711 , 134–141 (2011).
Sage, E. & Harrison, L. Clustered DNA lesion repair in eukaryotes: relevance to mutagenesis and cell survival. Mutat. Res . 711 , 123–133 (2011).
Nickoloff, J. A., Sharma, N. & Taylor, L. Clustered DNA double-strand breaks: biological effects and relevance to cancer radiotherapy. Genes (Basel) . 11 , JanPMC7017136 (2020).
De Bont, R. & van Larebeke, N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19 , 169–185 (2004).
Sangaletti, S. et al. Intra-tumour heterogeneity of diffuse large B-cell lymphoma involves the induction of diversified stroma-tumour interfaces. EBioMedicine 61 , 103055 (2020).
Naval-Sanchez, M. et al. Selection signatures in tropical cattle are enriched for promoter and coding regions and reveal missense mutations in the damage response gene HELB. Genet Sel. Evol. 52 , 27 (2020).
Alhmoud, J. F., Woolley, J. F., Al Moustafa, A. E. & Malki, M. I. DNA damage/repair management in cancers. Cancers (Basel) . 12 , 1050 (2020).
Pilie, P. G., Tang, C., Mills, G. B. & Yap, T. A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16 , 81–104 (2019).
Article PubMed CAS PubMed Central Google Scholar
Kitagishi, Y., Kobayashi, M. & Matsuda, S. Defective DNA repair systems and the development of breast and prostate cancer (review). Int J. Oncol. 42 , 29–34 (2013).
Ijsselsteijn, R., Jansen, J. G. & de Wind, N. DNA mismatch repair-dependent DNA damage responses and cancer. DNA Repair (Amst.) 93 , 102923 (2020).
Gupta, D. & Heinen, C. D. The mismatch repair-dependent DNA damage response: mechanisms and implications. DNA Repair (Amst.) 78 , 60–69 (2019).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534 , 47–54 (2016).
Okoyo, C. et al. Prevalence, intensity and associated risk factors of soil-transmitted helminth and schistosome infections in Kenya: Impact assessment after five rounds of mass drug administration in Kenya. PLoS Negl. Trop. Dis. 14 , e0008604 (2020).
Collins, P. L. et al. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nat. Commun. 11 , 3158 (2020).
Podhorecka, M., Skladanowski, A. & Bozko, P. H2AX phosphorylation: its role in DNA damage response and cancer therapy. J. Nucleic Acids . 2010 , 920161 (2010).
Chen, K. H. et al. Analysis of DNA damage responses after boric acid-mediated boron neutron capture therapy in hepatocellular carcinoma. Anticancer Res. 39 , 6661–6671 (2019).
Monteiro, F. L. et al. Expression and functionality of histone H2A variants in cancer. Oncotarget 5 , 3428–3443 (2014).
Baptistella, A. R. et al. Rab5C enhances resistance to ionizing radiation in rectal cancer. J. Mol. Med (Berl.). 97 , 855–869 (2019).
Pucci, S. et al. Ku70, Ku80, and sClusterin: a cluster of predicting factors for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Int J. Radiat. Oncol. Biol. Phys. 97 , 381–388 (2017).
O’Connor, M. J. Targeting the DNA damage response in cancer. Mol. Cell. 60 , 547–560 (2015).
Pott, P. [The first description of an occupational cancer in 1777 (scrotal cancer, cancer of chimney sweeps)]. Bull. Soc. Liban. Hist. Med . 11 , 98–101, (1993).
Kaufmann, B. P. & Gay, H. Frequency of recessive lethals induced in Drosophila by near infra-red rays and X-rays. Anat. Rec. 96 , 530 (1946).
Herskowitz, I. H. The relationship of x-ray induced recessive lethals to chromosomal breakage. Am. Nat. 80 , 588–592 (1946).
Boveri, T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J. Cell Sci. 121 (Suppl 1), 1–84 (2008).
Burdette, W. J. The significance of mutation in relation to the origin of tumors: a review. Cancer Res. 15 , 201–226 (1955).
Dean, C. J., Feldschreiber, P. & Lett, J. T. Repair of x-ray damage to the deoxyribonucleic acid in Micrococcus radiodurans. Nature 209 , 49–52 (1966).
Brunk, C. F. & Hanawalt, P. C. Repair of damaged DNA in a eucaryotic cell: Tetrahymena pyriformis. Science 158 , 663–664 (1967).
Chen, Z., Xu, X. S., Yang, J. & Wang, G. Defining the function of XPC protein in psoralen and cisplatin-mediated DNA repair and mutagenesis. Carcinogenesis 24 , 1111–1121 (2003).
Cleaver, J. E. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc. Natl Acad. Sci. Usa. 63 , 428–435 (1969).
Fadeel, B. & Orrenius, S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J. Intern Med 258 , 479–517 (2005).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26 , 239–257 (1972).
Bishop, J. M. Enemies within: the genesis of retrovirus oncogenes. Cell 23 , 5–6 (1981).
Murphree, A. L. & Benedict, W. F. Retinoblastoma: clues to human oncogenesis. Science 223 , 1028–1033 (1984).
Chen, P. L., Chen, Y. M., Bookstein, R. & Lee, W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science 250 , 1576–1580 (1990).
Kinzler, K. W. & Vogelstein, B. Cancer-susceptibility genes. Gatekeepers caretakers. Nat. 386 , 761–763 (1997).
Sharan, S. K. et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 386 , 804–810 (1997).
Milner, J. et al. Transcriptional activation functions in BRCA2. Nature 386 , 772–773 (1997).
Karlsson, A. et al. Defective double-strand DNA break repair and chromosomal translocations by MYC overexpression. Proc. Natl Acad. Sci. Usa. 100 , 9974–9979 (2003).
Vafa, O. et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 9 , 1031–1044 (2002).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434 , 864–870 (2005).
Pearl, L. H. et al. Therapeutic opportunities within the DNA damage response. Nat. Rev. Cancer 15 , 166–180 (2015).
Lord, C. J. & Ashworth, A. The DNA damage response and cancer therapy. Nature 481 , 287–294 (2012).
Lee, T. H., Choi, J. Y., Park, J. M. & Kang, T. H. Posttranscriptional control of the replication stress response via TTP-mediated Claspin mRNA stabilization. Oncogene 39 , 3245–3257 (2020).
Glineburg, M. R., Johns, E. & Johnson, F. B. Deletion of ULS1 confers damage tolerance in sgs1 mutants through a Top3-dependent D-loop mediated fork restart pathway. DNA Repair (Amst.) 78 , 102–113 (2019).
Joshi, R. R., Ali, S. I. & Ashley, A. K. DNA Ligase IV prevents replication fork stalling and promotes cellular proliferation in triple negative breast cancer. J. Nucleic Acids 2019 , 9170341 (2019).
Beecher, M. et al. Expanding molecular roles of UV-DDB: Shining light on genome stability and cancer. DNA Repair (Amst.). 94 , 102860 (2020).
Pospiech, K., Pluciennik, E. & Bednarek, A. K. WWOX tumor suppressor gene in breast cancer, a historical perspective and future directions. Front Oncol. 8 , 345 (2018).
Jeggo, P. A., Pearl, L. H. & Carr, A. M. DNA repair, genome stability and cancer: a historical perspective. Nat. Rev. Cancer 16 , 35–42 (2016).
Bilusic, M. et al. Molecular profiling of exceptional responders to cancer therapy. Oncologist , 26 , 186–195 (2020).
Gotting, I., Jendrossek, V. & Matschke, J. A new twist in protein kinase B/Akt signaling: role of altered cancer cell metabolism in Akt-mediated therapy resistance. Int J Mol Sci . 21 , 8563 (2020).
Sheth, V. S. & Gauthier, J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transpl. 56 , 552–566 (2020).
Luo, G. F., Chen, W. H., Zeng, X. & Zhang, X. Z. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem. Soc. Rev . (2020).
Pal, D. et al. TGF-beta reduces DNA ds-break repair mechanisms to heighten genetic diversity and adaptability of CD44+/CD24- cancer cells. Elife . 6 , (2017).
Rossi, F. et al. Differences and similarities between cancer and somatic stem cells: therapeutic implications. Stem Cell Res Ther. 11 , 489 (2020).
Beheshtirouy, S., Mirzaei, F., Eyvazi, S. & Tarhriz, V. Recent advances on therapeutic peptides for breast cancer treatment. Curr. Protein Pept. Sci . (2020).
Wang, S., Shi, Y. & Han, X. [Advances in drug resistance mechanisms and prognostic markers of targeted therapy in ALK-positive non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi 23 , 1014–1022 (2020).
Kumar, V., Yadavilli, S. & Kannan, R. A review on RNAi therapy for NSCLC: Opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 13 , e1677, (2020).
Jiang, H., Chen, H. & Chen, N. Construction and validation of a seven-gene signature for predicting overall survival in patients with kidney renal clear cell carcinoma via an integrated bioinformatics analysis. Anim. Cells Syst. (Seoul.) 24 , 160–170 (2020).
Caravagna, G., Sanguinetti, G., Graham, T. A. & Sottoriva, A. The MOBSTER R package for tumour subclonal deconvolution from bulk DNA whole-genome sequencing data. BMC Bioinform. 21 , 531 (2020).
Nie, Y. H. et al. Analysis of mRNA expression patterns in peripheral blood cells of 3 patients with cancer after the first fraction of 2 Gy irradiation: an integrated case report and systematic review. Dose Response 17 , 1559325819833474 (2019).
Liu, X. D. et al. Integrated analysis of lncRNA-mRNA co-expression networks in the alpha-particle induced carcinogenesis of human branchial epithelial cells. Int J. Radiat. Biol. 95 , 144–155 (2019).
Nastasi, C., Mannarino, L. & D’Incalci, M. DNA damage response and immune defense. Int. J. Mol. Sci . 21 , 7504 (2020).
Roger, E. et al. Maintenance therapy for ATM-deficient pancreatic cancer by multiple DNA damage response interferences after platinum-based chemotherapy. Cells . 9 , 2110 (2020).
Perkhofer, L. et al. DNA damage repair as a target in pancreatic cancer: state-of-the-art and future perspectives. Gut , 70 , 606–61 (2020).
Hanawalt, P. C. Historical perspective on the DNA damage response. DNA Repair (Amst.). 36 , 2–7 (2015).
Brown, E. J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14 , 397–402 (2000).
Leach, F. S. et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75 , 1215–1225 (1993).
Friedberg, E. C. A history of the DNA repair and mutagenesis field: I. The discovery of enzymatic photoreactivation. DNA Repair (Amst.). 33 , 35–42 (2015).
Friedberg, E. C. Nucleotide excision repair of DNA: The very early history. DNA Repair (Amst.). 10 , 668–672 (2011).
Setlow, R. B. & Carrier, W. L. The disappearance of thymine dimers from DNA: an error-correcting mechanism. 1963. DNA Repair (Amst.) 2 , 1274–1279 (2003).
CAS Google Scholar
Kabat, S. & Visser, D. W. The incorporation of aminodeoxyuridine into deoxyribonucleic acid of escherichia coli 15t. Biochim Biophys. Acta 80 , 680–682 (1964).
Sachsenmaier, C. et al. Damage to DNA by UV light and activation of transcription factors. Biochem Pharmacol. 47 , 129–136 (1994).
Cleaver, J. R. & Painter, R. B. Evidence for repair replication of HeLa cell DNA damaged by ultraviolet light. Biochim Biophys. Acta 161 , 552–554 (1968).
Gellert, M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc. Natl Acad. Sci. USA 57 , 148–155 (1967).
Lindahl, T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc. Natl Acad. Sci. Usa. 71 , 3649–3653 (1974).
Wagner, R. Jr & Meselson, M. Repair tracts in mismatched DNA heteroduplexes. Proc. Natl Acad. Sci. USA 73 , 4135–4139 (1976).
Radman, M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 5A , 355–367 (1975).
Crowley, D. J. & Hanawalt, P. C. Induction of the SOS response increases the efficiency of global nucleotide excision repair of cyclobutane pyrimidine dimers, but not 6-4 photoproducts, in UV-irradiated Escherichia coli. J. Bacteriol. 180 , 3345–3352 (1998).
Hanawalt, P. C. & Spivak, G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 9 , 958–970 (2008).
Ford, J. M. & Hanawalt, P. C. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc. Natl Acad. Sci. USA 92 , 8876–8880 (1995).
Deger, N. et al. Drosophila, which lacks canonical transcription-coupled repair proteins, performs transcription-coupled repair. J. Biol. Chem. 294 , 18092–18098 (2019).
Kobaisi, F. et al. Signaling pathways, chemical and biological modulators of nucleotide excision repair: the faithful shield against UV genotoxicity. Oxid. Med Cell Longev. 2019 , 4654206 (2019).
Baddock, H. T. et al. TheSNM1A DNA repair nuclease. DNA Repair (Amst.). 95 , 102941 (2020).
Sale, J. E. & Translesion, D. N. A. synthesis and mutagenesis in eukaryotes. Cold Spring Harb. Perspect. Biol. 5 , a012708 (2013).
Cortez, D. Preventing replication fork collapse to maintain genome integrity. DNA Repair (Amst.). 32 , 149–157 (2015).
Bailey, R., Priego Moreno, S. & Gambus, A. Termination of DNA replication forks: "Breaking up is hard to do". Nucleus 6 , 187–196 (2015).
Friedberg, E. C. A brief history of the DNA repair field. Cell Res. 18 , 3–7 (2008).
Lai, Y., Beaver, J. M., Laverde, E. & Liu, Y. Trinucleotide repeat instability via DNA base excision repair. DNA Repair (Amst.) 93 , 102912 (2020).
Sassa, A. & Odagiri, M. Understanding the sequence and structural context effects in oxidative DNA damage repair. DNA Repair (Amst.) 93 , 102906 (2020).
Szewczuk, M., Boguszewska, K., Kazmierczak-Baranska, J. & Karwowski, B. T. The role of AMPK in metabolism and its influence on DNA damage repair. Mol. Biol. Rep. 47 , 9075–9086 (2020).
Kajitani, G. S. et al. Transcription blockage by DNA damage in nucleotide excision repair-related neurological dysfunctions. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2020.10.009 (2020).
Sena, L. A. et al. Tumor frameshift mutation proportion predicts response to immunotherapy in mismatch repair-deficient prostate cancer. Oncologist. 26 , e270–e278 (2020).
Latham, A. et al. Characterization and clinical outcomes of DNA mismatch repair deficient (MMR-D) small bowel adenocarcinoma. Clin. Cancer Res , 27 , 1429–1437 (2020).
Gachechiladze, M. et al. Predictive and prognostic value of DNA damage response associated kinases in solid tumors. Front Oncol. 10 , 581217 (2020).
Yoshioka, K. I. & Matsuno, Y. Genomic destabilization and its associated mutagenesis increase with senescence-associated phenotype expression. Cancer Sci . 112 , 515–522 (2020).
Rzeszutek, I. & Betlej, G. The role of small noncoding RNA in DNA double-strand break repair. Int. J. Mol. Sci . 21 , 8039 (2020).
Couve, S. et al. Direct DNA lesion reversal and excision repair in escherichia coli. EcoSal Plus . 5 , 26442931 (2013).
Dalhus, B., Laerdahl, J. K., Backe, P. H. & Bjoras, M. DNA base repair-recognition and initiation of catalysis. FEMS Microbiol. Rev. 33 , 1044–1078 (2009).
Dinant, C., Houtsmuller, A. B. & Vermeulen, W. Chromatin structure and DNA damage repair. Epigenetics Chromatin 1 , 9 (2008).
Park, H. W., Kim, S. T., Sancar, A. & Deisenhofer, J. Crystal structure of DNA photolyase from Escherichia coli. Science 268 , 1866–1872 (1995).
Huang, Y. et al. Crystal structure of cryptochrome 3 from Arabidopsis thaliana and its implications for photolyase activity. Proc. Natl Acad. Sci. USA. 103 , 17701–17706 (2006).
Hearst, J. E. The structure of photolyase: using photon energy for DNA repair. Science 268 , 1858–1859 (1995).
Eker, A. P., Quayle, C., Chaves, I. & van der Horst, G. T. DNA repair in mammalian cells: Direct DNA damage reversal: elegant solutions for nasty problems. Cell Mol. Life Sci. 66 , 968–980 (2009).
Ragg, S. et al. Direct reversal of DNA damage by mutant methyltransferase protein protects mice against dose-intensified chemotherapy and leads to in vivo selection of hematopoietic stem cells. Cancer Res. 60 , 5187–5195 (2000).
Yi, C. & He, C. DNA repair by reversal of DNA damage. Cold Spring Harb. Perspect. Biol. 5 , a012575 (2013).
Coyne, G. O. et al. Phase I trial of TRC102 (methoxyamine HCl) in combination with temozolomide in patients with relapsed solid tumors and lymphomas. Oncotarget 11 , 3959–3971 (2020).
Sinitsky, M. Y. et al. Mitomycin C induced genotoxic stress in endothelial cells is associated with differential expression of proinflammatory cytokines. Mutat. Res. 858-860 , 503252 (2020).
Klawitter, J. et al. Cyclophilin D knockout protects the mouse kidney against cyclosporin A-induced oxidative stress. Am. J. Physiol. Ren. Physiol. 317 , F683–F694 (2019).
Flitton, M. et al. Interaction of nutrition and genetics via DNMT3L-mediated DNA methylation determines cognitive decline. Neurobiol. Aging 78 , 64–73 (2019).
Yang, Z. et al. C8-substituted imidazotetrazine analogs overcome temozolomide resistance by inducing DNA adducts and DNA damage. Front Oncol. 9 , 485 (2019).
Chu, C. W. et al. GSK3betamediated Ser156 phosphorylation modulates a BH3like domain in BCL2L12 during TMZinduced apoptosis and autophagy in glioma cells. Int J. Mol. Med. 42 , 905–918 (2018).
Chen, F. et al. Adaptive Response Enzyme AlkB Preferentially Repairs 1-Methylguanine and 3-Methylthymine Adducts in Double-Stranded DNA. Chem. Res Toxicol. 29 , 687–693 (2016).
Schoonhoven, N. M. et al. Altering residue 134 confers an increased substrate range of alkylated nucleosides to the E. coli OGT protein. Molecules . 22 , 1948 (2017).
Denisov, A. Y. et al. Structural basis of interstrand cross-link repair by O(6)-alkylguanine DNA alkyltransferase. Org. Biomol. Chem. 15 , 8361–8370 (2017).
Taira, K. et al. Distinct pathways for repairing mutagenic lesions induced by methylating and ethylating agents. Mutagenesis 28 , 341–350 (2013).
Pegg, A. E. Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools. Chem. Res Toxicol. 24 , 618–639 (2011).
Wibley, J. E., Pegg, A. E. & Moody, P. C. Crystal structure of the human O(6)-alkylguanine-DNA alkyltransferase. Nucleic Acids Res . 28 , 393–401 (2000).
Vechtomova, Y. L., Telegina, T. A. & Kritsky, M. S. Evolution of proteins of the DNA photolyase/cryptochrome family. Biochem. (Mosc.) 85 , S131–S153 (2020).
Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 103 , 2203–2237 (2003).
Zhong, D. Electron transfer mechanisms of DNA repair by photolyase. Annu. Rev. Phys. Chem. 66 , 691–715 (2015).
Essen, L. O. & Klar, T. Light-driven DNA repair by photolyases. Cell Mol. Life Sci. 63 , 1266–1277 (2006).
Schleicher, E. et al. Electron nuclear double resonance differentiates complementary roles for active site histidines in (6-4) photolyase. J. Biol. Chem. 282 , 4738–4747 (2007).
Yamamoto, J. et al. Loss of fourth electron-transferring tryptophan in animal (6-4) Photolyase Impairs DNA Repair Activity in Bacterial Cells. Biochemistry 56 , 5356–5364 (2017).
Zhang, M., Wang, L. & Zhong, D. Photolyase: dynamics and electron-transfer mechanisms of DNA repair. Arch. Biochem Biophys. 632 , 158–174 (2017).
Kim, S. T. et al. Characterization of (6-4) photoproduct DNA photolyase. J. Biol. Chem. 269 , 8535–8540 (1994).
Guo, X. et al. Dynamics and mechanism of UV-damaged DNA repair in indole-thymine dimer adduct: molecular origin of low repair quantum efficiency. J. Phys. Chem. B. 119 , 3446–3455 (2015).
Liu, Z. et al. Electron tunneling pathways and role of adenine in repair of cyclobutane pyrimidine dimer by DNA photolyase. J. Am. Chem. Soc. 134 , 8104–8114 (2012).
Benjdia, A. DNA photolyases and SP lyase: structure and mechanism of light-dependent and independent DNA lyases. Curr. Opin. Struct. Biol. 22 , 711–720 (2012).
Benjdia, A. et al. Structural insights into recognition and repair of UV-DNA damage by Spore Photoproduct Lyase, a radical SAM enzyme. Nucleic Acids Res. 40 , 9308–9318 (2012).
Kiontke, S. et al. Crystal structures of an archaeal class II DNA photolyase and its complex with UV-damaged duplex DNA. EMBO J. 30 , 4437–4449 (2011).
Falnes, P. O., Johansen, R. F. & Seeberg, E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419 , 178–182 (2002).
Trewick, S. C. et al. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419 , 174–178 (2002).
Zhan, G. et al. Radioprotective effects on late third-instar bactrocera dorsalis (Diptera: Tephritidae) larvae in low-oxygen atmospheres. Insects . 11 , 526 (2020).
Wityk, P., Piatek, R., Nowak, R. & Kostrzewa-Nowak, D. Generation and characterization of a DNA-GCN4 oligonucleotide-peptide conjugate: the impact DNA/protein interactions on the sensitization of DNA. Molecules . 25 , 3630 (2020).
Forster, J. C., Douglass, M. J. J., Phillips, W. M. & Bezak, E. Stochastic multicellular modeling of x-ray irradiation, DNA damage induction, DNA free-end misrejoining and cell death. Sci. Rep. 9 , 18888 (2019).
Piekna-Przybylska, D. Reporter assays for BER pathway. Methods Mol. Biol. 1999 , 145–160 (2019).
Wang, K., Maayah, M., Sweasy, J. B. & Alnajjar, K. S. The role of cysteines in the structure and function of OGG1. J. Biol. Chem . 296, 100093 (2020).
Kimura, Y., Kajimoto, S., Yamamoto, Y. & Tanaka, N. Enzymatic characteristics of Nudix hydrolase 2 (Nud2), an 8-oxo-dGTP hydrolase from Myxococcus xanthus. J. Gen. Appl Microbiol. 66 , 46–50 (2020).
Whitaker, A. M. & Freudenthal, B. D. APE1: A skilled nucleic acid surgeon. DNA Repair (Amst.) 71 , 93–100 (2018).
Hendershot, J. M. & O’Brien, P. J. Search for DNA damage by human alkyladenine DNA glycosylase involves early intercalation by an aromatic residue. J. Biol. Chem. 292 , 16070–16080 (2017).
Owiti, N. et al. Def1 and Dst1 play distinct roles in repair of AP lesions in highly transcribed genomic regions. DNA Repair (Amst.). 55 , 31–39 (2017).
Parsons, J. L. & Dianov, G. L. Co-ordination of base excision repair and genome stability. DNA Repair (Amst.) 12 , 326–333 (2013).
Dianov, G. L. & Hubscher, U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 41 , 3483–3490 (2013).
Liu, C. et al. Individualized genetic network analysis reveals new therapeutic vulnerabilities in 6,700 cancer genomes. PLoS Comput Biol. 16 , e1007701 (2020).
Cai, Y., Geacintov, N. E. & Broyde, S. Variable impact of conformationally distinct DNA lesions on nucleosome structure and dynamics: Implications for nucleotide excision repair. DNA Repair (Amst.) 87 , 102768 (2020).
Yudkina, A. V., Dvornikova, A. P. & Zharkov, D. O. Variable termination sites of DNA polymerases encountering a DNA-protein cross-link. PLoS One 13 , e0198480 (2018).
Spivak, G. Nucleotide excision repair in humans. DNA Repair (Amst.). 36 , 13–18 (2015).
Brevik, A. et al. Both base excision repair and nucleotide excision repair in humans are influenced by nutritional factors. Cell Biochem Funct. 29 , 36–42 (2011).
Ye, N., Bianchi, M. S., Bianchi, N. O. & Holmquist, G. P. Adaptive enhancement and kinetics of nucleotide excision repair in humans. Mutat. Res. 435 , 43–61 (1999).
Guillotin, D. & Martin, S. A. Exploiting DNA mismatch repair deficiency as a therapeutic strategy. Exp. Cell Res. 329 , 110–115 (2014).
Huang, Y. & Li, G. M. DNA mismatch repair in the chromatin context: Mechanisms and therapeutic potential. DNA Repair (Amst.) 93 , 102918 (2020).
Chakraborty, U., Dinh, T. A. & Alani, E. Genomic instability promoted by overexpression of mismatch repair factors in yeast: a model for understanding cancer progression. Genetics 209 , 439–456 (2018).
Chakraborty, U. & Alani, E. Understanding how mismatch repair proteins participate in the repair/anti-recombination decision. FEMS Yeast Res . 16 , fow071 (2016).
He, D., Li, T., Sheng, M. & Yang, B. Exonuclease 1 (Exo1) participates in mammalian non-homologous end joining and contributes to drug resistance in ovarian cancer. Med. Sci. Monit. 26 , e918751 (2020).
Bowen, N. & Kolodner, R. D. Reconstitution of Saccharomyces cerevisiae DNA polymerase epsilon-dependent mismatch repair with purified proteins. Proc. Natl Acad. Sci. USA 114 , 3607–3612 (2017).
Guan, J. et al. MLH1 deficiency-triggered DNA hyperexcision by exonuclease 1 activates the cGAS-STING pathway. Cancer Cell. 39 , 109–121 e105 (2021).
Motegi, A., Masutani, M., Yoshioka, K. I. & Bessho, T. Aberrations in DNA repair pathways in cancer and therapeutic significances. Semin Cancer Biol. 58 , 29–46 (2019).
Boyle, K. M. & Barton, J. K. Targeting DNA mismatches with rhodium metalloinsertors. Inorg. Chim. Acta 452 , 3–11 (2016).
Dieckman, L. Something’s gotta give: How PCNA alters its structure in response to mutations and the implications on cellular processes. Prog. Biophys. Mol. Biol. 163 , 46–59 (2020).
Wang, H. et al. Mechanisms used by DNA MMR system to cope with Cadmium-induced DNA damage in plants. Chemosphere 246 , 125614 (2020).
Bradford, K. C. et al. Dynamic human MutSalpha-MutLalpha complexes compact mismatched DNA. Proc. Natl Acad. Sci. Usa. 117 , 16302–16312 (2020).
Sharma, R., Lewis, S. & Wlodarski, M. W. DNA repair syndromes and cancer: insights into genetics and phenotype patterns. Front Pediatr. 8 , 570084 (2020).
Nicolas, E., Golemis, E. A. & Arora, S. POLD1: central mediator of DNA replication and repair, and implication in cancer and other pathologies. Gene 590 , 128–141 (2016).
Li, Z., Pearlman, A. H. & Hsieh, P. DNA mismatch repair and the DNA damage response. DNA Repair (Amst.) 38 , 94–101 (2016).
Xiong, J., Zhang, J. & Li, H. Identification of G2 and S phase-expressed-1 as a potential biomarker in patients with prostate cancer. Cancer Manag Res 12 , 9259–9269 (2020).
Liu, A., Yoshioka, K., Salerno, V. & Hsieh, P. The mismatch repair-mediated cell cycle checkpoint response to fluorodeoxyuridine. J. Cell Biochem 105 , 245–254 (2008).
Allmann, S. et al. Benzo[a]pyrene represses DNA repair through altered E2F1/E2F4 function marking an early event in DNA damage-induced cellular senescence. Nucleic Acids Res. 48 , 12085–12101 (2020).
Suwala, A. K. et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol . 141 , 85–100 (2020).
Brown, J. S., O’Carrigan, B., Jackson, S. P. & Yap, T. A. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 7 , 20–37 (2017).
Mei, C. et al. The role of single strand break repair pathways in cellular responses to camptothecin induced DNA damage. Biomed. Pharmacother. 125 , 109875 (2020).
Gaziev, A. I. [DNA damage in cells exposed to ionizing radiation]. Radiats Biol. Radioecol. 39 , 630–638 (1999).
Richardson, C., Moynahan, M. E. & Jasin, M. Homologous recombination between heterologs during repair of a double-strand break. Suppression of translocations in normal cells. Ann. N. Y Acad. Sci. 886 , 183–186 (1999).
Yao, Y. et al. ATM promotes RAD51-mediated meiotic DSB repair by inter-sister-chromatid recombination in arabidopsis. Front Plant Sci. 11 , 839 (2020).
Kumar, A., Purohit, S. & Sharma, N. K. Aberrant DNA double-strand break repair threads in breast carcinoma: orchestrating genomic insult survival. J. Cancer Prev. 21 , 227–234 (2016).
Trenner, A. & Sartori, A. A. Harnessing DNA double-strand break repair for cancer treatment. Front Oncol. 9 , 1388 (2019).
Gomez-Mejiba, S. E. & Ramirez, D. C. Trapping of DNA radicals with the nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide and genotoxic damage: Recent advances using the immuno-spin trapping technology. Mutat. Res. 782 , 108283 (2019).
Dasika, G. K. et al. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18 , 7883–7899 (1999).
Lai, T. H. et al. HDAC inhibition induces MicroRNA-182, which targets RAD51 and impairs HR repair to sensitize cells to sapacitabine in acute myelogenous leukemia. Clin. Cancer Res. 22 , 3537–3549 (2016).
Sinha, A. et al. RAD51-mediated DNA homologous recombination is independent of PTEN mutational status. Cancers (Basel) . 12 , 3178 (2020).
Allera-Moreau, C. et al. DNA replication stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients. Oncogenesis 1 , e30 (2012).
Atwell, S. et al. Probing Rad51-DNA interactions by changing DNA twist. Nucleic Acids Res. 40 , 11769–11776 (2012).
Dhingra, N., Wei, L. & Zhao, X. Replication protein A (RPA) sumoylation positively influences the DNA damage checkpoint response in yeast. J. Biol. Chem. 294 , 2690–2699 (2019).
Gavande, N. S. et al. Structure-guided optimization of replication protein A (RPA)-DNA Interaction Inhibitors. ACS Med Chem. Lett. 11 , 1118–1124 (2020).
Lyu, K., Kumagai, A. & Dunphy, W. G. RPA-coated single-stranded DNA promotes the ETAA1-dependent activation of ATR. Cell Cycle 18 , 898–913 (2019).
Prados-Carvajal, R., Rodriguez-Real, G., Gutierrez-Pozo, G. & Huertas, P. CtIP-mediated alternative mRNA splicing finetunes the DNA damage response. RNA , (2020).
Mozaffari, N. L., Pagliarulo, F. & Sartori, A. A. Human CtIP: A ’double agent’ in DNA repair and tumorigenesis. Semin Cell Dev. Biol. 113 , 47–56 (2020).
Batenburg, N. L. et al. CSB interacts with BRCA1 in late S/G2 to promote MRN- and CtIP-mediated DNA end resection. Nucleic Acids Res. 47 , 10678–10692 (2019).
Soria-Bretones, I. et al. DNA end resection requires constitutive sumoylation of CtIP by CBX4. Nat. Commun. 8 , 113 (2017).
Caston, R. A. et al. The multifunctional APE1 DNA repair-redox signaling protein as a drug target in human disease. Drug Discov. Today 26 , 218–228 (2020).
Koike, M. & Koike, A. The Ku70-binding site of Ku80 is required for the stabilization of Ku70 in the cytoplasm, for the nuclear translocation of Ku80, and for Ku80-dependent DNA repair. Exp. Cell Res. 305 , 266–276 (2005).
Inagawa, T. et al. C-terminal extensions of Ku70 and Ku80 differentially influence DNA end binding properties. Int. J. Mol. Sci . 21 , 6725 (2020).
Jin, S. & Weaver, D. T. Double-strand break repair by Ku70 requires heterodimerization with Ku80 and DNA binding functions. EMBO J. 16 , 6874–6885 (1997).
Shibata, A. & Jeggo, P. A. Roles for the DNA-PK complex and 53BP1 in protecting ends from resection during DNA double-strand break repair. J. Radiat. Res 61 , 718–726 (2020).
Hammel, M. et al. Visualizing functional dynamicity in the DNA-dependent protein kinase holoenzyme DNA-PK complex by integrating SAXS with cryo-EM. Prog. Biophys. Mol. Biol . 20 , S0079–6107 (2020).
Medunjanin, S. et al. DNA-PK: gatekeeper for IKKgamma/NEMO nucleocytoplasmic shuttling in genotoxic stress-induced NF-kappaB activation. Cell Mol. Life Sci. 77 , 4133–4142 (2020).
Su, Y. et al. Association of LIG4 and XRCC4 gene polymorphisms with the risk of human glioma in a Chinese population. Int J. Clin. Exp. Pathol. 8 , 2057–2062 (2015).
PubMed PubMed Central Google Scholar
Gomes, B. C. et al. The role of common variants of non-homologous end-joining repair genes XRCC4, LIG4 and Ku80 in thyroid cancer risk. Oncol. Rep. 24 , 1079–1085 (2010).
Liu, Y. et al. Polymorphisms of LIG4 and XRCC4 involved in the NHEJ pathway interact to modify risk of glioma. Hum. Mutat. 29 , 381–389 (2008).
Piccinno, R., Minneker, V. & Roukos, V. 53BP1-DNA repair enters a new liquid phase. EMBO J. 38 , e102871 (2019).
Hwang, J. W. et al. PRMT5 promotes DNA repair through methylation of 53BP1 and is regulated by Src-mediated phosphorylation. Commun. Biol. 3 , 428 (2020).
Spies, J. et al. 53BP1 nuclear bodies enforce replication timing at under-replicated DNA to limit heritable DNA damage. Nat. Cell Biol. 21 , 487–497 (2019).
Shibata, A. & Jeggo, P. A. Roles for 53BP1 in the repair of radiation-induced DNA double strand breaks. DNA Repair (Amst.) 93 , 102915 (2020).
Zhao, L. et al. The determinant of DNA repair pathway choices in ionising radiation-induced DNA double-strand breaks. Biomed Res Int 2020 , 4834965 (2020).
Scully, R., Panday, A., Elango, R. & Willis, N. A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 20 , 698–714 (2019).
Truong, L. N. et al. Microhomology-mediated end joining and homologous recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl Acad. Sci. USA. 110 , 7720–7725 (2013).
Ahrabi, S. et al. A role for human homologous recombination factors in suppressing microhomology-mediated end joining. Nucleic Acids Res. 44 , 5743–5757 (2016).
Sallmyr, A. & Tomkinson, A. E. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J. Biol. Chem. 293 , 10536–10546 (2018).
Huang, Y. et al. Poly(ADP-ribose) polymerase-1 promotes recruitment of meiotic recombination-11 to chromatin and DNA double-strand break repair in Ku70-deficient breast cancer cells. FASEB J . 6 , fj201800092R, (2018).
Soni, A. et al. Requirement for Parp-1 and DNA ligases 1 or 3 but not of Xrcc1 in chromosomal translocation formation by backup end joining. Nucleic Acids Res. 42 , 6380–6392 (2014).
Ghezraoui, H. et al. Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol. Cell. 55 , 829–842 (2014).
Mateos-Gomez, P. A. et al. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature 518 , 254–257 (2015).
Iliakis, G., Mladenov, E. & Mladenova, V. Necessities in the processing of DNA double strand breaks and their effects on genomic instability and cancer. Cancers (Basel) . 11 , 1671 (2019).
Haince, J. F. et al. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 283 , 1197–1208 (2008).
Wang, M. et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 34 , 6170–6182 (2006).
Wang, H. et al. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res. 65 , 4020–4030 (2005).
Malaby, A. W., Martin, S. K., Wood, R. D. & Doublie, S. Expression and structural analyses of human DNA polymerase theta (POLQ). Methods Enzymol. 592 , 103–121 (2017).
Chan, S. H., Yu, A. M. & McVey, M. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in Drosophila. PLoS Genet 6 , e1001005 (2010).
Decottignies, A. Alternative end-joining mechanisms: a historical perspective. Front Genet. 4 , 48 (2013).
Zhao, F., Kim, W., Kloeber, J. A. & Lou, Z. DNA end resection and its role in DNA replication and DSB repair choice in mammalian cells. Exp. Mol. Med . 52 , 1705–1714 (2020).
Averbeck, N. B. et al. DNA end resection is needed for the repair of complex lesions in G1-phase human cells. Cell Cycle 13 , 2509–2516 (2014).
Peng, H., Zhang, S. & Chen, X. Monitoring 5'-end resection at site-specific double-strand breaks by southern blot analysis. Methods Mol. Biol. 2196 , 245–255 (2021).
Symington, L. S. & Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet 45 , 247–271 (2011).
Tomimatsu, N. et al. Phosphorylation of EXO1 by CDKs 1 and 2 regulates DNA end resection and repair pathway choice. Nat. Commun. 5 , 3561 (2014).
Cremona, C. A. et al. Extensive DNA damage-induced sumoylation contributes to replication and repair and acts in addition to the mec1 checkpoint. Mol. Cell. 45 , 422–432 (2012).
Robert, T. et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature 471 , 74–79 (2011).
Jimeno, S. et al. Neddylation inhibits CtIP-mediated resection and regulates DNA double strand break repair pathway choice. Nucleic Acids Res. 43 , 987–999 (2015).
Bunting, S. F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141 , 243–254 (2010).
Callen, E. et al. 53BP1 enforces distinct pre- and post-resection blocks on homologous recombination. Mol. Cell. 77 , 26–38 e27 (2020).
Wang, H. et al. The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair. PLoS Genet. 9 , e1003277 (2013).
Jachimowicz, R. D. & Reinhardt, H. C. UBQLN4 promotes non-homologous end joining by repressing DNA end-resection. Mol. Cell Oncol. 6 , 1575692 (2019).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature 518 , 258–262 (2015).
Howard, S. M., Yanez, D. A. & Stark, J. M. DNA damage response factors from diverse pathways, including DNA crosslink repair, mediate alternative end joining. PLoS Genet 11 , e1004943 (2015).
Unno, J. et al. FANCD2 binds CtIP and regulates DNA-end resection during DNA interstrand crosslink repair. Cell Rep. 7 , 1039–1047 (2014).
Iliakis, G., Murmann, T. & Soni, A. Alternative end-joining repair pathways are the ultimate backup for abrogated classical non-homologous end-joining and homologous recombination repair: Implications for the formation of chromosome translocations. Mutat. Res Genet Toxicol. Environ. Mutagen 793 , 166–175 (2015).
Janssen, A. et al. A single double-strand break system reveals repair dynamics and mechanisms in heterochromatin and euchromatin. Genes Dev. 30 , 1645–1657 (2016).
Liao, S., Tammaro, M. & Yan, H. The structure of ends determines the pathway choice and Mre11 nuclease dependency of DNA double-strand break repair. Nucleic Acids Res 44 , 5689–5701 (2016).
Azenha, D., Lopes, M. C. & Martins, T. C. Claspin: From replication stress and DNA damage responses to cancer therapy. Adv. Protein Chem. Struct. Biol. 115 , 203–246 (2019).
Tian, H. et al. DNA damage response-a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 358 , 8–16 (2015).
Neizer-Ashun, F. & Bhattacharya, R. Reality CHEK: Understanding the biology and clinical potential of CHK1. Cancer Lett. 497 , 202–211 (2021).
Zhang, Y. & Hunter, T. Roles of Chk1 in cell biology and cancer therapy. Int J. Cancer 134 , 1013–1023 (2014).
Peddibhotla, S., Lam, M. H., Gonzalez-Rimbau, M. & Rosen, J. M. The DNA-damage effector checkpoint kinase 1 is essential for chromosome segregation and cytokinesis. Proc. Natl Acad. Sci. USA 106 , 5159–5164 (2009).
Carrassa, L., Colombo, I., Damia, G. & Bertoni, F. Targeting the DNA damage response for patients with lymphoma: Preclinical and clinical evidences. Cancer Treat. Rev. 90 , 102090 (2020).
Khanna, A. DNA damage in cancer therapeutics: a boon or a curse? Cancer Res. 75 , 2133–2138 (2015).
Kaemmerer, E., Loessner, D. & Avery, V. M. Addressing the tumour microenvironment in early drug discovery: a strategy to overcome drug resistance and identify novel targets for cancer therapy. Drug Discov. Today , 26 , 663–676 (2020).
Karanika, S. et al. DNA damage response and prostate cancer: defects, regulation and therapeutic implications. Oncogene 34 , 2815–2822 (2015).
Cazaux, C. [Genetic instability as a driver for oncogenesis]. Bull. Cancer 97 , 1241–1251 (2010).
Contreras, H. R., Lopez-Moncada, F. & Castellon, E. A. Cancer stem cell and mesenchymal cell cooperative actions in metastasis progression and hormone resistance in prostate cancer: Potential role of androgen and gonadotropinreleasing hormone receptors (Review). Int J. Oncol. 56 , 1075–1082 (2020).
Zeng, X. et al. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharm. Res. 163 , 105320 (2020).
Anichini, A., Perotti, V. E., Sgambelluri, F. & Mortarini, R. Immune escape mechanisms in non small cell lung cancer. Cancers (Basel) . 12 , 3605 (2020).
Xia, W. Y. et al. Radiotherapy for non-small cell lung cancer in the immunotherapy era: the opportunity and challenge-a narrative review. Transl. Lung Cancer Res. 9 , 2120–2136 (2020).
Chakravarty, D., Huang, L., Kahn, M. & Tewari, A. K. Immunotherapy for metastatic prostate cancer: current and emerging treatment options. Urol. Clin. North Am. 47 , 487–510 (2020).
Keam, S. et al. Enhancing the efficacy of immunotherapy using radiotherapy. Clin. Transl. Immunol. 9 , e1169 (2020).
Carlson, R. D., Flickinger, J. C., Jr & Snook, A. E. Talkin’ toxins: from Coley’s to modern cancer immunotherapy. Toxins (Basel) . 12 , 241 (2020).
Coley, W. B. I. I. Contribution to the Knowledge of Sarcoma. Ann. Surg. 14 , 199–220 (1891).
Coley, W. B. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus erysipelas and the bacillus prodigiosus). Proc. R. Soc. Med. 3 , 1–48 (1910).
Johnston, B. J. & Novales, E. T. Clinical effect of Coley’s toxin. II. A seven-year study. Cancer Chemother. Rep. 21 , 43–68 (1962).
Bonnichon, P. et al. [History of cancer and chemotherapy before chemotherapy]. Hist. Sci. Med. 48 , 469–474 (2014).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1 , 405–413 (1994).
Walunas, T. L., Bakker, C. Y. & Bluestone, J. A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med 183 , 2541–2550 (1996).
Kim, M. T. et al. Enhancing dendritic cell-based immunotherapy with IL-2/monoclonal antibody complexes for control of established tumors. J. Immunol. 195 , 4537–4544 (2015).
Kasagi, S., Kawano, S. & Kumagai, S. PD-1 and autoimmunity. Crit. Rev. Immunol. 31 , 265–295 (2011).
Kwon, E. D. et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl Acad. Sci. Usa. 94 , 8099–8103 (1997).
Hurwitz, A. A. et al. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J. Neuroimmunol. 73 , 57–62 (1997).
Dunn, G. P. et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3 , 991–998 (2002).
Hsieh, E. M. & Rouce, R. H. Chimeric antigen receptor T cells for mature B-cell lymphoma and Burkitt lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2020 , 487–493 (2020).
Baxevanis, C. N., Fortis, S. P., Ardavanis, A. & Perez, S. A. Exploring essential issues for improving therapeutic cancer vaccine trial design. Cancers (Basel) . 12 , 2908 (2020).
Hwang, J. K., Hong, J. & Yun, C. O. Oncolytic viruses and immune checkpoint inhibitors: preclinical developments to clinical trials. Int J Mol Sci . 21 , 8627 (2020).
Hargadon, K. M., Gyorffy, B. & McGee, T. J. Genomic and transcriptional changes in IFNgamma pathway genes are putative biomarkers of response to ipilimumab immunotherapy in melanoma patients. Expert Rev Clin Immunol. https://doi.org/10.1080/1744666X.2021.1847644 (2020).
Wright, K. FDA approves nivolumab plus ipilimumab for previously untreated unresectable malignant pleural mesothelioma. Oncol. (Williston Park). 34 , 502–503 (2020).
Google Scholar
Fessas, P. et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer . 8 , e001033 (2020).
Koch, M. S., Lawler, S. E. & Chiocca, E. A. HSV-1 oncolytic viruses from bench to bedside: an overview of current clinical trials. Cancers (Basel) . 12 , 3514(2020).
Muller, L. et al. Past, present and future of oncolytic reovirus. Cancers (Basel) . 12 , 3219 (2020).
Hamid, O., Ismail, R. & Puzanov, I. Intratumoral Immunotherapy-Update 2019. Oncologist 25 , e423–e438 (2020).
Broman, K. K. & Zager, J. S. An evaluation of talimogene laherparepvec for the treatment of melanoma. Expert Opin. Biol. Ther. 20 , 9–14 (2020).
Mulbauer, G. D. & Matthew, H. W. T. Biomimetic scaffolds in skeletal muscle regeneration. Discoveries (Craiova) 7 , e90 (2019).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359 , 97–103 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359 , 91–97 (2018).
Reislander, T., Groelly, F. J. & Tarsounas, M. DNA Damage and Cancer Immunotherapy: A STING in the Tale. Mol. Cell 80 , 21–28 (2020).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548 , 466–470 (2017).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548 , 461–465 (2017).
Unterholzner, L. & Dunphy, G. cGAS-independent STING activation in response to DNA damage. Mol. Cell Oncol. 6 , 1558682 (2019).
Dunphy, G. et al. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-kappaB Signaling after Nuclear DNA Damage. Mol. Cell. 71 , 745–760 e745 (2018).
Storozynsky, Q. & Hitt, M. M. The impact of radiation-induced DNA damage on cGAS-STING-mediated immune responses to cancer. Int. J. Mol. Sci . 21 , 8877 (2020).
Almine, J. F. et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 8 , 14392 (2017).
Kikuchi, T. et al. A subset of patients with MSS/MSI-low-colorectal cancer showed increased CD8(+) TILs together with up-regulated IFN-gamma. Oncol. Lett. 18 , 5977–5985 (2019).
Takeda, K. et al. IFN-gamma is required for cytotoxic T cell-dependent cancer genome immunoediting. Nat. Commun. 8 , 14607 (2017).
Coquel, F. et al. [SAMHD1 acts at stalled replication forks to prevent interferon induction]. C. R. Biol. 343 , 9–21 (2020).
Erdal, E. et al. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 31 , 353–369 (2017).
Tarsounas, M. & Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 21 , 284–299 (2020).
Fu, J., Mao, J. & Wang, C. The microRNA-152/human leukocyte antigen-G axis affects proliferation and immune escape of non-small cell lung cancer cells. J. Int Med Res 48 , 300060520970758 (2020).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8 , 1069–1086 (2018).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39 , 98–106 (2016).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19 , 133–150 (2019).
Gasser, S. & Raulet, D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 16 , 344–347 (2006).
Gasser, S. & Raulet, D. H. The DNA damage response arouses the immune system. Cancer Res. 66 , 3959–3962 (2006).
Nakad, R. & Schumacher, B. DNA damage response and immune defense: links and mechanisms. Front Genet 7 , 147 (2016).
Cerboni, C. et al. The DNA damage response: a common pathway in the regulation of NKG2D and DNAM-1 ligand expression in normal, infected, and cancer cells. Front Immunol. 4 , 508 (2014).
Raulet, D. H., Marcus, A. & Coscoy, L. Dysregulated cellular functions and cell stress pathways provide critical cues for activating and targeting natural killer cells to transformed and infected cells. Immunol. Rev. 280 , 93–101 (2017).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436 , 1186–1190 (2005).
Jinushi, M. et al. ATM-mediated DNA damage signals mediate immune escape through integrin-alphavbeta3-dependent mechanisms. Cancer Res. 72 , 56–65 (2012).
Yang, H. et al. Beyond DNA Repair: DNA-PKcs in tumor metastasis, metabolism and immunity. Cancers (Basel) . 12 , 3389 (2020).
Li, H. et al. Pharmacological activation of p53 triggers anticancer innate immune response through induction of ULBP2. Cell Cycle 10 , 3346–3358 (2011).
Wang, Y., Shi, H., Meng, H. & Xu, J. Editorial: targeting the PD-1/PD-L1 cancer immune evasion axis: challenges and emerging strategies. Front Pharm. 11 , 591188 (2020).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348 , 56–61 (2015).
Facchini, G. et al. Advanced/metastatic bladder cancer: current status and future directions. Eur. Rev. Med Pharm. Sci. 24 , 11536–11552 (2020).
Hu, Z. The future of immune checkpoint blockade immunotherapy: towards personalized therapy or towards combination therapy. J. Thorac. Dis. 9 , 4226–4229 (2017).
Innao, V., Allegra, A. G., Musolino, C. & Allegra, A. New frontiers about the role of human microbiota in immunotherapy: the immune checkpoint inhibitors and CAR T-Cell Therapy Era. Int. J. Mol. Sci. 21 , 8902 (2020).
Ponnusamy, L., Mahalingaiah, P. K. S. & Singh, K. P. Epigenetic reprogramming and potential application of epigenetic-modifying drugs in acquired chemotherapeutic resistance. Adv. Clin. Chem. 94 , 219–259 (2020).
Tazzite, A., Jouhadi, H., Benider, A. & Nadifi, S. BRCA mutational status is a promising predictive biomarker for platinum- based chemotherapy in triple-negative breast cancer. Curr. Drug Targets 21 , 962–973 (2020).
Crusz, S. M. & Miller, R. E. Targeted therapies in gynaecological cancers. Histopathology 76 , 157–170 (2020).
Klinakis, A., Karagiannis, D. & Rampias, T. Targeting DNA repair in cancer: current state and novel approaches. Cell Mol. Life Sci. 77 , 677–703 (2020).
Rose, M. et al. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev. Biol. 8 , 564601 (2020).
Harrision, D., Gravells, P., Thompson, R. & Bryant, H. E. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP) - Function in Genome Maintenance and Relevance of Inhibitors for Anti-cancer Therapy. Front Mol. Biosci. 7 , 191 (2020).
Cerrato, A., Morra, F. & Celetti, A. Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic. J. Exp. Clin. Cancer Res 35 , 179 (2016).
Fisher, A. E., Hochegger, H., Takeda, S. & Caldecott, K. W. Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol. Cell Biol. 27 , 5597–5605 (2007).
Sugimura, K. et al. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J. Cell Biol. 183 , 1203–1212 (2008).
Dockery, L. E., Gunderson, C. C. & Moore, K. N. Rucaparib: the past, present, and future of a newly approved PARP inhibitor for ovarian cancer. Onco Targets Ther. 10 , 3029–3037 (2017).
Preiss, J., Schlaeger, R. & Hilz, H. Specific inhibition of poly adpribose polymerase by thymidine and nicotinamide in HeLa cells. FEBS Lett. 19 , 244–246 (1971).
Lemjabbar-Alaoui, H., Peto, C. J., Yang, Y. W. & Jablons, D. M. AMXI-5001, a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers. Am. J. Cancer Res 10 , 2649–2676 (2020).
Geenen, J. J. J., Linn, S. C., Beijnen, J. H. & Schellens, J. H. M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharmacokinet. 57 , 427–437 (2018).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 , 913–917 (2005).
Kaufman, B. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 33 , 244–250 (2015).
Pujade-Lauraine, E. et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1274–1284 (2017).
Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med . 377 , 1700, (2017).
Jang, A., Sartor, O., Barata, P. C. & Paller, C. J. Therapeutic potential of PARP inhibitors in the treatment of metastatic castration-resistant prostate cancer. Cancers (Basel) . 12 , 3467 (2020).
Oza, A. M. et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 147 , 267–275 (2017).
Gonzalez-Martin, A. et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 381 , 2391–2402 (2019).
Mirza, M. R. et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 375 , 2154–2164 (2016).
Ettl, J. et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann. Oncol. 29 , 1939–1947 (2018).
Baxter, P. A. et al. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: a Pediatric Brain Tumor Consortium study. Neuro Oncol. 22 , 875–885 (2020).
Luo, J. et al. Fluzoparib increases radiation sensitivity of non-small cell lung cancer (NSCLC) cells without BRCA1/2 mutation, a novel PARP1 inhibitor undergoing clinical trials. J. Cancer Res Clin. Oncol. 146 , 721–737 (2020).
Abbotts, R. & Madhusudan, S. Human AP endonuclease 1 (APE1): from mechanistic insights to druggable target in cancer. Cancer Treat. Rev. 36 , 425–435 (2010).
Tell, G., Damante, G., Caldwell, D. & Kelley, M. R. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid. Redox Signal 7 , 367–384 (2005).
Herring, C. J. et al. Levels of the DNA repair enzyme human apurinic/apyrimidinic endonuclease (APE1, APEX, Ref-1) are associated with the intrinsic radiosensitivity of cervical cancers. Br. J. Cancer 78 , 1128–1133 (1998).
Wang, D. et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 66 , 298–304 (2009).
Felix, F. A. et al. DNA base excision repair and nucleotide excision repair proteins in malignant salivary gland tumors. Arch. Oral. Biol. 121 , 104987 (2021).
Manoel-Caetano, F. S. et al. Hydrogen peroxide and Helicobacter pylori extract treatment combined with APE1 knockdown induce DNA damage, G2/M arrest and cell death in gastric cancer cell line. DNA Repair (Amst.). 96 , 102976 (2020).
Kabzinski, J., Walczak, A., Mik, M. & Majsterek, I. Sirt3 regulates the level of mitochondrial DNA repair activity through deacetylation of NEIL1, NEIL2, OGG1, MUTYH, APE1 and LIG3 in colorectal cancer. Pol. Przegl Chir. 92 , 1–4 (2019).
Laev, S. S., Salakhutdinov, N. F. & Lavrik, O. I. Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorg. Med. Chem. 25 , 2531–2544 (2017).
Gordon, M. S. et al. A phase 1 study of TRC102, an inhibitor of base excision repair, and pemetrexed in patients with advanced solid tumors. Invest N. Drugs 31 , 714–723 (2013).
Franchi, L. P., de Freitas Lima, J. E. B., Piva, H. L. & Tedesco, A. C. The redox function of apurinic/apyrimidinic endonuclease 1 as key modulator in photodynamic therapy. J. Photochem Photobio. B 211 , 111992 (2020).
Madhusudan, S. et al. Isolation of a small molecule inhibitor of DNA base excision repair. Nucleic Acids Res. 33 , 4711–4724 (2005).
Nobler, M. P. & Scher, A. J. Lucanthone as a radiosensitizing agent in the treatment of carcinoma of the cervix. Int J. Radiat. Oncol. Biol. Phys. 4 , 1039–1044 (1978).
Carew, J. S. et al. Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J. Biol. Chem. 286 , 6602–6613 (2011).
Cook, J. A., Jordan, P., Woodstock, L. & Pilgrim, V. A controlled trial of hycanthone and placebo in schistosomiasis mansoni in St. Lucia. Ann. Trop. Med Parasitol. 71 , 197–202 (1977).
Del Rowe, J. D. et al. Accelerated regression of brain metastases in patients receiving whole brain radiation and the topoisomerase II inhibitor, lucanthone. Int J. Radiat. Oncol. Biol. Phys. 43 , 89–93 (1999).
Mendez, F., Goldman, J. D. & Bases, R. E. Abasic sites in DNA of HeLa cells induced by lucanthone. Cancer Invest 20 , 983–991 (2002).
Fishel, M. L. & Kelley, M. R. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol. Asp. Med 28 , 375–395 (2007).
Ding, J. et al. Ref-1/APE1 as a transcriptional regulator and novel therapeutic target in pediatric t-cell leukemia. Mol. Cancer Ther. 16 , 1401–1411 (2017).
Guerreiro, P. S. et al. The APE1 redox inhibitor E3330 reduces collective cell migration of human breast cancer cells and decreases chemoinvasion and colony formation when combined with docetaxel. Chem. Biol. Drug Des. 90 , 561–571 (2017).
London, R. E. XRCC1 - Strategies for coordinating and assembling a versatile DNA damage response. DNA Repair (Amst.). 93 , 102917 (2020).
Abbotts, R. & Wilson, D. M. 3rd Coordination of DNA single strand break repair. Free Radic. Biol. Med 107 , 228–244 (2017).
Iyama, T. & Wilson, D. M. 3rd DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst.) 12 , 620–636 (2013).
Ferri, A., Stagni, V. & Barila, D. Targeting the DNA damage response to overcome cancer drug resistance in glioblastoma. Int. J. Mol. Sci . 21 , 4910 (2020).
O’Connor, E. et al. Mutations in XRCC1 cause cerebellar ataxia and peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry 89 , 1230–1232 (2018).
Zhang, N. et al. Pharmacogenetic association between XRCC1 polymorphisms and response to platinum-based chemotherapy in asian patients with NSCLC: a meta-analysis. Biomed. Res Int. 2020 , 3520764 (2020).
Mani, R. S. et al. Domain analysis of PNKP-XRCC1 interactions: Influence of genetic variants of XRCC1. J. Biol. Chem. 294 , 520–530 (2019).
Xie, X. et al. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung V5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother. Oncol. 154 , 187–193 (2020).
Raturi, V. et al. Prospective evaluation of XRCC-1 Arg194Trp polymorphism as bio-predictor for clinical outcome in locally advanced laryngeal cancer undergoing cisplatin-based chemoradiation. Head. Neck. 42 , 1045–1056 (2020).
Nanda, S. S. et al. Evaluation of XRCC1 gene polymorphism as a biomarker in head and neck cancer patients undergoing chemoradiation therapy. Int J. Radiat. Oncol. Biol. Phys. 101 , 593–601 (2018).
Figg, W. D. et al. Phase II study of satraplatin and prednisone in patients with metastatic castration-resistant prostate cancer: a pharmacogenetic assessment of outcome and toxicity. Clin. Genitourin. Cancer 11 , 229–237 (2013).
Ott, K. et al. DNA repair gene and MTHFR gene polymorphisms as prognostic markers in locally advanced adenocarcinoma of the esophagus or stomach treated with cisplatin and 5-fluorouracil-based neoadjuvant chemotherapy. Ann. Surg. Oncol. 18 , 2688–2698 (2011).
Vaezi, A., Feldman, C. H. & Niedernhofer, L. J. ERCC1 and XRCC1 as biomarkers for lung and head and neck cancer. Pharmgenomics Pers. Med 4 , 47–63 (2011).
Huang, M. Y. et al. Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet Genomics. 21 , 18–25 (2011).
Tung, C. L. et al. Down-regulation of ERK1/2 and AKT-mediated X-ray repair cross-complement group 1 protein (XRCC1) expression by Hsp90 inhibition enhances the gefitinib-induced cytotoxicity in human lung cancer cells. Exp. Cell Res. 334 , 126–135 (2015).
Davis, A. J., Lee, K. J. & Chen, D. J. The N-terminal region of the DNA-dependent protein kinase catalytic subunit is required for its DNA double-stranded break-mediated activation. J. Biol. Chem. 288 , 7037–7046 (2013).
Neal, J. A. et al. Unraveling the complexities of DNA-dependent protein kinase autophosphorylation. Mol. Cell Biol. 34 , 2162–2175 (2014).
Dobbs, T. A., Tainer, J. A. & Lees-Miller, S. P. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair (Amst.) 9 , 1307–1314 (2010).
Article PubMed Central CAS Google Scholar
Sun, X. et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat. Commun. 11 , 6182 (2020).
Li, Y., Goronzy, J. J. & Weyand, C. M. DNA damage, metabolism and aging in pro-inflammatory T cells: Rheumatoid arthritis as a model system. Exp. Gerontol. 105 , 118–127 (2018).
Yu, Z. J. et al. [Expression of DNA-PK in hepato- and cholangio-neoplasms and its significance]. Zhonghua Gan Zang Bing Za Zhi 12 , 652–655 (2004).
An, J. et al. Silencing of DNA-PKcs alters the transcriptional profile of certain signal transduction genes related to proliferation and differentiation in HeLa cells. Int J. Mol. Med. 16 , 455–462 (2005).
An, J. et al. Downregulation of c-myc protein by siRNA-mediated silencing of DNA-PKcs in HeLa cells. Int J. Cancer 117 , 531–537 (2005).
Guo, Z. et al. HUWE1-dependent DNA-PKcs neddylation modulates its autophosphorylation in DNA damage response. Cell Death Dis. 11 , 400 (2020).
Goldberg, F. W. et al. The Discovery of 7-Methyl-2-[(7-methyl[1,2,4]triazolo[1,5-a]pyridin-6-yl)amino]−9-(tetrahydro-2H-p yran-4-yl)−7,9-dihydro-8H-purin-8-one (AZD7648), a Potent and Selective DNA-Dependent Protein Kinase (DNA-PK) Inhibitor. J Med Chem 63 , 3461–3471 (2020).
Dylgjeri, E. et al. Pleiotropic impact of DNA-PK in cancer and implications for therapeutic strategies. Clin. Cancer Res. 25 , 5623–5637 (2019).
Wu, Z. X. et al. M3814, a DNA-PK inhibitor, modulates ABCG2-mediated multidrug resistance in lung cancer cells. Front Oncol. 10 , 674 (2020).
Fok, J. H. L. et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 10 , 5065 (2019).
Khan, A. J. et al. VX-984 is a selective inhibitor of non-homologous end joining, with possible preferential activity in transformed cells. Oncotarget 9 , 25833–25841 (2018).
Timme, C. R. et al. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic xenografts. Mol. Cancer Ther. 17 , 1207–1216 (2018).
Berzosertib Is Safe, with Signs of Efficacy against Advanced Solid Tumors. Cancer Discov . 10 , 1250, (2020).
McMullen, M. et al. DUETTE: a phase II randomized, multicenter study to investigate the efficacy and tolerability of a second maintenance treatment in patients with platinum-sensitive relapsed epithelial ovarian cancer, who have previously received poly(ADP-ribose) polymerase (PARP) inhibitor maintenance treatment. Int J. Gynecol. Cancer 30 , 1824–1828 (2020).
Bradbury, A., Hall, S., Curtin, N. & Drew, Y. Targeting ATR as cancer therapy: a new era for synthetic lethality and synergistic combinations? Pharm. Ther. 207 , 107450 (2020).
Ellenberger, T. & Tomkinson, A. E. Eukaryotic DNA ligases: structural and functional insights. Annu. Rev. Biochem 77 , 313–338 (2008).
Britton, S. et al. ATM antagonizes NHEJ proteins assembly and DNA-ends synapsis at single-ended DNA double strand breaks. Nucleic Acids Res. 48 , 9710–9723 (2020).
Tomkinson, A. E. & Naila, T. Altered DNA ligase activity in human disease. Mutagenesis 35 , 51–60 (2020).
Riyazuddin, M. et al. Elucidation of pharmacokinetics of novel DNA ligase I inhibitor, S012-1332 in rats: Integration of in vitro and in vivo findings. J. Pharm. Biomed. Anal. 162 , 205–214 (2019).
Howes, T. R. L. et al. Structure-activity relationships among DNA ligase inhibitors: Characterization of a selective uncompetitive DNA ligase I inhibitor. DNA Repair (Amst.). 60 , 29–39 (2017).
Hussain, M. K. et al. A novel benzocoumarin-stilbene hybrid as a DNA ligase I inhibitor with in vitro and in vivo anti-tumor activity in breast cancer models. Sci. Rep. 7 , 10715 (2017).
Chen, X. et al. Rational design of human DNA ligase inhibitors that target cellular DNA replication and repair. Cancer Res. 68 , 3169–3177 (2008).
Srivastava, M. et al. An inhibitor of nonhomologous end-joining abrogates double-strand break repair and impedes cancer progression. Cell 151 , 1474–1487 (2012).
Ray, U. et al. Identification and characterization of novel SCR7-based small-molecule inhibitor of DNA end-joining, SCR130 and its relevance in cancer therapeutics. Mol. Carcinog. 59 , 618–628 (2020).
Huang, R., Xiang, J. & Zhou, P. Vitamin D, gut microbiota, and radiation-related resistance: a love-hate triangle. J. Exp. Clin. Cancer Res . 38 , 493 (2019).
Drullinsky, P. R. & Hurvitz, S. A. Mechanistic basis for PI3K inhibitor antitumor activity and adverse reactions in advanced breast cancer. Breast Cancer Res Treat. 181 , 233–248 (2020).
Tan, A. C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 11 , 511–518 (2020).
Dent, S. et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann. Oncol. 32 , 197–207 (2021).
Bertho, M. et al. A pharmacokinetic evaluation of alpelisib for the treatment of HR+, HER2-negative, PIK3CA-mutated advanced or metastatic breast cancer. Expert Opin. Drug Metab. Toxicol. 17 , 139–152 (2021).
Matasar, M. J. et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(21)00145-5 (2021).
Leenhardt, F., Alexandre, M. & Jacot, W. Alpelisib for the treatment of PIK3CA-mutated, hormone receptor-positive, HER2-negative metastatic breast cancer. Expert Opin. Pharmacother. 22 , 667–675 (2021).
Garrido-Castro, A. C. et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 22 , 120 (2020).
Kaklamani, V. G., Richardson, A. L. & Arteaga, C. L. Exploring biomarkers of phosphoinositide 3-kinase pathway activation in the treatment of hormone receptor positive, human epidermal growth receptor 2 negative advanced breast cancer. Oncologist 24 , 305–312 (2019).
Dreyling, M. et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 35 , 3898–3905 (2017).
Buckley, A. M., Lynam-Lennon, N., O’Neill, H. & O'Sullivan, J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat. Rev. Gastroenterol. Hepatol. 17 , 298–313 (2020).
Verma, N. & Tiku, A. B. Significance and nature of bystander responses induced by various agents. Mutat. Res 773 , 104–121 (2017).
Hendijani, F. & Javanmard, S. H. Dual protective and cytotoxic benefits of mesenchymal stem cell therapy in combination with chemotherapy/radiotherapy for cancer patients. Crit. Rev. Eukaryot. Gene Expr. 25 , 203–207 (2015).
Tao, Y. et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur. J. Cancer 141 , 21–29 (2020).
Kunos, C. A. et al. Randomized phase II trial of triapine-cisplatin-radiotherapy for locally advanced stage uterine cervix or vaginal cancers. Front Oncol. 9 , 1067 (2019).
Ciszewski, W. M., Tavecchio, M., Dastych, J. & Curtin, N. J. DNA-PK inhibition by NU7441 sensitizes breast cancer cells to ionizing radiation and doxorubicin. Breast Cancer Res. Treat. 143 , 47–55 (2014).
Sun, X. et al. Identification and characterization of a small inhibitory peptide that can target DNA-PKcs autophosphorylation and increase tumor radiosensitivity. Int J. Radiat. Oncol. Biol. Phys. 84 , 1212–1219 (2012).
Wu, Y. H. et al. A novel histone deacetylase inhibitor TMU-35435 enhances etoposide cytotoxicity through the proteasomal degradation of DNA-PKcs in triple-negative breast cancer. Cancer Lett. 400 , 79–88 (2017).
Klein, C. et al. Overcoming hypoxia-induced tumor radioresistance in non-small cell lung cancer by targeting DNA-dependent protein kinase in combination with carbon ion irradiation. Radiat. Oncol. 12 , 208 (2017).
Dong, W. et al. End processing factor APLF promotes NHEJ efficiency and contributes to TMZ- and ionizing radiation-resistance in glioblastoma cells. Onco Targets Ther. 13 , 10593–10605 (2020).
Zhou, W. et al. Thymine DNA glycosylase-regulated TAZ promotes radioresistance by targeting nonhomologous end joining and tumor progression in esophageal cancer. Cancer Sci. 111 , 3613–3625 (2020).
Cleary, J. M., Aguirre, A. J., Shapiro, G. I. & D’Andrea, A. D. Biomarker-guided development of DNA repair inhibitors. Mol. Cell 78 , 1070–1085 (2020).
Helleday, T. et al. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 8 , 193–204 (2008).
Jin, J. et al. Methylation-associated silencing of miR-193b improves the radiotherapy sensitivity of esophageal cancer cells by targeting cyclin D1 in areas with zinc deficiency. Radiother. Oncol. 150 , 104–113 (2020).
Ali, R. M. M., McIntosh, S. A. & Savage, K. I. Homologous recombination deficiency in breast cancer: Implications for risk, cancer development, and therapy. Genes Chromosomes Cancer 60 , 358–372 (2020).
Belli, C., Duso, B. A., Ferraro, E. & Curigliano, G. Homologous recombination deficiency in triple negative breast cancer. Breast 45 , 15–21 (2019).
Oei, A. L. et al. Enhancing synthetic lethality of PARP-inhibitor and cisplatin in BRCA-proficient tumour cells with hyperthermia. Oncotarget 8 , 28116–28124 (2017).
Parsels, L. A. et al. Combinatorial efficacy of olaparib with radiation and ATR inhibitor requires PARP1 protein in homologous recombination proficient pancreatic cancer. Mol. Cancer Ther. 20 , 0365 (2020).
Estrada-Bernal, A. et al. MEK inhibitor GSK1120212-mediated radiosensitization of pancreatic cancer cells involves inhibition of DNA double-strand break repair pathways. Cell Cycle 14 , 3713–3724 (2015).
Makita, N. et al. Inhibitory effects of valproic acid in DNA double-strand break repair after irradiation in esophageal squamous carcinoma cells. Oncol. Rep. 34 , 1185–1192 (2015).
Shoji, M. et al. Valproic acid, a histone deacetylase inhibitor, enhances radiosensitivity in esophageal squamous cell carcinoma. Int J. Oncol. 40 , 2140–2146 (2012).
Urick, M. E. et al. Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition. Clin. Cancer Res. 17 , 5038–5047 (2011).
Wu, C. et al. Phase I Trial of trametinib with neoadjuvant chemoradiation in patients with locally advanced rectal cancer. Clin. Cancer Res. 26 , 3117–3125 (2020).
Chang, L. et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 5 , e1437 (2014).
Goglia, A. G. et al. Identification of novel radiosensitizers in a high-throughput, cell-based screen for DSB repair inhibitors. Mol. Cancer Ther. 14 , 326–342 (2015).
Young, M. J. Off-target effects of drugs that disrupt human mitochondrial DNA maintenance. Front Mol. Biosci. 4 , 74 (2017).
Muresanu, C. et al. Updated understanding of cancer as a metabolic and telomere-driven disease, and proposal for complex personalized treatment, a hypothesis. Int. J. Mol. Sci . 21 , 6521 (2020).
Saini, N. & Gordenin, D. A. Somatic mutation load and spectra: A record of DNA damage and repair in healthy human cells. Environ. Mol. Mutagen 59 , 672–686 (2018).
Hadwiger, L. A. & Tanaka, K. DNA damage and chromatin conformation changes confer nonhost resistance: a hypothesis based on effects of anti-cancer agents on plant defense responses. Front Plant Sci. 9 , 1056 (2018).
Sharma, A., Sharma, K. L., Bansal, C. & Kumar, A. Updates on "Cancer Genomics and Epigenomics". World J. Clin. Oncol. 11 , 890–897 (2020).
Hasanvand, M. et al. Dose-response meta-analysis of arsenic exposure in drinking water and intelligence quotient. J. Environ. Health Sci. Eng. 18 , 1691–1697 (2020).
Schiewer, M. J. et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2 , 1134–1149 (2012).
Mills, K. D. Tumor suppression: putting p53 in context. Cell Cycle 12 , 3461–3462 (2013).
Kraljevic, S. et al. Casting light on molecular events underlying anti-cancer drug treatment: what can be seen from the proteomics point of view? Cancer Treat. Rev. 32 , 619–629 (2006).
Papaleo, E. Investigating Conformational dynamics and allostery in the p53 DNA-binding domain using molecular simulations. Methods Mol. Biol. 2253 , 221–244 (2021).
Sun, Q. et al. Therapeutic Implications of p53 Status on Cancer Cell Fate Following Exposure to Ionizing Radiation and the DNA-PK Inhibitor M3814. Mol. Cancer Res 17 , 2457–2468 (2019).
Lajud, S. A. et al. Dual disruption of DNA repair and telomere maintenance for the treatment of head and neck cancer. Clin. Cancer Res. 20 , 6465–6478 (2014).
Patnaik, A. et al. Safety and clinical activity of a new anti-PD-L1 antibody as monotherapy or combined with targeted therapy in advanced solid tumors: the PACT phase Ia/Ib trial. Clin Cancer Res. 27 , 1267–1277 (2020).
Herbst, R. S. et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 383 , 1328–1339 (2020).
Powles, T. et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383 , 1218–1230 (2020).
Powles, T. et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 21 , 1574–1588 (2020).
Voltin, C. A. et al. Early response to first-line anti-PD-1 treatment in hodgkin lymphoma: a PET-based analysis from the prospective, randomized phase II NIVAHL trial. Clin. Cancer Res. 27 , 402–407 (2020).
Rischin, D. et al. PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol. Oncol. 159 , 322–328 (2020).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383 , 2207–2218 (2020).
Oaknin, A. et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol . 6 , 1766–1722 (2020).
Schouten, R. D. et al. Nivolumab in pre-treated advanced non-small cell lung cancer: long term follow up data from the Dutch expanded access program and routine clinical care. Transl. Lung Cancer Res. 9 , 1736–1748 (2020).
Calabro, L. et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 14 , 1104–1111 (2013).
Kamath, S. D. et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist 25 , e808–e815 (2020).
Eikesdal, H. P. et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.11.009 (2020).
Tampaki, E. C. et al. Efficacy and safety of neoadjuvant treatment with bevacizumab, liposomal doxorubicin, cyclophosphamide and paclitaxel combination in locally/regionally advanced, HER2-negative, grade III at premenopausal status breast cancer: a phase II study. Clin. Drug Investig. 38 , 639–648 (2018).
Leone, J. P. et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res. 22 , 131 (2020).
Litton, J. K. et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann. Oncol. 31 , 1526–1535 (2020).
Dieras, V. et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21 , 1269–1282 (2020).
Vinayak, S. et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 5 , 1132–1140 (2019).
O’Shaughnessy, J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364 , 205–214 (2011).
Riches, L. C. et al. Pharmacology of the ATM Inhibitor AZD0156: potentiation of irradiation and olaparib responses preclinically. Mol. Cancer Ther. 19 , 13–25 (2020).
Gatti-Mays, M. E. et al. A phase II single arm pilot study of the CHK1 inhibitor prexasertib (LY2606368) in BRCA wild-type, advanced triple-negative breast cancer. Oncologist 25 , 1013–e1824 (2020).
Oza, A. M. et al. Patient-centered outcomes in ARIEL3, a Phase III, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma. J. Clin. Oncol. 38 , 3494–3505 (2020).
Mirza, M. R. et al. Long-term safety in patients with recurrent ovarian cancer treated with niraparib versus placebo: Results from the phase III ENGOT-OV16/NOVA trial. Gynecol. Oncol. 159 , 442–448 (2020).
Zsiros, E. et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: a phase 2 nonrandomized clinical trial. JAMA Oncol. 7 , 78–85 (2021).
Friedlander, M. et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol . https://doi.org/10.1016/S1470-2045(21)00098-X (2021).
De Simone, M. et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with oxaliplatin, cisplatin, and doxorubicin in patients with peritoneal carcinomatosis: an open-label, single-arm, phase ii clinical trial. Biomedicines . 8 , 102 (2020).
Iyer, G. et al. Multicenter prospective phase II trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J. Clin. Oncol. 36 , 1949–1956 (2018).
Colombo, R. et al. Neoadjuvant short-term intensive intravesical mitomycin C regimen compared with weekly schedule for low-grade recurrent non-muscle-invasive bladder cancer: preliminary results of a randomised phase 2 study. Eur. Urol. 62 , 797–802 (2012).
Choueiri, T. K. et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J. Clin. Oncol. 32 , 1889–1894 (2014).
Zhang, T. et al. The ATM inhibitor KU55933 sensitizes radioresistant bladder cancer cells with DAB2IP gene defect. Int J. Radiat. Biol. 91 , 368–378 (2015).
Segovia, C. et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat. Med. 25 , 1073–1081 (2019).
Munemoto, Y. et al. A phase II trial to evaluate the efficacy of panitumumab combined with fluorouracil-based chemotherapy for metastatic colorectal cancer: the PF trial. Cancer Chemother. Pharmacol. 81 , 829–838 (2018).
Kim, S. Y. et al. A phase II study of S-1 plus irinotecan and oxaliplatin in heavily-treated patients with metastatic colorectal cancer. Invest N. Drugs 27 , 269–274 (2009).
Ando, K. et al. Efficacy and feasibility of S-1 plus oxaliplatin (C-SOX) for treating patients with stage III colon cancer (KSCC1303): final analysis of 3-year disease-free survival. Int J. Clin. Oncol. 25 , 1115–1122 (2020).
Chen, E. X. et al. A Phase I study of olaparib and irinotecan in patients with colorectal cancer: Canadian Cancer Trials Group IND 187. Invest N. Drugs 34 , 450–457 (2016).
Gallia, G. L. et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial. Neurooncol Adv. 3 , vdaa154 (2021).
Wang, Y. et al. A randomized, double-blind, placebo-controlled study of B-cell lymphoma 2 homology 3 mimetic gossypol combined with docetaxel and cisplatin for advanced non-small cell lung cancer with high expression of apurinic/apyrimidinic endonuclease 1. Invest N. Drugs 38 , 1862–1871 (2020).
Provencio, M. et al. Phase II clinical trial with metronomic oral vinorelbine and tri-weekly cisplatin as induction therapy, subsequently concomitant with radiotherapy (RT) in patients with locally advanced, unresectable, non-small cell lung cancer (NSCLC). Analysis of survival and value of ctDNA for patient selection. Lung Cancer 153 , 25–34 (2021).
Owonikoko, T. K. et al. Phase 2 study of talazoparib in patients with homologous recombination repair-deficient squamous cell lung cancer: Lung-MAP substudy S1400G. Clin. Lung Cancer 10 , S1525-7304(21)00004-8(2021).
Farago, A. F. et al. Combination olaparib and temozolomide in relapsed small-cell. Lung Cancer Cancer Discov. 9 , 1372–1387 (2019).
Royer, B. et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase ii study of the intergroupe francophone du myelome. J. Clin. Oncol. 34 , 2125–2132 (2016).
van Tilburg, C. M. et al. Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma, or leukemia. Clin. Epigenetics. 11 , 188 (2019).
Li, X. et al. High PARP-1 expression predicts poor survival in acute myeloid leukemia and PARP-1 inhibitor and SAHA-bendamustine hybrid inhibitor combination treatment synergistically enhances anti-tumor effects. EBioMed. 38 , 47–56 (2018).
Ghelli Luserna Di Rora, A. et al. Prexasertib, a Chk1/Chk2 inhibitor, increases the effectiveness of conventional therapy in B-/T- cell progenitor acute lymphoblastic leukemia. Oncotarget 7 , 53377–53391 (2016).
Ikeda, M. et al. A Phase I/II trial of continuous hepatic intra-arterial infusion of 5-fluorouracil, mitoxantrone and cisplatin for advanced hepatocellular carcinoma. Jpn J. Clin. Oncol. 47 , 512–519 (2017).
El Dika, I. et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med 9 , 7453–7459 (2020).
Bridgewater, J. A. et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 21 , 398–411 (2020).
Correction to Lancet Oncol 2019; 20: 663-73. Lancet Oncol . 20 , e242, (2019).
Gabrielson, A. et al. Phase II study of temozolomide and veliparib combination therapy for sorafenib-refractory advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 76 , 1073–1079 (2015).
Niesvizky, R. et al. Phase 1/2 study of cyclin-dependent kinase (CDK)4/6 inhibitor palbociclib (PD-0332991) with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. Leuk. Lymphoma 56 , 3320–3328 (2015).
Khashab, T. et al. Long-term overall- and progression-free survival after pentostatin, cyclophosphamide and rituximab therapy for indolent non-Hodgkin lymphoma. Br. J. Haematol. 185 , 670–678 (2019).
Cohen, J. B. et al. A phase II study of bortezomib added to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in patients with previously untreated indolent non-Hodgkin’s lymphoma. Br. J. Haematol. 171 , 539–546 (2015).
Schaefer, N. G., James, E. & Wahl, R. L. Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma. Nucl. Med Commun. 32 , 1046–1051 (2011).
Flaig, T. W. et al. A phase II trial of dexamethasone, vitamin D, and carboplatin in patients with hormone-refractory prostate cancer. Cancer 107 , 266–274 (2006).
Buonerba, C. et al. Phase II trial of cisplatin plus prednisone in docetaxel-refractory castration-resistant prostate cancer patients. Cancer Chemother. Pharmacol. 67 , 1455–1461 (2011).
Pishvaian, M. J. et al. A Phase I/II Study of Veliparib (ABT-888) in Combination with 5-Fluorouracil and Oxaliplatin in Patients with Metastatic Pancreatic Cancer. Clin. Cancer Res. 26 , 5092–5101 (2020).
Okano, N. et al. Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J. Clin. Oncol. 25 , 1835–1843 (2020).
Wang-Gillam, A. et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 108 , 78–87 (2019).
Bendell, J. et al. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann. Oncol. 26 , 804–811 (2015).
Download references
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82073486, U1803124, 31870847, and 81842033).
Author information
Authors and affiliations.
Department of Occupational and Environmental Health, Xiangya School of Public Health, Central South University, Changsha, Hunan, China
Ruixue Huang
Department of Radiation Biology, Beijing Key Laboratory for Radiobiology, Beijing Institute of Radiation Medicine, AMMS, Beijing, China
Ping-Kun Zhou
You can also search for this author in PubMed Google Scholar
Contributions
P.K.Z. contributed to the study concept and critical design of the study. R.X.H. went through the literatures and analyzed and interpreted the data. R.X.H. wrote the initial manuscript, P.K.Z. critically reviewed and revised the final manuscript. All the authors read and approved the manuscript.
Corresponding author
Correspondence to Ping-Kun Zhou .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Huang, R., Zhou, PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Sig Transduct Target Ther 6 , 254 (2021). https://doi.org/10.1038/s41392-021-00648-7
Download citation
Received : 24 January 2021
Revised : 28 April 2021
Accepted : 13 May 2021
Published : 09 July 2021
DOI : https://doi.org/10.1038/s41392-021-00648-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Identification of a dna damage repair-related lncrna signature for predicting the prognosis and immunotherapy response of hepatocellular carcinoma.
- Chunyan Zhang
BMC Genomics (2024)
Pancancer analysis of the prognostic and immunological role of FANCD2: a potential target for carcinogenesis and survival
- Xiangzhen Fan
BMC Medical Genomics (2024)
Unveiling the mechanisms and challenges of cancer drug resistance
- Sameer Ullah Khan
- Kaneez Fatima
- Fayaz Malik
Cell Communication and Signaling (2024)
Development and validation of a cuproptosis-related prognostic model for acute myeloid leukemia patients using machine learning with stacking
- Xichao Wang
- Suning Chen
Scientific Reports (2024)
Single-mitosis dissection of acute and chronic DNA mutagenesis and repair
- Paul Adrian Ginno
- Helena Borgers
- Duncan T. Odom
Nature Genetics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

DNA repair mechanisms
Jul 18, 2014
1.18k likes | 3.48k Views
DNA repair mechanisms. Dr.S.Chakravarty MBBS , MD. DNA Damage . During replication – corrected by proof reading Environmental factors : physical and chemical factors – corrected by DNA repair mechanisms. Already proof reading !! Why Repair ?. Learning objectives.
Share Presentation
- main control point
- cdk4 complex
- tumor suppressor gene p53
- blood vessels

Presentation Transcript
DNA repair mechanisms Dr.S.Chakravarty MBBS , MD
DNA Damage • During replication – corrected by proof reading • Environmental factors : physical and chemical factors – corrected by DNA repair mechanisms.
Already proof reading !!Why Repair ?
Learning objectives • Outline the Causes and Types of DNA damage that can occur in the living cell • Describe in detail the 4 types of DNA repair mechanisms • List the Key enzymes associated with DNA damage repair mechanisms and their defects
Types of DNA damage 1. Single base alteration : • Deamination of cytosine to uracil • Depurination • Demination of adenine to hypoxanthine Deamination of Cytosine to Uracil Mutations , wrong reading of the Genetic code
Two base alteration : • UV light induced thymine – thymidine dimers Adjacent Thymidine dimers
3. Chain breaks: • Ionizing radiation • Radioactive disintegration • Oxidative free radicals 4. Cross linkage : Chemicals - vinyl chloride and hydrogen peroxide, and Polycyclic aromatic hydrocarbons found in smoke
DNA repair • Mismatch repair • Base excision repair • Nucleotide excision repair • Double stranded break repair
Mismatch repair • Mismatch repair is strand specific. • G2 PHASE OF CELL CYCLE …CORRECTS DNA REPLICATION ERRORS • Mismatch repair machinery can distinguish between template and newly synthesized strands. HOW DOES THIS HAPPEN ? • An enzyme called as the DAM methylasemethylates DNA at N6 position for all adenines within 5’ GATC sequences • Newly synthesized strands are not methylated immediately. This allows to distinguish b/w the two.
PankajaNaik , Biochemistry , 3rd Edition
Harper’s Biochemistry 26th edition
Faulty mismatch repair • HNPCC – hereditary non polyposis colon cancer (LYNCH syndrome) • Autosomal Dominant • 60% due to defect in hMSH-2 gene or hMLH1 • Mutations happen in microsatellite repeat region leading to instability • Risk of other cancers (Liver) Microsatellites are di and tri nucleotide repeats throughout the genome in non coding sections.(Normal phenomenon) Microsatellite instability :- Cells which lack mismatch repair The number of microsatellites differ in the mutated cells
Base excision repair • Correction for Spontaneous deamination or depurination. • G1 phase of cell cycle • Identified by DNA glycosylase and removed. • The attached sugar and phosphate are removed by Apurinic or Apyrimidinic endonucleases. Harper’s Biochemistry 26th edition
Action of DNA glycosylases. These enzymes hydrolyze the glycosidic bond of their corresponding altered base (red)
Nucleotide excision repair • Mechanism used to replace DNA upto 30 base pairs. • Excision endonuclease (exinuclease) identifies and cleaves a fragment of DNA of around 20 -25 base pairs. • G1 phase of cell cycle
Nucleotide excision repair EXCISION ENDONUCLEASE (EXCINUCLEASE)
Xerodermapigmentosa • Autosomal recessive • Defect in nucleotide excision repair (XPA and XPC) gene • Extreme UV light sensitivity • Excessive freckling • Corneal ulcerations • Multiple skin cancers
Double strand break repair • Free radical and ionizing radiation cause double strand breaks. • Ku protein and DNA dependent protein kinaseare involved in repair. • It is also physiologically seen in immunoglobulin gene rearrangement.
Non homologous end-joining (NHEJ).
Fanconi anemia • FA is the result of a genetic defect in a cluster of proteins responsible for DNA repair. • 1 per 350,000 births with a higher frequency in Ashkenazi Jews and Afrikaners in South Africa • Stunted growth • Skeletal deformities – digits • Café-au-lait spots • Myelodysplastic syndromes • Acute myelogenous leukemia • Bone marrow failure
Bloom syndrome • Characterized by a high frequency of breaks and rearrangements in an affected chromosomes. • Mutations in the BLMgene • Short stature, characteristic long face, butterfly shaped rash, micrognathia, hypogonadism
Ataxia telengiectasia • Defect in the ATM gene, responsible for recognizing and correcting errors in duplicating DNA when cells divide. • Cerebellar ataxia • Chromosomal instability • Telengiectasias of blood vessels • Immunodeficiency
Mechanism of DNA repair Type of Defect Repair mechsnism: Mismatch repair Base excision repair Nucleotide excision repair Double stranded break repair Copying errors during replication Spontaneous chemical or radiation damage to a single base Chemical or radiation to a segment Ionising radiation, chemotheray and free radicals
Autosomal recessive:F - Fanconi anemiaA - Ataxia telangiectasiaB - Bloom syndromeX - Xerodermapigmentosum Autosomal dominant:C- hereditary non-polyposisColorectal Cancer
Question 1 Patients with hereditary nonpolyposis colon cancer [HNPCC(114500)] have genes with microsatellite instability, that is, many regions containing abnormal, small loops of unpaired DNA. This is a result of a mutation affecting a. Mismatch repair b. Chain break repair c. Base excision repair d. Depurination repair e. Nucleotide excision repair
Question 2 Following ultraviolet damage of DNA in skin a. A specific excinuclease detects damaged areas b. Purinedimers are formed c. Both strands are cleaved d. Endonuclease removes the strand e. DNA hydrolysis does not occur
Question 3 Xerodermapigmentosum is an inherited human skin disease that causes a variety of phenotypic changes in skin cells exposed to sunlight. The molecular basis of the disease appears to be Rapid water loss caused by defects in the cell membrane permeability b. The inactivation of temperature-sensitive transport enzymes in sunlight c. The induction of a virulent provirus on ultraviolet exposure d. The inability of the cells to synthesize carotenoid-type compounds e. A defect in an excision-repair system that removes thymine dimers from DNA
Question 4 • Dyskeratosis congenital is a genetic condition with impaired proliferation of stem cells. The defect has been traced to an inadequate production of an enzyme required for chromosome duplication in nuclei of dividing cells. This enzyme contains a single stranded RNA.What is the deficient enzyme? • A) DNA POLYMEASE δ • B)DNA LIGASE • C)PRIMASE • D)TELOMERASE • E)DNA POLYMERASE γ
Cell Cycle and its importance
Learning Objectives • Briefly describe the stages of cell cycle and its check points • Explain the importance of P53 and Rb proteins in cell cycle regulation
Cell cycle CELLULAR DIVISION Interphase - Cell spends almost 90% of its time in this phase
How long does the cell cycle last? • Well, it depends on the cell type !! • Stem cells, embryonic cells- a few hours • Some cells divide very slowlyNeurons • Some cells divide when inducedLymphocytes
Stages of cell cycle • G0 stage : • Post mitotic stage • Quiescent or senescent stage • Permanent stage for fully differentiated cells: Ex: Neurons, Beta cells. • Interphase : G1 – Phase m RNA transcription S – Phase G2 – Phase Replicated DNA is assessed Protein synthesis Preparatory phase: Accumulation of Nutrients Replication phase
G1 phase • Ending of previous mitosis to beginning of DNA synthesis. • Increased biosynthetic activity of the cells • Rapid growth • Enzymes required for the S phase are synthesized. • Average Duration - 6-12 hours
S phase • DNA synthesis • Doubling of DNA content – replication • Low protein synthesis. • but histone production is normal. • Average Time – 6-8 hours
G2 phase • Microtubule formation: • Further protein and organelle synthesis • Average Time – 3-4 hrs M phase • Divided into Prophase, Metaphase, Anaphase, Telophase – karyokinesis • Cytokinesis – division of cytoplasm
The Check Points
Regulation of cell cycle • Cyclins - Regulatory subunits • Cyclin dependent kinases – catalytic subunits • phosphorylation of proteins which drives the cell cycle.
Regulators of cell cycle:
Cell cycle Control Cyclin D binds to existing CDK4, forming the active cyclin D-CDK4 complex. Cyclin D-CDK4 complex in turn phosphorylates the retinoblastoma susceptibility protein (Rb) hyperphosphorylatedRbdissociates from the E2F/DP1/Rbcomplex (which was bound to the E2F responsive genes, effectively "blocking" them from transcription), activating E2F G2/M check point M phase Rb Cyclin B CDK-1 (+) Cyclin D CDK 6,4 (+) G1 G2 Rb DISSOCIATES P (+) Cyclin E CDK-2 S (+) G1/S check point Cyclin A CDK-2
Inhibitors of cell cycle: Central role of p53 INK4a/ARF locus ARF tumor suppressor (-) (-) MDM-2 RbPhosphorylation (-) ATM gene (+) (+) p53 BAX, BAD, BIM (+) (+) (+) p21 p27 p57 Apoptosis Inhibition of cell cycle MDM -murine double minute ATM-Ataxia telangiectasia mutated
TUMOR SUPPRESSOR GENE P53 p53 : most common gene involved in cancers • Tumor suppressor gene altered in many cancers • Causes cell arrest and apoptosis • Stimulates apoptosis by stimulating BAX gene. • MDM-2 regulates p53 by feedback. • Main control point at G1/S checkpoint and component of G2/M checkpoint
Inhibitors of cell cycle CIP/KIP family (cyclin-dependent kinases inhibitors) INK4/ARF family Examples – p21,p27 and p57 Blocks the cell cycle by binding to Cyclin /CDK complexes. Activated by p53 – tumor suppressor gene Examples - p16 and p14 Promotes the inhibitory effects of Rb gene. Increase p53 levels by inhibiting MDM-2 protein.
ATM gene • Ataxia telengiectasia mutated gene: • Activated by DNA breaks in cell cycle • Acts through p53 at G1/S phase • At G2/M phase – Disrupts cyclin B –CDK-1 complex. • ATM additionally phosphorylates MDM2 and p53
Colorectal Tumorigenesis 1When Ras(Rat sarcoma) is 'switched on' by incoming signals, it subsequently switches on other proteins, which ultimately turn on genes involved in cell growth, differentiation and survivalunintended and overactive signalling inside the cell, even in the absence of incoming signals 2
Colorectal tumorigenesis
- More by User

710 views • 48 slides

DNA Repair.
472 views • 12 slides

DNA repair. Mutación y reparación del DNA. Why repair DNA?. Replication error. OH. H +. 1. Errors in DNA replication 2. Endogenous DNA damage and mutagens 3. Environmental insults to DNA 4. Un-repaired damage leads to: -mistakes in RNA/protein synthesis
723 views • 45 slides
DNA repair. CMB Pp 209-215. DNA repair. Damaged DNA must be repaired If the damage is passed on to subsequent generations, then we use the evolutionary term - mutation . It must take place in the germ cells - the gametes - eggs and sperm
493 views • 27 slides

DNA Repair. The Ultimate Antiaging Tool. By: LifeSpan International, Inc. www.lifespaninternational.com. DNA IN THE “NEWS”. On The Pill: Inner and Outer Beauty Products; NY Post – March 6, 2005
474 views • 33 slides

DNA Repair. DNA Damage Tolerance and Repair. 1-Dealing with Problems occurring during DNA replication. • Mutations resulting from errors made during DNA replication Mismatch Repair Pathway . • Ribonucleotides incorporated during DNA replication .
743 views • 28 slides

Chapter 12 Molecular Mechanisms of Mutation and DNA Repair
Chapter 12 Molecular Mechanisms of Mutation and DNA Repair. Mutations . A mutation is any heritable change in the genetic material Mutations are classified in a variety of ways Most mutations are spontaneous — they are random, unpredictable events
1.16k views • 61 slides

DNA metabolism DNA replication DNA repair DNA recombination
DNA metabolism DNA replication DNA repair DNA recombination. Map of E.coli chromosome. Meselson-Stahl experiment – DNA replication is semi conservative. DNA replication. Nucleases (eg DNases) degrade DNA
680 views • 25 slides

DNA Repair. Uracil-DNA Glycosylase. DNA is continually assaulted by damaging agents (oxygen free radicals, ultraviolet light, toxic chemicals). Fortunately, the cell has multiple mechanisms to identify and correct mutations. Yeasts have 50 different enzymes involved in DNA repair.
625 views • 25 slides

MUTATIONS AND DNA REPAIR MECHANISMS
ECDA August 2009. MUTATIONS AND DNA REPAIR MECHANISMS.
494 views • 30 slides

Biological repair mechanisms
Biological repair mechanisms.
604 views • 17 slides

DNA Repair. Dr Derakhshandeh-Peykar, PhD. For DNA. information must be transmitted intact to daughter cells. Accuracy is maintained by:. 1- High fidelity in replication 3’- exonuclease activity of DNA pol I Uracil-DNA N-glycosylase pathway (corrects mutations from deamination of
707 views • 40 slides

Repair mechanisms
Repair mechanisms. 1. Reversal of damage 2. Excision repair 3. Mismatch repair 4. Recombination repair 5. Error-prone repair 6. Restriction-modification systems. 1. Reversal of damage. Enzymatically un-do the damage a) Photoreactivation b) Removal of methyl groups. 5. 5. 6. 6.
1.13k views • 26 slides

DNA Repair. Drosophila BLM in Double-Strand Break Repair. Bloom Syndrome: Men & Flys. BLM is Bloom Syndrome Gene Predisposition to cancer BLM is a RecQ-type Helicase Involved in synthesis dependent DS-break repair
254 views • 9 slides

DNA Repair. Dr Derakhshandeh. For DNA. information must be transmitted intact to daughter cells. Accuracy is maintained by:. 1- High fidelity in replication 3’- exonuclease activity of DNA pol I Uracil-DNA N-glycosylase pathway (corrects mutations from deamination of
780 views • 38 slides

DNA REPAIR. DNA is the only biological molecule that is repaired DNA damage Alteration to the chemical structure of DNA Mutation Change in the sequence of DNA. DNA REPAIR. NATURE OF DNA DAMAGE. Loss of bases Modification of bases Inter/intra-strand crosslinks
1.26k views • 36 slides

Molecular Mechanisms of Mutations and DNA Repair
Molecular Mechanisms of Mutations and DNA Repair. Genetics Spring 2014. Outline. Types of Mutation and their Molecular Basis.
713 views • 30 slides

DNA Recombination Mechanisms
DNA Recombination Mechanisms. AHMP 5405. Objectives. List the major classes of mobile genetic elements (we went over this before) Describe the process of general recombination Diagram the process of gene conversion via Holliday junctions
590 views • 40 slides

12 Molecular Mechanisms of Mutation and DNA Repair
12 Molecular Mechanisms of Mutation and DNA Repair. Mutations . A mutation is any heritable change in the genetic material Mutations are classified in a variety of ways Most mutations are spontaneous : they are random, unpredictable events
589 views • 39 slides

DNA Repair. Stable, but fragile. Types of damage experience by DNA Ionizing radiation can break DNA backbone chemicals, some made by cell metabolism ultraviolet radiation: pyrimidine dimers thermal energy can depurinate adenine & guanine warm-blooded mammals lose ~10,000 bases/day.
424 views • 23 slides

BIOCHEMISTRY
638 views • 49 slides

DNA repair. Lecture 13 Pp 209-215. DNA repair. Damaged DNA must be repaired If the damage is passed on to subsequent generations, then we use the evolutionary term - mutation . It must take place in the germ cells - the gametes - eggs and sperm
330 views • 27 slides
- Search Menu
- Advance Articles
- Collections
- Focus Collections
- Teaching Tools in Plant Biology
- Browse by cover
- High-Impact Research
- Author Guidelines
- Quick and Simple Author Support
- Focus Issues Call for Papers
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Why Publish with Us?
- About The Plant Cell
- About The American Society of Plant Biologists
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
From the archives: on dna maintenance – swi/snf chromatin remodeling complexes, dna damage repair, and transposon excision repair mechanisms.
- Article contents
- Figures & tables
- Supplementary Data
Peng Liu, From the archives: On DNA maintenance – SWI/SNF chromatin remodeling complexes, DNA damage repair, and transposon excision repair mechanisms, The Plant Cell , 2024;, koae127, https://doi.org/10.1093/plcell/koae127
- Permissions Icon Permissions
Article PDF first page preview
Email alerts, citing articles via.
- Recommend to Your Librarian
- Advertising & Corporate Services
- Awards & Funding
- Plant Science Today
- Plant Biology Meeting
- Meeting Management Services
- Plant Science Research Weekly
- Taproot: A Plantae Podcast
Affiliations
- Online ISSN 1532-298X
- Print ISSN 1040-4651
- Copyright © 2024 American Society of Plant Biologists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice

This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Cockayne syndrome: New insights into cellular DNA repair mechanism
Cockayne syndrome is a severe autosomal recessive disorder caused by defective DNA repair mechanisms. People with the disease have much reduced life expectancy and suffer from facial deformities; growth failure; neurological, cognitive, and sensory impairments; bone, joint, and muscle deformities; kidney problems; and premature aging. Like xeroderma pigmentosum (XP), Cockayne syndrome (CS) is a disease where elements of nucleotide excision repair (NER) do not work properly. The purpose of this repair mechanism is to remove DNA damage caused by ultraviolet (UV) light, chemicals, and various other environmental factors.
Researchers from the group of biochemist Professor Julian Stingele from LMU's Gene Center Munich have now uncovered important details about the role of the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. These genes encode two enzymes associated with DNA repair. The results of their work have been published in the journal Nature Cell Biology . "Our data point to a new, previously unknown function of these two genes and their gene products in the repair of covalent DNA-protein interactions in the course of transcription," reports Stingele, referring to the cytotoxic, biologically undesirable crosslinking of proteins to DNA.
An obstacle for transcription
In collaboration with researchers from the University of Cambridge, the scientists demonstrated that DNA-protein crosslinks present a physical obstacle to further transcription. Arresting transcription brings CS proteins to the blockade sites. "Our results indicate that CSB and CSA then initiate the transcription-coupled repair of the toxic DNA-protein crosslinks," says Stingele. "This previously unrecognized cellular function of CS proteins leads to the marking of the DNA damage -- and thence to its enzymatic breakdown."
The study also revealed that this newly discovered function of CS proteins works independently of classic TC-NER (transcription-coupled nucleotide excision repair) enzymes, which are deployed, among other things, for repairing DNA damage caused by UV light -- and the absence of which leads to xeroderma pigmentosum. "The fact that CS proteins have additional functions is noteworthy. This discovery could help to explain the pathological differences between xeroderma pigmentosum and Cockayne syndrome," says Stingele. CS is a more severe and more multifaceted disorder than XP, with complex and incompletely understood causes. As their next step, Stingele's research group plans to decode the exact process of CS-protein-mediated repair.
- Biochemistry Research
- Biotechnology
- Developmental Biology
- Cell Biology
- New Species
- Biotechnology and Bioengineering
- Molecular biology
- Genetic recombination
Story Source:
Materials provided by Ludwig-Maximilians-Universität München . Note: Content may be edited for style and length.
Journal Reference :
- Christopher J. Carnie, Aleida C. Acampora, Aldo S. Bader, Chimeg Erdenebat, Shubo Zhao, Elnatan Bitensky, Diana van den Heuvel, Avital Parnas, Vipul Gupta, Giuseppina D’Alessandro, Matylda Sczaniecka-Clift, Pedro Weickert, Fatih Aygenli, Maximilian J. Götz, Jacqueline Cordes, Isabel Esain-Garcia, Larry Melidis, Annelotte P. Wondergem, Simon Lam, Maria S. Robles, Shankar Balasubramanian, Sheera Adar, Martijn S. Luijsterburg, Stephen P. Jackson, Julian Stingele. Transcription-coupled repair of DNA–protein cross-links depends on CSA and CSB . Nature Cell Biology , 2024; DOI: 10.1038/s41556-024-01391-1
Cite This Page :
Explore More
- Fossil Frogs Share Their Skincare Secrets
- Fussy Eater? Most Parents Play Short Order Cook
- Precise Time Measurement: Superradiant Atoms
- Artificial Cells That Act Like Living Cells
- Affordable and Targeted Anticancer Agent
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
Trending Topics
Strange & offbeat.
- Open access
- Published: 24 April 2024
Modulating DNA damage response in uveal melanoma through embryonic stem cell microenvironment
- Yingxu Zhang 1 ,
- Jinbiao Zheng 1 ,
- Minyu Chen 1 ,
- Shulun Zhao 1 ,
- Ruiqian Ma 1 ,
- Wenwei Chen 1 &
- Jiahui Liu 1
BMC Cancer volume 24 , Article number: 519 ( 2024 ) Cite this article
Metrics details
Uveal melanoma (UVM) is the most common primary intraocular tumor in adults, with a median survival of 4–5 months following metastasis. DNA damage response (DDR) upregulation in UVM, which could be linked to its frequent activation of the PI3K/AKT pathway, contributes to its treatment resistance. We have reported that embryonic stem cell microenvironments (ESCMe) can revert cancer cells to less aggressive states through downregulation of the PI3K signaling, showing promise in modulating the DDR of UVM.
Since nonhomologous end joining (NHEJ) is the main DNA repair mechanism in UVM, this study utilized gene expression analysis and survival prognosis analysis to investigate the role of NHEJ-related genes in UVM based on public databases. Xenograft mouse models were established to assess the therapeutic potential of ESC transplantation and exposure to ESC-conditioned medium (ESC-CM) on key DNA repair pathways in UVM. Quantitative PCR and immunohistochemistry were used to analyze NHEJ pathway-related gene expression in UVM and surrounding normal tissues. Apoptosis in UVM tissues was evaluated using the TUNEL assay.
PRKDC, KU70, XRCC5, LIG4 and PARP1 showed significant correlations with UM progression. High expression of PRKDC and XRCC5 predicted poorer overall survival, while low PARP1 and XRCC6 expression predicted better disease-free survival in UVM patients. ESCMe treatment significantly inhibited the NHEJ pathway transcriptionally and translationally and promoted apoptosis in tumor tissues in mice bearing UVM. Furthermore, ESC transplantation enhanced DDR activities in surrounding normal cells, potentially mitigating the side effects of cancer therapy. Notably, direct cell-to-cell contact with ESCs was more effective than their secreted factors in regulating the NHEJ pathway.
Conclusions
Our results suggest that NHEJ-related genes might serve as prognostic markers and therapeutic targets in UVM. These findings support the therapeutic potential of ESC-based therapy in enhancing UVM sensitivity to radiochemotherapy and improving treatment outcomes while minimizing damage to healthy cells.
Peer Review reports
Uveal melanoma (UVM) is the most common primary intraocular malignancy in adults. As a highly aggressive form of ocular cancer originating in uveal melanocytes, it poses a significant threat to ocular vision and even causes cancer-related death. Although radiation and surgery are effective therapeutic strategies for primary tumors, up to 50% of patients subsequently develop metastasis [ 1 ]. UVM is highly susceptible to metastasis to the liver with a median survival of 6–12 months [ 2 , 3 ]. Despite advancements in treatment modalities, the prognosis for patients with metastatic UVM remains poor because of its treatment resistance, making it one of the few cancers with stable mortality over the past three decades [ 4 ].
Radiation or genotoxic chemotherapy exerts lethal effects by causing DNA damage. Activation of the DNA damage response (DDR) in cancer cells can ensue [ 5 ]. Recent evidence has begun to suggest a consensus on the upregulation of DDR proteins in UVM [ 6 , 7 , 8 ], potentially explaining the well-documented resistance of UVM to radiotherapy and chemotherapy. If DNA repair is incomplete, cancer cells cannot maintain genome integrity and undergo apoptosis and death [ 9 , 10 ], showing that defective DDR may be an attractive target for UVM treatment.
Studies have shown that the embryonic microenvironment can revert cancer cells into non-cancerous or less aggressive states, such as metastatic melanoma cells and myeloid leukemia cells [ 11 , 12 ]. These discoveries have garnered attention regarding the embryonic microenvironment in the field of cancer treatment. As embryonic stem cells (ESCs) can provide and maintain a microenvironment similar to the embryonic microenvironment, they have been shown to have suppressive effects on a variety of tumors [ 13 , 14 ]. Similarly, we have previously established an embryo-like microenvironment using mouse ESCs and indicated that such a microenvironment can effectively induce cell cycle arrest and cell apoptosis of UVM cells both in vitro and in vivo through inhibition of PI3K signaling without damage to normal somatic cells. [ 15 ] As inhibition of the PI3K-AKT pathway can downregulate DNA damage repair factors, increasing the sensitivity of cancer cells to radiochemotherapy [ 16 ], ESCMe has the potential to modulate the DDR of tumors.
Due to the pivotal role of DDR as a determinant of resistance to therapy in UVM [ 7 , 8 , 9 ], inhibition of DNA repair mechanisms can render cancer cells more vulnerable to genotoxic insults, leading to increased DNA damage accumulation and compromised cell survival. This study used bioinformatic methods to detect the expression of the core DDR factors, aiming to screen the genes closely related to the development and prognosis of UVM through clinical big data. Furthermore, we sought to investigate the hypothesis that ESCMe can inhibit the DDR of UVM in vivo using xenograft mouse models. We focused on assessing the impact of ESCMe on key DNA repair pathways in UVM. Additionally, the effects of different ESCMe, including ESCs and ESC-conditioned medium (ESC-CM), on DNA repair modulation were also explored. By elucidating the interactions between ESCMe and DNA repair processes, the findings from this study hold the potential to pave the way for the development of innovative strategies that enhance the efficacy of conventional cancer treatments, such as chemotherapy and radiotherapy.
Gene expression analysis
The UALCAN portal ( http://ualcan.path.uab.edu/analysis-prot.html ) [ 14 ] was utilized to explore transcription in UVM subgroups based on clinicopathological features in the TCGA database. We also collected a publicly available RNA-seq dataset (Accession ID: E-GEOD-22138) [ 15 ] from BioStudies [ 16 ], and explored the relationship between gene expression and metastasis.
Survival prognosis analysis
TIMER ( https://cistrome.shinyapps.io/timer/ ), UALCAN, and GEPIA 2 ( http://gepia2.cancer-pku.cn/#index ) databases can be used to perform survival analysis of specific genes. UVM patients were divided into low and high expression groups based on the values of mRNA expression and validated by survival curves. The methods used were overall survival (OS) and disease-free survival (DFS) with a cutoff of 50%.
Cell cultures
Human uveal melanoma cell (C918) was obtained from KeyGen Biotechnology Company (China) and cultured in RPMI 1640 medium (Corning, USA) supplemented with 1% penicillin–streptomycin (Gibco, Japan) and 10% fetal bovine serum (FBS; Corning). Mouse ESCs were gifts from Professor Peng Xiang from Sun Yat-sen University. ESCs were cultured by knockout Dulbecco’s modified Eagle’s medium (Gibco) containing 10% FBS, 0.1 mM non-essential amino acid (Gibco), 1% GlutaMAX media (Gibco), 0.055 mM 2-mercaptoethanol (Gibco), 5 × 10 5 units leukemia inhibitory factor (Millipore, USA), and 1% penicillin–streptomycin.
Animal experiments
We injected 1 × 10 6 C918 cells subcutaneously into the right flanks of male Balb/c nude mice as previously described [ 17 ]. We collected ESC-CM from cultured ESCs every day and then filtered through a 0.22-mm filter (Millex, USA). ESC-CM was stored at –20 °C. ESCs were collected after culture and resuspended in PBS prior to injection into tumors. Mice were randomized to receive treatment with ESCs, ESC-CM, or phosphate-buffered saline (PBS) when the tumor volume reached 150 mm 3 . ESCs (5 × 10 5 cells/tumor in 200 μl PBS), ESC-CM (200 μl/tumor), or PBS (200 μl/tumor) was administrated at 2 different sites peritumorally every 7 days. When ESCs are differentiated, their ability to reverse tumor is significantly reduced or completely abolished. ESCs with a suicide gene, herpes simplex virus thymidine kinase (HSV-TK), controlled by ganciclovir (GCV), were constructed previously. GCV (Sigma, 2 mg/mouse in 200 μl PBS) was injected intraperitoneally on day 5 of every treatment cycle to eliminate the differentiated ESCs and avoid the formation of teratomas. We have previously demonstrated the elimination of ESCs by GCV. After 3 treatment cycles, the mice were euthanized in an enclosed chamber filled with carbon dioxide, followed by cervical dislocation, and their tumor tissues and surrounding skin tissues were examined.
We used RNeasy Fibrous Tissue Mini kit (Qiagen) to isolate the RNA from the UVM tissues and surrounding skin tissues following the manufacturer’s instructions, then quantified the total RNA by absorption at 260 nm as previously described [ 17 ]. Next, a PrimeScript™ RT Master Mix (Takara, Japan) was used to generate cDNA, which was used for qPCR with SYBR® Premix Ex Taq™ (Takara) in a StepOnePlus thermal cycler (ABI, USA). The GAPDH gene was served as the internal reference.
Immunohistochemical (IHC) staining
Immunohistochemistry was performed for tumor and skin tissues according to the standard procedure using the following primary antibodies: XRCC6 (ab92450; Abcam), LIG4 (ab193353; Abcam), PARP1 (ab191217; Abcam), DNA-PK (#38168; Cell Signaling) and XRCC5 (WH0007520M2; Sigma-Aldrich). Slides were imaged on a Pannoramic Digital Slide Scanner (3DHISTECH, Hungary) and analyzed by Image-Pro Plus 6.0 (Media Cybemetics, USA). Paraffin sections were deparaffinized and hydrated in PBS. Following blocking of endogenous peroxidase, sections were pretreated with appropriate buffer, if necessary. Thereafter, sections were incubated with primary antibody at 4˚C overnight. Following washing three times with PBS, sections were subsequently incubated with MAX-PO. Peroxidase activity was visualized with diaminobenzidine (DAB).
Terminal dUTP nick end‐labelling (TUNEL) assay
TUNEL assay was used to detect apoptosis in tumor tissue. It was performed following the manufacturer’s (KeyGen’s) instructions and analyzed by Image-Pro Plus 6.0.
Statistical analysis
The survival times were compared using Kaplan–Meier analysis, and the p value was calculated using the log-rank test. Statistical analysis was performed using GraphPad Prism software. A 2-tailed unpaired Student t-test was used for analyses comparing only 2 groups, and analysis of variance and an appropriate post hoc test were used for analyses comparing more than 2 groups. Statistical significance was set at p < 0.05.
Correlation between NHEJ pathway-related genes and clinicopathological parameters in patients with UVM
DDR is a complex network of pathways and proteins, and alterations in any components of the DDR may also contribute to the overall DNA repair deficiency observed in UVM. Since nonhomologous end joining (NHEJ) is the main DNA repair mechanism in UVM [ 7 ], we focused on the expression of NHEJ pathway-related genes in UVM subgroups based on individual cancer stages in the data of UALCAN database. As shown in Fig. 1 A, for patients with UVM, DNA-PK (alias PRKDC), XRCC6, XRCC5, LIG4 and PARP1 were markedly statistically significant during the transition from stage 3 to stage 4. We also analyzed the relationship of NHEJ pathway-related genes expression and tumor metastasis of UVM patients in a BioStudies database and found that expression of XRCC6, PRKDC and PARP1 was significantly higher in patients with metastasis than in those without metastasis (Fig. 1 B). These data strongly suggested that the NHEJ pathway plays a significant role in the UVM progression.
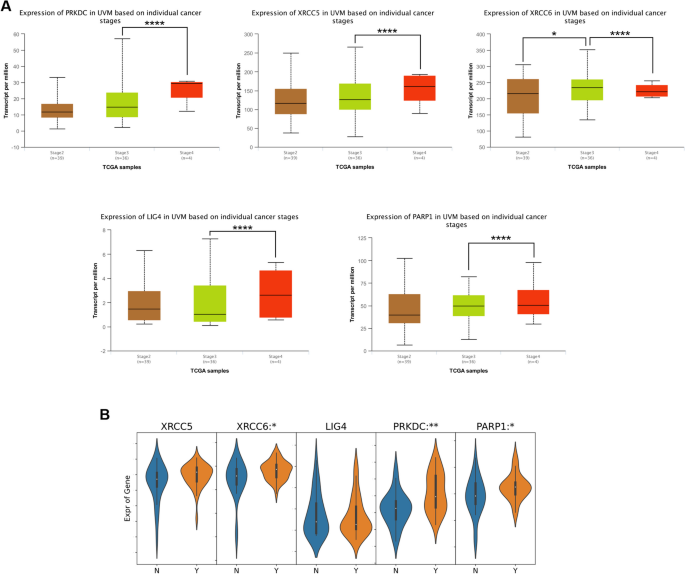
Correlation between NHEJ pathway-related genes and clinicopathological characteristics in patients with UVM. A The expression of NHEJ pathway-related genes in different tumor stages of UVM patients (UALCAN). B The relative expression level of NHEJ pathway-related genes in UVM patients with metastasis (Y group, n = 35) and without metastasis (N group, n = 26) (BioStudies database: E-GEOD-22138). The p value was set at 0.05. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001, **** represents p < 0.0001
The prognostic value of DDR related genes in UVM
We investigated the association of NHEJ pathway-related genes expression and prognosis of UVM patients in TIMER, UALCAN and GEPIA databases. The Kaplan–Meier curve analyses showed that high expression of PRKDC and XRCC5 was markedly associated with lower OS in UVM patients (Figs. 2 , 3 and 4 ). We also used the GEPIA dataset to compare the mRNA expression of the above genes to the DFS of patients with UVM. DFS curves are presented in Fig. 5 . The results showed that the UVM patients with low expression of PARP1 and XRCC6 predicted better DFS. Taken together, survival analysis showed that decreased expression of NHEJ pathway-related genes was significantly associated with improved prognosis in UVM patients.
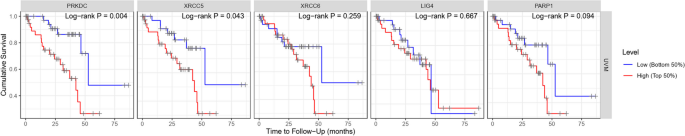
The prognostic value of NHEJ pathway-related genes in UVM patients (80 patients with 23 dying) (TIMER). Patients were divided into high expression group (red, n = 40) and low expression group (blue, n = 40) according to the median amount of gene expression. The overall survival curves comparing patients with high and low expressions of NHEJ pathway-related genes were plotted. The threshold of p value is 0.05
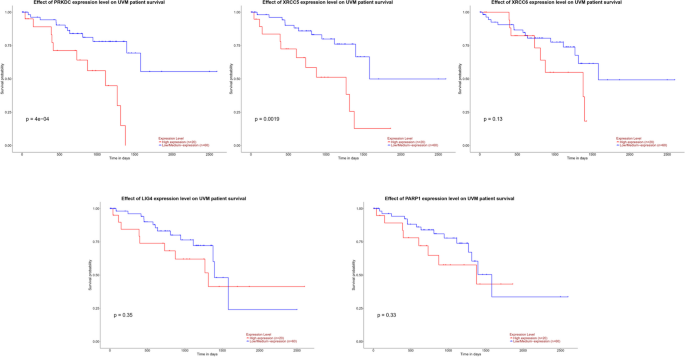
The prognostic value of NHEJ pathway-related genes in UVM patients (UALCAN). The overall survival curves comparing patients with high (red) and low (blue) expression of NHEJ pathway-related genes were plotted. The threshold of p value is 0.05
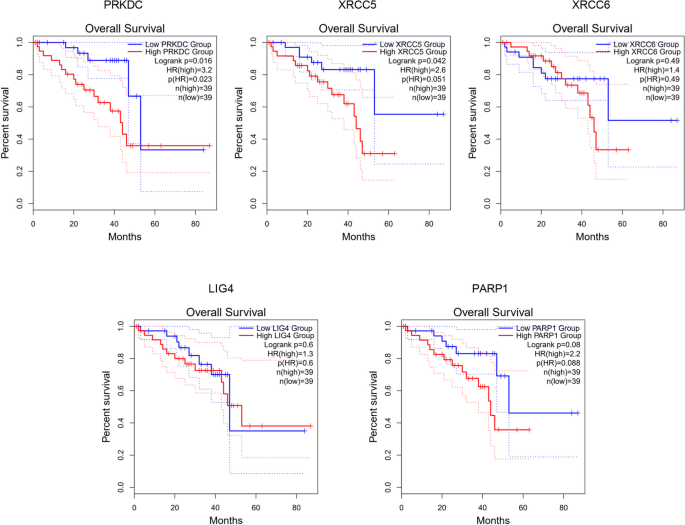
The prognostic value of NHEJ pathway-related genes in UVM patients (GEPIA). The overall survival curves comparing patients with high (red) and low (blue) expression of NHEJ pathway-related genes were plotted. The threshold of p value is 0.05
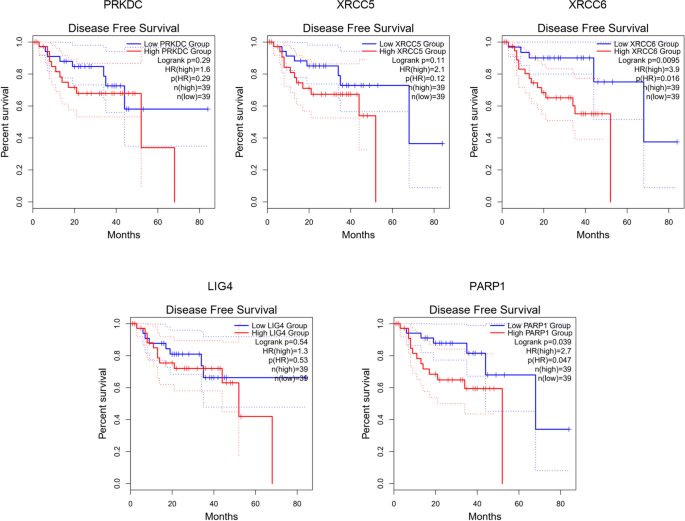
The prognostic value of NHEJ pathway-related genes in UVM patients (GEPIA). The disease-free survival curves comparing patients with high (red) and low (blue) expression of NHEJ pathway-related genes were plotted. The threshold of p value is 0.05
ESCMe inhibits the NHEJ pathway in UVM cells
As reported in our previous article [ 17 ], we transplanted ESCs into mice bearing UVM cancer to recapitulate the early embryonic microenvironment and demonstrated that ESCs can reverse the malignant phenotype of C918 cells. Now we performed a quantitative gene expression analysis of the key NHEJ pathway-related genes in UVM tumors. PRKDC, XRCC6, XRCC5 and PARP1 were significantly downregulated in tumor tissue of mice treated with ESCs and ESC-CM compared with those of mice treated with PBS (Fig. 6 ). Immunohistochemistry analysis revealed that the expression levels of all these NHEJ pathway-related factors were pronouncedly decreased after ESC treatment whereas only the expression levels of XRCC5, XRCC6, LIG4 and PARP1 decreased in the tumors in the ESC-CM treated mice compared with those from the control group. Our results indicate that the expression of nearly all these genes was significantly altered in the ESC treatment group compared with the ESC-CM treatment group, suggesting that the direct cell–cell contact approach with ESCs is more effective than their secreted factors in suppressing the NHEJ pathway. Taken together, these results suggest that the ESCMe significantly inhibited the NHEJ pathway of UVM in vivo.
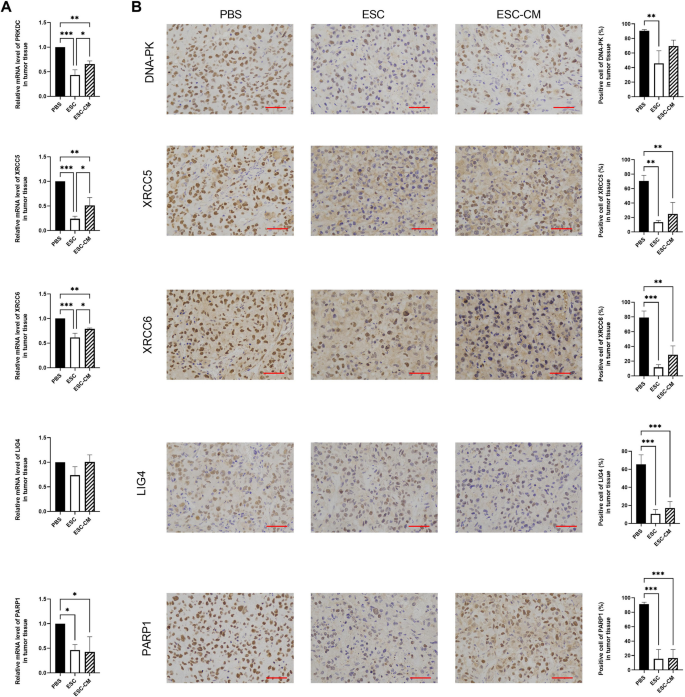
ESCMe inhibits the NHEJ pathway of UVM cells in vivo. A Expression of NHEJ pathway-related genes in UVM tissues, as assessed by RT-qPCR. B Staining of NHEJ pathway-related factors in UVM tissues obtained from mice 21 days after treatment with PBS, ESCs, or ESC-CM. Data are means ± SDs. * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001. Scale bar, 50 μm
ESCMe promotes apoptosis in UVM tissues
Recent studies have demonstrated that UVM had a reliance for NHEJ in terms of double strand break (DSB) repair and NHEJ is vital to the survival of UVM. If the NHEJ pathway is impaired or overwhelmed, it can lead to persistent DNA damage and activation of apoptotic pathways, resulting in cell death. To determine whether ESCMe treatment affects cell apoptosis in UVM tissues, we performed the TUNEL assay. TUNEL-labeled cells were sporadically positive in the tumor tissue of PBS-treated mice, and the number of positive cells increased only after ESC treatment (Fig. 7 ). Compared with the control group, the number of apoptotic cells in the ESC-CM treatment group increased, but the difference was not statistically significant. Therefore, ESC treatment can trigger apoptosis in mice bearing UVM cancer, which may be related to the downregulation of the NHEJ pathway in the tumor.
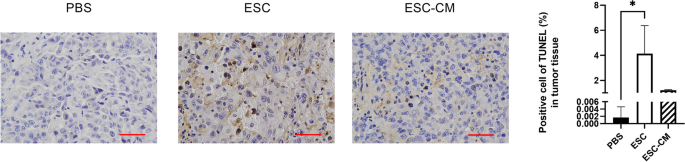
TUNEL staining in UVM tissues. Data are means ± SDs. * P < 0.05. Scale bar, 50 μm
ESCMe improves the DDR activities of normal cells
Administration of toxic drugs, such as DDR targeted agents and/or high dosage for inducing cell death can cause severe side effects and circumscribe their use as viable therapeutic strategies. To investigate whether ESC treatment impairs the DDR activities of normal cells, we examined the key genes involved in the NHEJ pathway of surrounding normal tissue. Following ESC treatment, while the expressions of NHEJ pathway-related genes in tumors were reduced, whereas the levels of XRCC6 and PARP1 were clearly enhanced in surrounding skin tissue (Fig. 8 ). The expression levels of XRCC6 and PARP1 showed no obvious change transcriptionally and translationally in ESC-CM treated skin tissue. Combined with our previous research results showing that ESCs could suppress the aggressive phenotype of tumor cells while promoting the proliferation of normal somatic cells, upregulation of DDR activities by ESC treatment could efficiently remove DNA damage from normal cells, contributing to accelerating cell cycle progression and promoting cell proliferation.
ESCMe improves the DDR activities of normal tissue. A Expression of NHEJ pathway-related genes in skin tissues, as assessed by RT-qPCR. B Staining of NHEJ pathway-related factors in skin tissues obtained from mice 21 days after treatment with PBS, ESCs, or ESC-CM. Data are means ± SDs. * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001. Scale bar, 50 μm
UVM is notoriously resistant to both radiation and DNA-damaging agents, which is closely related to its high DDR activities, underlining the importance of targeting the DNA repair capacity as a potentially effective therapeutic option, either alone or as a sensitizer for other treatments [ 6 , 7 , 8 ]. The present study showed that a low level of NHEJ pathway-related genes predicted better prognosis in UVM patients and ESCMe could significantly impair the DDR in UVM tissues by downregulating the NHEJ pathway. Previously, we found that the ESCMe suppressed the proliferation, invasiveness, and tumorigenicity of UVM [ 17 ]. Combining these results, we can conclude that the ESCMe could inhibit various malignant phenotypes of UVM, indicating a promising and attractive prospect of ESC-based therapy to enhance the sensitivity of UVM to radiochemotherapy and improve treatment outcomes.
DSBs, which can be induced by radiation and other types of genotoxic noxae or during replication are mainly repaired by homologous recombination (HR) and NHEJ [ 18 , 19 , 20 ]. Evidence suggests that NHEJ activity was upregulated and was the main mechanism for repairing DNA damage in UVM [ 7 ]. The inhibition of the NHEJ protein DNA-PK is even lethal to UVM. In the previous study, we demonstrated that UVM cells treated with ESCs had higher expression levels of p21 [ 17 ]. p21 induction is essential for the onset of cell cycle arrest in the DDR, arresting cells at the G1/S transition and giving cells time to repair critical damage [ 21 ]. If DNA repair is incomplete, such as when NHEJ-related factors are decreased or DNA damage is extensive, the repair of DSBs can be compromised and the cell undergoes apoptosis [ 9 , 10 ]. The data presented here, demonstrating the inhibition of functional NHEJ in UVM tissue of ESC-treated mice, was confirmed by gene expression analysis of PCR and immunohistochemical detection. It would therefore be reasonable to consider that the increase in tumor apoptosis by ESCs may be implicated in the upregulation of p21 and downregulation of NHEJ.
Apart from its important role in NHEJ, PARP-1 also engages in other DNA repair mechanisms, such as base excision repair (BER), nucleotide excision repair (NER), DNA mismatch repair (MMR), and maintenance of replication fork stability [ 22 , 23 , 24 ], regulating cell death in the event of excessive damage. Several studies have indeed reported that an elevated level of PARP-1 expression was observed in UVM and was associated with a shorter overall survival time and disease-free survival time [ 6 , 8 , 25 , 26 ]. It is possible that PARP-1 inhibition can suppress damaged DNA repair and improve tumor killing [ 27 ]. Therefore, induced downregulation of PARP-1 by ESCs could lead to multiple compromised DNA repair pathways and a reduced ability to repair DNA damage, suggesting ESCs as an attractive therapeutic option alone or in combination with other treatments of UVM.
Although DDR inhibitors have shown promising results in cancer treatment, they can disrupt the normal DNA repair mechanisms in healthy cells, leading to the accumulation of DNA damage, causing cell cycle arrest or apoptosis, potentially interfering with the ability of normal tissues to regenerate and repair, especially in rapidly dividing tissues such as the bone marrow, skin, and gastrointestinal tract [ 28 , 29 , 30 ]. These limit the clinical application of DDR inhibitors [ 31 ]. Surprisingly, this study shows that while the expression of NHEJ pathway-related genes in tumors decreased, the levels of XRCC6 and PARP1 were enhanced in the surrounding skin tissue, indicating an upregulation of DDR activities. This enhanced DDR response may efficiently remove DNA damage and maintain genome stability in normal cells, avoiding apoptotic pathways triggered by DNA damage, facilitating faster cell cycle turnover and promoting cell proliferation [ 32 ]. This was consistent with our previous research showing that ESCs could markedly suppress tumor growth and enhance the proliferation of adjacent skin tissue [ 17 ]. By promoting efficient DNA repair, normal cells with elevated DDR levels are better equipped to cope with various genotoxic stresses, such as radiation or chemical-induced DNA damage. This increased resistance to DNA damage can contribute to the survival and maintenance of normal cells, potentially mitigating concerns about severe side effects of cancer therapy.
It is worth noting that the suppressive effects of the ESCMe on the DDR activities of UVM were much more significant in the ESC treatment group than in the ESC-CM treatment group, which was in agreement with our previous finding that ESC treatment showed a superior therapeutic effect to ESC-CM treatment in terms of proliferation, invasiveness, and tumorigenicity [ 17 ]. Direct cell-to-cell contact with ESCs appears to be more effective in inhibiting the NHEJ pathway and triggering apoptosis in tumor cells than exposure to their secreted factors. Furthermore, the secreted factors of ESCs showed no obvious effect on the DDR of normal cells. This may be due to the direct signal communication via cell–cell contact, which is the main mechanism by which ESC influence the DDR activity of both cancer cells and normal cells.
Nevertheless, this study has several limitations. First, this study only illustrated the effect of ESCMe on DDR in subcutaneous UVM models, the conditions of which could be different from that in orthotopic models. Further research is needed to explore the influence of ESCMe on orthotopic UVM models. Second, the experiments in this study were only performed on the C918 cell line. Further studies should be conducted in more UVM cell lines. Third, because of the lack of clinicopathological data on UVM in relevant databases such as TCGA, we were unable to find more data for effective analysis. Clinical data of more UVM patients need to be further collected.
Our study provides evidence that ESCs can effectively suppress the DDR activity of tumor cells in subcutaneous UVM models while promoting DNA repair in normal cells. These findings capitalize on the unique properties of ESCs in modulating the DDR response in tumors and normal tissues, supporting the potential therapeutic use of ESCs as a viable approach for UVM treatment while minimizing damage to healthy cells. Future studies of ESCs on orthotopic UVM models are needed to evaluate the role of ESCs in ocular environment and the mechanistic association with DDR activities. Such approaches could significantly impact cancer treatment paradigms and provide new avenues for improving patient outcomes in the battle against cancer.
Availability of data and materials
All data in the manuscript is available through the responsible corresponding author.
Abbreviations
- Embryonic stem cells
Phosphoinositide 3-kinase
Thymidine kinase
Embryonic stem cell microenvironment
TdT-mediated dUTP nick-end labeling
Reverse transcription polymerase chain reaction
Embryonic stem cell-conditioned medium
Phosphate-buffered saline
Herpes simplex virus thymidine kinase
Ganciclovir
- Uveal melanoma
- DNA damage response
- Nonhomologous end joining
The cancer genome atlas
Overall survival
Disease-free survival
Diaminobenzidine
Double strand break
Homologous recombination
Base excision repair
Nucleotide excision repair
DNA mismatch repair
Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101(1):38–44.
Article PubMed Google Scholar
Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118(9):1881–5.
Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–9.
Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, Ferrini S, Pfeffer U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017;36(1):109–40.
Article PubMed PubMed Central Google Scholar
Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15(3):166–80.
Article CAS PubMed Google Scholar
de Koning L, Decaudin D, El Botty R, Nicolas A, Carita G, Schuller M, Ouine B, Cartier A, Naguez A, Fleury J, et al. PARP inhibition increases the response to chemotherapy in uveal melanoma. Cancers. 2019;11(6):751.
Doherty RE, Bryant HE, Valluru MK, Rennie IG, Sisley K. Increased non-homologous end joining makes DNA-PK a promising target for therapeutic intervention in uveal melanoma. Cancers. 2019;11(9):1278.
Article CAS PubMed PubMed Central Google Scholar
Gajdzis M, Theocharis S, Klijanienko J, Cassoux N, Gardrat S, Donizy P, Kaczmarek R, Gajdzis P. The prognostic values of PARP-1 expression in uveal melanoma. Cells. 2021;10(2):285.
Radhakrishnan SK, Jette N, Lees-Miller SP. Non-homologous end joining: emerging themes and unanswered questions. DNA Repair. 2014;17:2–8.
Eriksson A, Lewensoh R, Larsson R, Nilsson A. DNA-dependent protein kinase in leukaemia cells and correlation with drug sensitivity. Anticancer Res. 2002;22(3):1787–93.
CAS PubMed Google Scholar
Bailey CM, Kulesa PM. Dynamic interactions between cancer cells and the embryonic microenvironment regulate cell invasion and reveal EphB6 as a metastasis suppressor. Mol Cancer Res. 2014;12(9):1303–13.
Dürr M, Harder F, Merkel A, Bug G, Henschler R, Müller AM. Chimaerism and erythroid marker expression after microinjection of human acute myeloid leukaemia cells into murine blastocysts. Oncogene. 2003;22(57):9185–91.
Giuffrida D, Rogers IM, Nagy A, Calogero AE, Brown TJ, Casper RF. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009;42(6):788–98.
Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. A three-dimensional model to study the epigenetic effects induced by the microenvironment of human embryonic stem cells. Stem cells (Dayton, Ohio). 2006;24(3):501–5.
Laurent C, Valet F, Planque N, Silveri L, Maacha S, Anezo O, Hupe P, Plancher C, Reyes C, Albaud B, et al. High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 2011;71(3):666–74. https://doi.org/10.1158/0008-5472.CAN-10-0605 . Epub 2010 Dec 6 PMID: 21135111.
McEntyre J, Sarkans U, Brazma A. The BioStudies database. Mol Syst Biol. 2015;11(12):847.
Liu J, Huang Z, Yang L, Wang X, Wang S, Li C, Liu Y, Cheng Y, Wang B, Sang X, et al. Embryonic stem cells modulate the cancer-permissive microenvironment of human uveal melanoma. Theranostics. 2019;9(16):4764–78.
Götting I, Jendrossek V. A new twist in protein Kinase B/Akt signaling: role of altered cancer cell metabolism in Akt-mediated therapy resistance. Int J Mol Sci. 2020;21(22):8563.
Woodbine L, Brunton H, Goodarzi AA, Shibata A, Jeggo PA. Endogenously induced DNA double strand breaks arise in heterochromatic DNA regions and require ataxia telangiectasia mutated and Artemis for their repair. Nucleic Acids Res. 2011;39(16):6986–97.
Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417(3):639–50.
Hussain T, Saha D, Purohit G, Kar A, Kishore Mukherjee A, Sharma S, Sengupta S, Dhapola P, Maji B, Vedagopuram S, et al. Transcription regulation of CDKN1A (p21/CIP1/WAF1) by TRF2 is epigenetically controlled through the REST repressor complex. Sci Rep. 2017;7(1):11541.
Pazzaglia S, Pioli C. Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells. 2019;9(1):41.
Bryant HE, Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34(6):1685–91.
Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108(8):3406–11.
Molloy-Simard V, St-Laurent JF, Vigneault F, Gaudreault M, Dargis N, Guérin MC, Leclerc S, Morcos M, Black D, Molgat Y, et al. Altered expression of the poly(ADP-ribosyl)ation enzymes in uveal melanoma and regulation of PARG gene expression by the transcription factor ERM. Invest Ophthalmol Vis Sci. 2012;53(10):6219–31.
Géhl Z, Bai P, Bodnár E, Emri G, Remenyik É, Németh J, Gergely P, Virág L, Szabó É. Poly(ADP-ribose) in the skin and in melanomas. Histol Histopathol. 2012;27(5):651–9.
PubMed Google Scholar
Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2014;71(2):331–48.
Blanpain C, Mohrin M, Sotiropoulou PA, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8(1):16–29.
Vainshelbaum NM, Salmina K, Gerashchenko BI, Lazovska M, Zayakin P, Cragg MS, Pjanova D, Erenpreisa J. Role of the circadian clock “Death-Loop” in the DNA damage response underpinning cancer treatment resistance. Cells. 2022;11(5):880.
Lopacinska-Joergensen J, Oliveira D, Poulsen TS, Hoegdall CK, Hoegdall EV. Somatic variants in DNA damage response genes in ovarian cancer patients using whole-exome sequencing. Anticancer Res. 2023;43(5):1891–900.
Groelly FJ, Fawkes M, Dagg RA. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23(2):78–94.
Li P, Gao L, Cui T, Zhang W, Zhao Z, Chen L. Cops5 safeguards genomic stability of embryonic stem cells through regulating cellular metabolism and DNA repair. Proc Natl Acad Sci USA. 2020;117(5):2519–25.
Download references
Acknowledgements
The authors thank Professor Andy Peng Xiang, from the Center for Stem Cell Biology and Tissue Engineering, the Key Laboratory for Stem Cells and Tissue Engineering, Ministry of Education, Sun Yat-Sen University for providing mouse ESCs.
This research was supported by the National Natural Science Foundation of China (82102993) and Guangdong Basic and Applied Basic Research Foundation (2023A1515012454).
Author information
Authors and affiliations.
Ophthalmology Department, The Tenth Affiliated Hospital, Southern Medical University (Dongguan People’s Hospital), 78 Wandao Road, Dongguan, 523000, China
Yingxu Zhang, Jinbiao Zheng, Minyu Chen, Shulun Zhao, Ruiqian Ma, Wenwei Chen & Jiahui Liu
You can also search for this author in PubMed Google Scholar
Contributions
Y. Z. and J. L. conceived and designed the study. Y. Z., J. Z., M. C. and J. L. participated in designing the experiments and writing the manuscript. Y. Z. and J. Z. conducted gene expression analysis and survival prognosis analysis based on public databases. S. Z. and J. L. conducted animal experiments. W. C. and R. M. prepared the RNA, performed the RNA integrity analysis, and conducted immunohistochemistry. M. C. assisted with the TUNEL assay. S. Z. and J. L. made the charts and performed statistical analyses. All authors reviewed the manuscript.
Corresponding author
Correspondence to Jiahui Liu .
Ethics declarations
Ethical approval and consent to participate.
The animal experiments conformed to the Guide for the Care and Use of Laboratory Animals. Animal study has been approved by the Animal Ethics Committee of Zhongshan School of Medicine, Sun Yat-sen University. All experiments were performed in accordance with relevant guidelines and regulations. The manuscript reporting adheres to the ARRIVE guidelines for the reporting of animal experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, Y., Zheng, J., Chen, M. et al. Modulating DNA damage response in uveal melanoma through embryonic stem cell microenvironment. BMC Cancer 24 , 519 (2024). https://doi.org/10.1186/s12885-024-12290-x
Download citation
Received : 04 September 2023
Accepted : 19 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s12885-024-12290-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Microenvironment
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier Sponsored Documents

Balancing DNA repair to prevent ageing and cancer
Eleanor rachel stead.
a UCL Cancer Institute, Paul O'Gorman Building, University College London, 72 Huntley Street London, London WC1E 6DD, UK
Ivana Bjedov
b University College London, Department of Medical Physics and Biomedical Engineering, Malet Place Engineering Building, Gower Street, London WC1E 6BT, UK
DNA damage is a constant stressor to the cell. Persistent damage to the DNA over time results in an increased risk of mutation and an accumulation of mutations with age. Loss of efficient DNA damage repair can lead to accelerated ageing phenotypes or an increased cancer risk, and the trade-off between cancer susceptibility and longevity is often driven by the cell's response to DNA damage. High levels of mutations in DNA repair mutants often leads to excessive cell death and stem cell exhaustion which may promote premature ageing. Stem cells themselves have distinct characteristics that enable them to retain low mutation rates. However, when mutations do arise, stem cell clonal expansion can also contribute to age-related tissue dysfunction as well as heightened cancer risk. In this review, we will highlight increasing DNA damage and mutation accumulation as hallmarks common to both ageing and cancer. We will propose that anti-ageing interventions might be cancer preventative and discuss the mechanisms through which they may act.
1. Types and sources of DNA damage
DNA is subject to constant assault, an estimated 70,000 lesions occur per day for a typical human cell [ 1 ]. This damage can originate from endogenous sources, such as reactive oxygen species (ROS), enzyme action and replication errors. Depurination, depyrimidination, single strand breaks (SSBs), 8-oxoG and cytosine deamination are the most common forms of DNA damage that arise spontaneously [ 1 ]. ROS causes direct modification to DNA bases by oxidation and results in conversion of guanine to 8oxoG. Depurination arises from spontaneous chemical reactions, typically hydrolysis, which breaks the labile glycosidic bonds between the DNA base and the deoxyribose creating an abasic site. These sites can result in mutations due to misincorporation by DNA polymerase or translesion DNA synthesis (TLS) [ 2 ]. Spontaneous, hydrolytic reactions such as deamination, can also cause mutations, for instance cytosine deamination converts it to uracil, while deaminated 5-methylcytosine becomes thymine. This process results in mutation accumulation over time and the mutational signature of 5-methyl-cytosine deamination shows a strong positive correlation with the age of when cancer is diagnosed [ 3 ]. During DNA replication the frequency of these spontaneous reactions increases due to the exposure of more vulnerable ssDNA [ 4 ]. Therefore, in scenarios where ssDNA is exposed for prolonged periods such as replication or transcriptional stress, the likelihood of damage occurring increases (see Fig. 1 ).

DNA damage and repair on DNA, cellular, and organismal level. DNA repair may differ if the damage is situated in the heterochromatin or euchromatin [ 5 , 6 ]. Heterochromatic regions have increased H3K9me3 mark, which is bound by HP1α (heterochromatin protein 1 alpha) [ 7 ]. Acetylation of histones in euchromatin increases chromatin availability [ 8 ]. Represented are different types of DNA repair, such as BER (base excision repair), GG-NER (global genomic nucleotide excision repair), ICLR (inter-strand crosslink repair), TC-NER (transcription coupled nucleotide excision repair), SSBR (single strand break repair), DSBR (double strand break repair), which can be repaired by HR (homologous recombination) or by either NHEJ (non-homologous end joining) or more mutagenic alt-NHEJ (alternative NHEJ) [ 9 ]. NHEJ is active throughout the cell cycle while HR is restricted to the late S and G2 phase. Replication errors are repaired by MMR (mismatch repair system) or are tolerated and bypassed by TLS (translesion synthesis repair). Transcription and replication make genome more vulnerable to damage and are associated with specialised types of repair. Upon DNA damage, damage sensors, such as PARP, mediate recruitment of transducer kinases ATM or ATR, whose activation leads to activation of DNA damage response to downstream proteins MDC1, BRCA1, 53BP1 and others. In presence of damage, ATM and ATR also activate Chk2 and Chk1, respectively [ 9 ]. CDC25 is one of the effector proteins that arrests cell cycle to allow damage repair [ 10 ]. p53 modifies transcription and thereby has a role in cell cycle arrest, apoptosis and senescence [ 11 ]. Mutations accumulate during ageing and are caused by different exposure to endogenous and exogenous factors, which produce DNA damage that can be repaired in error-free or error prone manner. Highly damaged cells are targeted for cell death, excess of which protects from cancer but depletes the stem cell pool and has pro-ageing effect [ 12 , 13 ]. Shown is clonal expansion of a mutated stem cell clone. If cell death is not induced in damaged and aberrant cells, then this increases chances clonal selection and expansion. Senescence is cancer protective but excess of prolonged senescence can promote both ageing and cancer via SASP (senescence associated secretory phenotype) [ 14 ].
Mitochondrial dysfunction and metabolic stress can result in increased production of reactive by-products such as ROS, lipid peroxides, oxidatively damaged proteins and aldehydes, of which lipid peroxidation may be particularly harmful due to its unique ability to propagate and amplify [ 15 , 16 ]. These reactive by-products, as well as endonuclease cleavage can cause endogenous SSBs and double strand breaks (DSBs) [ 16 , 17 ]. Another cause for SSBs and DSBs can be transcriptional stress due to abortive topoisomerase 2 action (TOP2CC cleavage complexes). The frequency of these events is increased in the presence of other lesions such as base cytotoxic modifications [ 18 ]. In addition, DNA damage lesions that are close in proximity, such as SSBs or closely spaced ongoing BER or NER on opposite strands, can further progress into DSB [ 19 ]. DSBs can also form when stalled replication forks collapse [ 20 ], further underlining how replication and transcriptional stress can contribute to endogenous DNA damage. The variety of causes for DSBs is important because, although this is the least frequent form of damage, DSBs are the most dangerous form of damage.
Multiple exogenous agents induce DNA damage, including UV light, ionising radiation, and chemical mutagens, such as polycyclic aromatic hydrocarbons present in tobacco smoke [ [21] , [22] , [23] ]. These agents cause chemical or physical modifications to the DNA often producing specific structures that are recognised by distinct repair enzymes. Interestingly, some cancer inducing chemicals do not seem to cause DNA mutations, but they induce selective constraint and expansion of existing clones instead, without generating specific mutation signatures [ 24 ]. Perhaps similarly, an “invisible” chemical or a metabolite, that does not leave fingerprints on DNA, could abet clonal expansion among hematopoietic stem cells (HSC) during ageing [ 25 ]. Another intriguing recent finding is that overexpression of some proteins, belonging to a variety of cellular functions, can also cause DNA damage and mutations [ 26 ].
Overall, DNA is continuously being damaged, often by unavoidable cellular metabolic processes. The challenge is to match a variety of different DNA lesions with the most adequate DNA repair enzymes and to coordinate the repair with other cellular activities.
2. How is DNA damage repaired? DNA damage repair pathways
Complex repair pathways have evolved to deal with the persistent problem of DNA damage [ 27 ]. They have been particularly well characterised in E. coli , yeast and mammals, and remarkable evolutionary conservation of repair enzymes have been observed between different organisms [ 28 , 9 ]. The repair pathway utilised depends on the type of damage incurred, the phase of the cell cycle and the availability of repair machinery. Below we describe DNA repair pathways, focusing mainly on mammalian systems.
Replication fidelity is preserved by the proofreading activity of polymerases [ 29 ], mismatch repair (MMR) [ [30] , [31] , [32] ], and regulation of the nucleotide pool's quality and quantity [ 33 ]. Lesions which block replicative DNA pol δ/ ε can be bypassed using translational DNA synthesis (TLS) polymerases [ [34] , [35] , [36] ] or repaired by template switching (TS) [ 37 , 38 ]. Both TLS and TS, can be mutagenic and can be thought of as damage tolerance instead of repair.
Lesions that distort the DNA helix, such as bulky adducts or UV-light induced pyrimidine-dimers, are repaired by nucleotide-excision repair (NER). Lesions that do not alter the helical structure of DNA, such as 8-oxoG, uracil or an AP (apurinic/apyrimidinic) site, are repaired by base excision repair (BER). BER also acts upon SSBs. DSBs are repaired by homologous recombination (HR), non-homologous end joining (NHEJ) or alternative NHEJ (Alt-NHEJ), a microhomology-based pathway. Different repair pathways are predominant in different phases of the cell cycle, for example HR requires the presence of homologous or sister chromosomes and is therefore active during late S and G2 phase [ 39 , 40 ]. Pathway activity is determined by CDK activity which regulates the expression of the required repair factors. For example, CDK2 activity is required for the expression of CtIP which promotes end resection, a key step for the initiation of HR [ 41 , 42 ].
NHEJ is divided into classical NHEJ (c-NHEJ) and alternative NHEJ (Alt-NHEJ). HR is divided into gene conversion HR (GC-HR) and single strand annealing (SSA-HR) [ 42 ]. HR requires a plethora of repair enzymes and factors which are often shared with other repair pathways such as the Fanconi anaemia (FA) pathways [ 43 ] which repair interstrand cross links (ICLs) and template switching [ 44 ] which relies on the use of HR machinery e.g. BRCA2 and Rad51 [ 45 ].
The switch from NHEJ to HR is influenced by the complexity of the break site, by the availability of DNA repair components and the compaction of the chromatin [ [46] , [47] , [48] , [49] ]. NHEJ is thought to be more erroneous than HR, however the cell repairs over 80% of DSBs by using NHEJ [ 46 , [50] , [51] , [52] ]. Even in G2 phase cells, NHEJ may be the first-choice pathway, as cells which lack DNA LigIV, a key component for NHEJ, not only exhibit a G1 phase repair defect but a G2 phase repair defect as well [ 46 , 48 ]. This may be due to the ease and speed of NHEJ. A subset of DSBs, around 15–20%, which cannot be repaired by NHEJ undergo end resection and repair by HR [ 46 , 53 ].
The choice between NHEJ and HR is also governed by levels of 53BP1. 53BP1 acts to restrain end resection whilst CtIP acts to promote it. However, 53BP1, along with its effector protein Shieldin [ 54 , 55 ] also determines whether HR is carried out by gene conversion (GC), typically thought of as an ‘error-free’ repair mechanism, or SSA which is a result of extensive end resection and is highly erroneous. In this way 53BP1 prevents excessive end resection and ensures the fidelity of HR [ 56 ]. Recent studies further indicate that HR can be mutagenic [ 40 , 57 , 58 ]. This is likely to be due to the involvement of translesion synthesis DNA polymerases such as Polζ [ [59] , [60] , [61] , [62] ] and because the ssDNA generated in the repair process is more at risk of being damaged [ 63 ].
The repair of DNA must happen within the broader context of the nuclear landscape/architecture; therefore, chromatin dynamics and modifications are also key player in the repair. The DNA damage response invokes a large array of histone modifications, e.g. poly-ADP ribose (PAR) chains, γH2AX, H2A-Ubiquitination. These facilitate repair in multiple ways, for example, reducing local rate of transcription, opening up compacted chromatin regions or serving as recruitment or signalling platforms for repair enzymes/processes [ 56 , [64] , [65] , [66] , [67] , [68] ]. High levels of chromatin accessibility and transcription are associated with fewer base-pair substitutions perhaps due to more efficient MMR or TC-NER [ [69] , [70] , [71] ]. Mutation rate in euchromatic, early replicating regions of the DNA is reduced compared to late-replicating, heterochromatic regions [ 72 ]. This may be due to early regions having more time to detect and repair faults while late-replicating regions are often rich in repetitive sequences which are problematic for polymerases. Chromatin accessibly is not, however, a guarantee of more proficient repair, as despite the DNA being accessible in transcriptionally active regions, the presence of DNA-binding factors such as transcription factors can cause exclusion of repair proteins [ 73 ]. Transcriptionally active and accessible regions tend to accumulate genomic rearrangements [ 74 ] and mutational hot spots [ 75 ], including regulatory regions [ 76 , 77 ].
Heterochromatic DSBs are preferentially repaired by HR [ 48 , 49 , 78 , 79 ]. The co-localisation of γH2AX, 53BP1, and MDC1 is exclusive to areas of H3K9me3, a mark of condensed chromatin. This suggests the assembly of these factors is promoted in heterochromatic regions and may contribute to the preference of HR in heterochromatic regions [ 80 ]. Within heterochromatin, DSB repair may rely on a specific HR pathway that is dependent on ATM and involves Artemis, 53BP1, RNF168 and RNF8 [ 81 ]. Repair efficiency has also been correlated with the mobility of DSBs. Heterochromatic DSBs are often extruded to the periphery of the heterochromatic domain to undergo repair [ 5 ]. However, extrusion of DSBs also occurs for irreparable DSBs. They are pushed out to the nuclear periphery as a last resort to prevent interference with undamaged DNA [ 6 ]. Recently, heterochromatin, specifically H3K9me3 marks, have been associated with mechanosensing. Nuclear softening driven by loss of H3K9me3, protects the cell from DNA damage induced by mechanical stress [ 82 ].
The cell is equipped with a remarkable set of tools to repair DNA. However, it is essential that the least mutagenic repair complex gets priority access to its cognate lesion, and that these repair enzymes, capable of cutting, resecting, and ligating DNA, are firmly controlled to avoid mutations and chromosomal aberrations.
3. How does the cell respond to DNA damage? DNA damage detection, checkpoint arrest, and choice of cell fate
A key element in DNA repair is damage detection. Major detectors of single and double strand DNA breaks are PARP1 (poly(ADP-ribose) polymerase 1) and PARP2 (poly(ADP-ribose) polymerase 2) enzymes, which signal broken DNA by decorating adjacent histones with poly(ADP-ribose) chains, that at the same time relax chromatin and increase access of DNA repair proteins to the damage [ 66 , 83 ]. Often, PARP enzymes are aided by PARP complex accessory proteins, such as HPF1 (histone PARylation factor 1), which limits PARylation to serine residues, a typical post-translational modification of DNA repair proteins, rather than other residues such as glutamate and aspartate [ [84] , [85] , [86] , [87] ]. PARylation is an early, brief event in DNA repair, which is terminated by removal of PAR chains by PARG and ARH3-mediated hydrolysis, once DDR factors are recruited to the lesion [ 66 , 83 ]. The importance of PARP enzymes in DNA repair is exploited in cancer therapy and PARP inhibitors have shown great success, specifically in HR-defective cancers [ 88 , 89 ]. A critical feature of PARP inhibitors is trapping PARP enzyme on broken DNA, which instead of initiating DNA repair generates further obstruction and damage and can lead to replication fork collapse. This creates an excess in the amount of substrates for recombination repair, which in HR-deficient cancer cells, such as BRCA1 mutated tumours, is limited to repair by lower-fidelity NHEJ, leading to chromosomal aberration such as radial chromosomes and selective death of tumour cells [ 88 , 89 ].
Another essential control of DNA damage response is brought about by phosphoinositide 3-kinase (PI3K)-related kinases: ATM, ATR and DNA-PKcs. These kinases are recruited to DNA breaks by their corresponding interacting proteins, for instance NSB1 from the Mre11/Rad50/NSB1 (MRN) complex recruits ATM double strand breaks. DSBs are also recognised by the Ku80 protein thereby aiding DNA-PKcs access to DNA damage. ATRIP, bound to RPA, is activating ATR kinase once replication forks are stalled [ 90 ]. These kinases provide a critical signalling cascade that orchestrates and activates a variety of DNA repair proteins that are specialised for given lesions [ 90 ].
The DNA repair process is helped by the cell cycle checkpoint arrest. The DNA damage response must be rapid and occurs before transition to the next phase of the cell cycle. Induction of checkpoint arrest relies on phosphorylation events such as ATR/ATM kinases acting to phosphorylate Chk1/2. Whereas maintenance of the checkpoint, whilst repair is occurring, relies on slower mechanisms involving transcription and expression of p53 and p21 [ 91 ].
Repair networks are brought back to homeostasis by the action of phosphatases, such as Wip1 and PP2A, to inactivate the DDR (DNA damage response). Depending on the level of DNA damage, several cycles or oscillations of effector proteins such as p53 [ 91 ] occur until the cell manages to repair the damage or a threshold is reached at which the cell commits to apoptosis, senescence or alternative cell death mechanisms due to prolonged activation of the DDR. The choice of cell fate, such as senescence, apoptosis, and quiescence, is governed by a combination of several factors, including the degree and type of damage, the activity of the p53-p21 axis, growth and survival signalling through PTEN-PI3K-AKT-mTOR and MAPK, the cell type, and the environment. The type and level of DNA damage, the efficiency of repair and the cellular response to this damage dictates whether an organism becomes more prone to cancer (by preserving mutated cell) or exhibits accelerated ageing phenotypes (by excessively eliminating cells with genomic aberations).
4. Somatic mutations are common characteristics of both cancer and ageing
Here we will present data showing how mutation accumulation is a common characteristic of both cancer and ageing [ 92 , 12 ], and some recent findings showing that non-tumorigenic normal tissue has surprisingly high mutation burden [ 93 ]. Cancer is a disease initiated and fuelled by genetic mutations, with multiple ‘hits’ required to malignantly transform a cell [ 93 , 94 ] and failure of DNA repair mechanisms may result in mutations [ 95 ]. In lung and skin cancers, mutation rates are dramatically increased by exposure to tobacco smoke and UV light, respectively [ 3 , 95 , 96 ]. Mutation rates in normal, somatic cells (B and T cells, fibroblasts, retinal and intestinal epithelium) is reported to be in the order of 2–10 mutations per cell division [ 97 ]. However, the incidence of cancer cannot be explained by this rate alone as the number of driver mutations generated would be insufficient to cause cancer. Instead, clonal expansion and hyper-mutation have been proposed to increase both the number of cells at risk and account for the discrepancy between mutation frequencies and cancer rates [ 98 , 99 ]. The predicted tissue-specific risk factor for cancer was proposed to be largely determined by stem cell endogenous replication error rates as opposed to exposure to exogenous factors [ 100 , 101 ], although there are other factors at play [ 102 , 103 ]. The exact number of driver mutations required to cause cancer is still unknown, and this may depend on the type of cancer and type of mutations acquired. Recent studies have shown that tobacco smoking, despite inducing a high mutational burden in the lung epithelial cells, leaves a population of quiescent cells which escape the high levels of DNA damage. These ‘protected’ cells go on to repopulate the lungs in those who stop smoking [ 104 ]. This highlights the cellular heterogeneity of mutation and how the selective process of regeneration can impact the mutational landscape of whole tissues over time.
Beyond cancer, accumulation of somatic mutations is thought to play a key role in ageing. Since mutations accumulate during ageing, this likely explains why ageing is the major risk factor for cancer [ 12 , 13 ]. The accumulation of somatic mutations in normal tissues is not well understood, they occur spontaneously throughout life and in a tissue-dependent manner. In the skin of the eyelid of normal, healthy persons, thousands of point mutations have been acquired by middle age and approximately 30% of cells have at least one driver mutation [ 105 ]. Daily exposure to sun light will have increased the number of mutations in the skin, however in the oesophagus, hundreds of clones are still present per square centimetre of tissue and these somatic mutations accumulate with age of the donor [ 106 ]. Recent single cell genome analysis of liver cells revealed the differentiated cells (hepatocytes) harbour higher levels of mutations accumulated with age compared to the adult stem cells [ 107 ], indicating certain cell populations, namely stem cells, are protected to an extent. This accumulation of mutations and clonal expansions in aged persons may contribute to the significant increase in cancer risk with age, from 2% risk at the age of 40, to a 50% risk by the age of 80 [ 93 ]. The accumulation of mutations and clonal expansion may lead to tissue dysfunction while changes in the tissue environment, such as inflammation, may drive further clonal selection and expansion [ [108] , [109] , [110] ]. While other unknown mechanisms which constrain clonal expansion may contribute to protection against cancer with age [ 111 ].
Overall, this common hallmark of cancer and ageing suggests that more crosstalk between these fields is urgently needed for better and faster understanding of the underlying causes of mutation accumulation.
5. Insights about cancer and ageing from DNA repair and growth signaling pathway mutants
Interesting insights about ageing and cancer could be gained by examining phenotypes of different DNA repair mutants, some of which are pro-ageing while others pro-cancer. In addition, in recent years there is evidence that down-regulation of growth pathway signalling can impact cancer and as well as ageing.
Several human disorders characterised by accelerated ageing are caused by deficiencies in DNA repair pathways. They often exhibit a high cancer incidence [ 112 , 113 ]. Examples include Werner and Bloom syndromes, both caused by mutations in the RecQ type helicases [ 114 ]. When modelled in yeast and mice, pro-ageing and pro-cancer phenotypes are observed as well as an increase in mutation frequency [ [114] , [115] , [116] , [117] , [118] ]. Pro-ageing and pro-cancer phenotypes are also seen in mice deficient in other key DNA damage repair proteins such as ATM [ 119 ] and p53 [ 120 ] suggesting that a functional DNA damage repair system is required for both cancer protection and longevity. Several studies in animal and cell models, in which the amount of DNA damage and mutations has been altered by deactivating or over-activing DNA repair genes, result in accelerated or decelerated ageing, respectively [ 12 , 13 ]. Overexpression of DNA repair genes in Drosophila including loki (Chk2), mei-41 (ATR) and WRN amongst others extend lifespan [ [121] , [122] , [123] , [124] ]. It should be noted however that overexpression of DNA repair enzymes led to either longer or shorter lifespan, depending on target tissue, level of expression and sex of the animals tested, therefore more investigation is needed to understand how DNA repair can be enhanced [ 123 ]. Given that the DNA repair process requires that numerous signalling proteins and repair enzymes act in concert, it is challenging to enhance DNA repair by overexpressing a single enzyme. More promising, albeit more pleiotropic, would be to alter some of the upstream regulatory pathways. For instance, enhanced capacity for DNA repair is reported in the long-lived Ames and Snell dwarf mice, in which IIS is reduced [ 125 , 126 ]. Ames dwarf mice also exhibit delayed accumulation of spontaneous mutations as do mice which are subject to caloric restriction, a regime which reduces IIS/mTOR signalling and extends lifespan [ 127 ]. Furthermore, in Snell dwarf and growth hormone receptor knock-out (GHR-KO) mice the downregulation of TORC1 activity was linked to upregulation of several proteins involved in DNA repair [ 128 ]. These data suggest that the down-regulation of IIS/mTOR signalling may promote longevity through upregulation of DNA repair pathways resulting in reduced age-associated mutation accumulation. Reduced cancer incidence is observed in the long-lived GHR-KO, Ames dwarf and Snell dwarf mice [ 129 , 130 ] as well as in mice treated with rapamycin [ 131 , 132 ]. However, other long-lived models, such as S6K1−/− mice do not show any difference in tumour incidence compared with controls [ 133 ] but they do show reduced incidence of other age-related pathologies. In yeast deletion of the S6K homologue (Sch 9) reduces genomic instability with age [ 134 ] and further deletion of homologues for TOR and Ras combined with the Sch 9 deletion produces a four-fold extension in lifespan with reduced age-related mutational frequency and genomic instability [ 135 ]. These studies suggest there is a strong link between the lifespan extending mechanisms of reduced IIS/mTOR signalling and genomic stability in old age which may explain the reduced cancer incidence often observed in long-lived animal models. Long-lived mutants are often also healthier, nevertheless it is important for future potential translational approaches that health improvements and healthspan, or the period of good health of individual mutants is also carefully characterised as well as longevity [ 136 ].
Interestingly, mouse models of accelerated-ageing syndromes which exhibit high levels of DNA damage (NER deficiency) also show attenuated IGF-1 signalling [ 137 ]. Initially this was puzzling because if IGF-1 signalling was reduced, why was lifespan not extended in these mice? It is now thought that cells respond to DNA damage by decreasing IGF-1 signalling to re-direct resources from growth to maintenance, therefore reducing IGF-1 signalling may act to enhance DNA damage repair in normal, healthy cells. For progeria-like syndromes however or DNA repair mutants the re-direction of resources is not enough, the high levels of DNA damage due to the deficiency in DNA repair ultimately leads to cell death, stem-cell functional decline and accelerated ageing [ 113 , 137 , 138 ]. However, when placing these NER deficient mice on caloric restriction, further downregulating IGF-1 signalling, this significantly extended their lifespan, health-span and increased genomic stability [ 139 ].
Comparative studies of several mammalian species have revealed positive correlations between DNA repair efficiency and lifespan [ [140] , [141] , [142] , [143] ]. The long-lived naked mole rat upregulates several genes involved in DNA damage repair resulting in more efficient base-excision repair (BER), mismatch repair (MMR), double strand break (DSB) repair and upregulation of the tumour suppressor gene, TP53, promoting cancer resistance [ [144] , [145] , [146] , [147] , [148] , [149] ]. Other mechanisms proposed for the naked mole rats cancer resistance include secretion of high molecular-mass hyaluronan which renders cells hypersensitive to contact inhibition, and these cells stop proliferating upon only a few cell-cell contacts [ 150 ].
p53 is a key player in the DNA damage response. In mice constant over-activation of p53 (p53+/mut) results in protection from cancer, likely due to heightened cell death but at the expense of a shorter lifespan [ 151 ], suggesting there is a trade-off between longevity and cancer protection, and that cancer protection can only be achieved at the expense of shorter lifespan. However, cancer protection and a normal lifespan is seen in super-p53 mice, which has an extra copy of p53 driven by the native promoter providing enhanced DNA repair capacity but only when required [ 152 ]. Interestingly, it is only when super-p53 mice have an additional copy of the tumour suppressor p19Arf that lifespan extension is achieved alongside cancer protection demonstrating that an anti-ageing and anti-cancer phenotype can be achieved, despite multiple examples of this trade-off [ 153 ]. The trade-off between pro-ageing but cancer protective mechanisms and anti-ageing yet pro-cancer phenotypes is often observed [ [13] , [154] ] and may be explained by differing responses to DNA damage. A response which leads to excessive apoptosis or senescence provides protection against mutation and therefore cancer, but at the cost of a pro-ageing phenotype due to stem cell pool depletion or accumulation of senescent cells. It should be noted that evidence for stem cell pool depletion is primarily observed in DNA repair mutant animals or in normal cells and animals that have been exposed to stressors or exogenous agents. The evidence regarding stem cells being depleted under the normal ageing process is scarce [ [155] , [156] , [157] ], and it is more likely that during normal ageing stem cell functional decline occurs [ 158 ].
On the other hand, a lack of cell death following DNA damage may promote longevity, but favours the accumulation of mutations and build-up of pre-malignant cells, resulting in elevated cancer risk. This trade-off phenomenon is exemplified by comparing Cockayne Syndrome and Xeroderma Pigmentosum (XP) both of which are a result of deficiencies in NER. Cockayne Syndrome is due to mutations in the CSA (Ercc8) or CSB (Ercc6) genes involved in the first steps of transcription-coupled repair (TC-NER). When cells with TC-NER deficiency sustain DNA damage they die due to transcriptional stress. This results in an accelerated ageing phenotype because the stem cell pool is depleted, but no cancer arises as the damaged cells are eliminated before they accrue mutations. In Xeroderma pigmentosum, cells are deficient in global-genome nucleotide excision repair (GG-NER) due to mutation of the XPC gene. TC-NER is still functional in these patients and promotes cell survival which delays premature ageing. However, due to the lack of GG-NER, lesions occurring in the non-transcribed genomic regions or in the template strand of active regions frequently result in mutations during replication. Therefore cancer incidence is high in XP patients [ 159 ].
In summary, defects in DNA damage repair can result in an accumulation of mutations or increased cell death, promoting cancer or ageing, respectively. These phenotypes lie at two ends of the spectrum with several possible intermediary phenotypes. In the context of translational medicine, the most interesting and relevant mutants are those displaying both cancer resistance and delayed ageing, for example the previously mentioned super-p53/Arf mice [ 153 ]. Another example is the long-lived C. elegans daf-2 (insulin receptor) mutant which shows resistance to lethal germ-line tumours caused by gld-1 mutation [ 160 ]. The mechanism behind such resistance is thought to be due to increased apoptosis, in which the daf-2 mutant background imposes a metabolic strain on the organism which results in selective apoptosis of the heavily, metabolically-demanding tumour cells but the surrounding normal tissue is not affected.
Growing evidence indicates cellular senescence occurring with age is a result of DNA damage accumulation [ [161] , [162] , [163] ]. Accumulation of senescent cells often results in an adverse senescence associated secretory phenotype (SASP) and can create a pro-tumorigenic environment [ 14 ]. The clearance of senescent cells extends lifespan and delays the onset of cancer [ [164] , [165] , [166] , [167] , [168] , [169] , [170] ].
During normal ageing the function and efficiency of several DNA repair pathways are thought to decline with age [ 171 ] including the p53 response [ 172 ]. A lack of efficient DNA damage repair combined with reduced apoptosis, due to an inefficient p53 response, may contribute to mutation accumulation with age. Overall, these studies demonstrate a functional DNA damage response is required to preserve genomic integrity which is essential for both longevity and cancer protection [ 173 ]. IIS/mTOR signalling regulates ageing and may contribute to some aspects of DNA damage repair and genomic stability. However, whilst it is expected that protection from DNA mutations will reduce cancer incidence, a careful balance is required between tumour suppression and maintenance of functional stem cell pool to ensure a long-life.
6. Stem cells in ageing and cancer
Stem cells play a critical part in renewing our tissues, but repeated cell divisions makes stem cells vulnerable to both transformation and cell death, depending on the type of mutations they accumulate [ 25 ]. Mutant clonal expansion and selection of stem cells occurs within the intestine and hematopoietic system with age [ [174] , [175] , [176] , [177] , [178] ]. In the intestine of humans and mice, this leads to clonal dominance of single ISCs within crypts [ [179] , [180] , [181] , [182] ]. Similarly, in human skin, there is also evidence of clonal dominance and prevalence of clones bearing mutations in NOTCH1, NOTCH2, TP53 and FAT1 [ 157 ]. Intriguingly, NOTCH1's fitness advantage is restricted to the normal oesophageal epithelium because this mutation is not overrepresented in oesophageal cancer [ 106 , 183 ].
Most evidence for the role of ISCs in ageing comes from the fruit fly, Drosophila . ISCs are regularly interspersed throughout the Drosophila gut, unlike mammalian ISCs which are located in crypts. Damage to the fly gut stimulates ISC proliferation to replace dead or dying cells [ [184] , [185] , [186] , [187] , [188] ]. At a young age this is a transient effect, and the stem cells return to quiescence but in aged guts ISC over-proliferation and an increased mis-differentiation is observed [ 184 , 189 , 190 ]. Preserving proliferative homeostasis in the Drosophila gut extends lifespan and reduces the incidence of hyperplasia with age [ 187 , 191 , 192 ] highlighting the importance of ISC quiescence in maintaining tissue integrity with age. These studies did not report directly on ISC genomic integrity, but DNA damage and somatic mutations do accumulate with age in Drosophila [ [193] , [194] , [195] ]. Genomic aberrations arising in the ISCs can drive gut neoplasia and dysplasia [ 25 , 196 , 197 ]. Therefore Drosophila , like humans, also exhibits an increased cancer risk with age [ 198 ]. In the crypts of mammalian intestines, the LGR5 + ISCs are highly proliferative [ 199 ], therefore unlike in Drosophila , quiescence is not important to maintain tissue integrity with age. Instead Wnt signalling appears to be the dominant factor regulating survival and regenerative capacity of ISCs in response to both DNA damage and ageing [ [200] , [201] , [202] , [203] ].
Replication stress is a key source of endogenous DNA damage contributing to both ageing and cancer progression, particularly when stem cells are affected. Ageing Haematopoietic Stem Cells (HSCs) are particularly vulnerable to replication stress, which has been attributed to reduced levels of mini-chromosome maintenance (MCM) helicase components [ 158 , 204 , 205 ]. The old quiescent HSCs have the DNA damage marker γH2AX primarily concentrated in the nucleolus, which houses the rDNA genes required for ribosome biogenesis. rDNA genes have several features, such as multiple repeat clusters and high transcription rates, that make them particularly vulnerable to DNA damage [ 206 ]. Dephosphorylation of γH2AX seems to be hampered in the old quiescent HSCs because of cytoplasmic mislocalisation of PP4c phosphatase, all of which may lead to a decrease in ribosomal biogenesis and therefore limits the functionality of aged HSCs and their ability to regenerate the blood-cell lineage [ 158 , 207 ] . Mutant HSC clones accumulate with age and contribute significantly to the age-related risk of leukaemia in humans [ 177 , 178 , 208 , 209 ].
HSCs enter quiescence when not actively required, however in this state they attenuate their DNA repair responses resulting in an accumulation DNA damage with age. It is only upon re-entry into the cell cycle that DNA repair occurs in these aged HSCs [ 210 ]. Whilst cycling, cells are more likely to experience mutations arising from replication errors [ 211 ], yet may benefit from HR of DSBs during the S and G2 phases. Quiescent cells, on the other hand, may rely on NHEJ, but evidence shows a preference for classical NHEJ and active suppression of the more erroneous alt-NHEJ [ 212 ] indicating an attempt to keep mutation rates low.
Replication stress can shorten lifespan in mice. Loss of MCM2 promotes premature ageing [ 213 ] consistent with observations in murine HSCs [ 158 , 207 ]. ATR deficient mice exhibit dramatically reduced regenerative capacity and accelerated ageing [ 204 , 205 ] which is reflected in the human disease, Seckel syndrome [ 214 ]. Replication stress is also observed in another accelerated ageing syndrome, Ruijs-Aalfs syndrome [ 215 ]. Here, loss of Spartan results in destabilized replication forks that is further aggravated by a lack of translesion synthesis [ [216] , [217] , [218] ].
The attrition of functional HSCs with age may underlie common age-related dysfunctions such as a poor immune system/response and poor wound healing/regenerative capacity. However , in C. elegans the loss of MCM2 actually extends lifespan [ 219 ] and in long-lived daf-2 mutants’, MCM2 expression is decreased compared to wild-types [ 220 ]. The differences between these studies on the relationship between MCM2 levels and ageing may be explained by the fact that C. elegans is largely a post mitotic organism, meaning they do not suffer from high levels of replication stress except in the germline. Alternatively, deficiency in one repair pathway may perhaps cause compensatory upregulation of a different repair pathway, leading to genome protection.
In summary, stem cells can accumulate DNA damage and mutations, leading to clonal selection and expansion, with age in a tissue dependent manner. Although stem cells may possess a level of inherent protection against mutations [ 104 , 107 ], on a population level, this may be contributed to by a low threshold for apoptosis due to increased expression of pro-apoptotic proteins as well as mitochondrial priming [ 221 ].
7. Chromatin status in ageing and cancer
The role of epigenetic modification in facilitating access to DNA damage and in having a more direct role in DNA repair, has been increasingly recognised [ [222] , [223] , [224] ]. Here we will describe the intricate relationship between epigenome and DNA repair which ultimately affects ageing and cancer.
Several changes to histone expression and methylation are observed upon ageing which may affect chromatin structure [ 225 ]. Ageing organisms and senescent cells exhibit reduced levels of repressive heterochromatic marks including H3K9me3, H3K27me3, and H4K20me3 [ [226] , [227] , [228] , [229] ] and an overall global loss and redistribution heterochromatin is a characteristic feature of ageing [ 230 , 231 ].
Levels of heterochromatin protein 1 (HP1α) are diminished in aged human cells and prematurely aged cells [ 232 , 233 ]. The level of H3K9me3 are reduced in both aged fibroblasts and fibroblasts isolated from patients with HGPS (Hutchinson-Gilford progeria syndrome), a premature ageing syndrome [ 234 ]. Cellular models of the accelerated ageing syndrome, Werner's, also report a global loss of H3K9me3 and an interaction with HP1α [ 235 ]. In C. elegans, lifespan is extended by inhibition of H3K27 demethylases but this lifespan extension could be mediated by the associated changes in IIS observed in these worms [ 236 ]. In Drosophila , a lack of functional HP1 reduces lifespan and overexpression of HP1 improves longevity [ 237 ] suggesting that increases heterochromatin do promote longevity in Drosophila . These studies suggest disorganisation and loss of heterochromatin promote ageing and maintenance of heterochromatin with age increases longevity.
Links between epigenetic modifications and lifespan were first highlighted in studies of yeast in which Sir2, an NAD + -dependent histone deacetylase, was overexpressed [ 238 , 239 ]. Additional evidence for the role of sirtuins in ageing comes from work on SIRT6. SIRT6 deficiency in mice results in premature ageing [ 240 ]. Overexpression of SIRT6 reduces genomic instability [ 241 ], increases DSB repair efficiency by both the HR and NHEJ pathways and extends lifespan [ 149 , 242 ]. Of the two activities associated with SIRT6, mono-ADP-ribosyl transferase and histone acetylase, the former is proposed to increase DSB repair efficiency via PARP1 activation [ 243 ]. The authors highlight how longevity across several species correlates with DSB repair efficiency and not with efficiency of other repair pathways such as NER. Instead NER efficiency shows correlation or coevolution with the level of sun exposure per species [ 149 ]. The deacetylase activity could still affect DNA damage via the level of chromatin compaction. A lack of deacetylase activity would relax the chromatin and potentially increase exposure to DNA damaging agents [ 244 ]. Since the histone deacetylase activity of sirtuns depends on NAD + levels, as does the activity of multiple DNA damage repair enzymes, including PARPs, it has been suggested that maintaining NAD + levels, which are known to decline with age, may protect the DNA and have anti-ageing effects [ [245] , [246] , [247] ].
Another epigenetic modification, DNA methylation, is also closely linked to ageing [ 248 ]. This has been exemplified through the recent advent of epigenetic ageing clocks used to predict biological age from the DNA methylome [ [249] , [250] , [251] ]. Well-known anti-aging interventions, such as dietary restriction, also have measurable effects on the epigenome [ 252 ]. Chromatin remodellers play key roles in DNA repair, genome stability and in preventing tumorigenesis [ 222 , 253 , 254 ]. Chromatin genes that are often mutated in cancer, for example H3.3 which is linked to paediatric glioblastoma [ 255 , 256 ], also have roles in lifespan regulation [ 257 , 258 ].
Overall, links between epigenetic modifications and ageing are complex due to the gene regulation that accompanies changes in chromatin packaging. However, it is clear that the regulation of the chromatin affects both DNA repair efficiency and subsequent mutation rate both of which contribute to ageing and cancer. Most studies indicate that maintenance of heterochromatin throughout ageing and efficient DSB repair promote longevity, yet how heterochromatin affects cancer risk/rate is not yet clear.
8. Summary and future prospects
Maintaining genome integrity is key for longevity and reduced cancer risk and we argue here that interventions that lower mutations are expected to improve ageing and delay cancer. It should be noted that in a controlled and limited way mutations can be beneficial. For instance, normal functioning of the adaptive immune system and somatic hypermutation process depend on mutations introduced by AID (activation-induced cytidine deaminase), which enables production of antibodies with greater antigen affinity [ 259 ]. In single cell organisms, such as bacteria, mutations enable survival in presence of antibiotics [ 260 ], and mutation frequency is sometimes adjusted depending on stressful conditions in the environment [ 261 ]. Despite mutations being essential for evolution, they are mostly detrimental, and repair of DNA damage must therefore occur effectively to prevent both mutations and excessive cell death, which would otherwise lead to increased cancer risk or accelerated ageing, respectively. The high mutational burden of normal, somatic cells was surprising [ [105] , [106] ], as is the discovery that particular populations of stem cells may be protected from DNA damage and mutation [ 104 , 107 ]. Both studies highlight how clonal selection and expansion are key factors in understanding cancer risk and ageing and are of interest for potential therapeutic interventions. Stem cells are key in the balance between cancer and ageing, it will be interesting to see whether alterations to chromatin status or reductions in replication and transcription stresses are present in ‘protected’ stem cells [ 262 ] and how this can be used for improvement of health and disease prevention. For understanding of high mutational burden in normal cells it will be important to clarify which endogenous and exogenous molecules are causing mutations [ 2 ] and some indication can be provided by mutational signatures [ 23 , 93 , 111 , 263 ]. Many commonly used laboratory chemicals that damage DNA, and which helped uncover details about DNA repair, are not necessarily chemicals that our cells most commonly encounter and more work on mutation causing agents in humans are needed [ 16 ]. Another future challenge would be to develop treatments that could boost error-free DNA repair. However, this is challenging because most DNA repair enzymes work in complexes and overexpression of one of them will not result in balanced and improved DNA repair. Expanding our knowledge around the enhancement of select aspects of DNA repair capacity and the mechanisms which can confer protection against DNA damage infliction will be critical to simultaneously promote longevity and reduce cancer risk.
Credit author statement
Eleanor Rachel Stead: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition, Ivana Bjedov: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition.
Acknowledgment
IB acknowledges funding from ERC StG 311331, ERC PoC 842174, CRUK-UCL Centre Award [C416/A25145], Radiation Research Unit at the Cancer Research UK City of London Centre Award [C7893/A28990], Royal Society Research Grant (RSG/R1/180431) and the Bill Lyons foundation. ERS is grateful for MRC PhD studentship funding.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.10A: DNA Repair
- Last updated
- Save as PDF
- Page ID 9295

Most mistakes during replication are corrected by DNA polymerase during replication or by post-replication repair mechanisms.
LEARNING OBJECTIVES
Explain how errors during replication are repaired
Key Takeaways
- Mismatch repair enzymes recognize mis-incorporated bases, remove them from DNA, and replace them with the correct bases.
- In nucleotide excision repair, enzymes remove incorrect bases with a few surrounding bases, which are replaced with the correct bases with the help of a DNA polymerase and the template DNA.
- When replication mistakes are not corrected, they may result in mutations, which sometimes can have serious consequences.
- Point mutations, one base substituted for another, can be silent (no effect) or may have effects ranging from mild to severe.
- Mutations may also involve insertions (addition of a base), deletion (loss of a base), or translocation (movement of a DNA section to a new location on the same or another chromosome ).
- mismatch repair : a system for recognizing and repairing some forms of DNA damage and erroneous insertion, deletion, or mis-incorporation of bases that can arise during DNA replication and recombination
- nucleotide excision repair : a DNA repair mechanism that corrects damage done by UV radiation, including thymine dimers and 6,4 photoproducts that cause bulky distortions in the DNA
Errors during Replication
DNA replication is a highly accurate process, but mistakes can occasionally occur as when a DNA polymerase inserts a wrong base. Uncorrected mistakes may sometimes lead to serious consequences, such as cancer. Repair mechanisms can correct the mistakes, but in rare cases mistakes are not corrected, leading to mutations; in other cases, repair enzymes are themselves mutated or defective.
Most of the mistakes during DNA replication are promptly corrected by DNA polymerase which proofreads the base that has just been added. In proofreading, the DNA pol reads the newly-added base before adding the next one so a correction can be made. The polymerase checks whether the newly-added base has paired correctly with the base in the template strand. If it is the correct base, the next nucleotide is added. If an incorrect base has been added, the enzyme makes a cut at the phosphodiester bond and releases the incorrect nucleotide. This is performed by the exonuclease action of DNA pol III. Once the incorrect nucleotide has been removed, a new one will be added again.

Some errors are not corrected during replication, but are instead corrected after replication is completed; this type of repair is known as mismatch repair. The enzymes recognize the incorrectly-added nucleotide and excise it; this is then replaced by the correct base. If this remains uncorrected, it may lead to more permanent damage. How do mismatch repair enzymes recognize which of the two bases is the incorrect one? In E. coli , after replication, the nitrogenous base adenine acquires a methyl group; the parental DNA strand will have methyl groups, whereas the newly-synthesized strand lacks them. Thus, DNA polymerase is able to remove the incorrectly-incorporated bases from the newly-synthesized, non-methylated strand. In eukaryotes, the mechanism is not very well understood, but it is believed to involve recognition of unsealed nicks in the new strand, as well as a short-term continuing association of some of the replication proteins with the new daughter strand after replication has been completed.

In another type of repair mechanism, nucleotide excision repair, enzymes replace incorrect bases by making a cut on both the 3′ and 5′ ends of the incorrect base. The segment of DNA is removed and replaced with the correctly-paired nucleotides by the action of DNA pol. Once the bases are filled in, the remaining gap is sealed with a phosphodiester linkage catalyzed by DNA ligase. This repair mechanism is often employed when UV exposure causes the formation of pyrimidine dimers.

DNA Damage and Mutations
Errors during DNA replication are not the only reason why mutations arise in DNA. Mutations, variations in the nucleotide sequence of a genome, can also occur because of damage to DNA. Such mutations may be of two types: induced or spontaneous. Induced mutations are those that result from an exposure to chemicals, UV rays, X-rays, or some other environmental agent. Spontaneous mutations occur without any exposure to any environmental agent; they are a result of natural reactions taking place within the body.
Mutations may have a wide range of effects. Some mutations are not expressed; these are known as silent mutations. Point mutations are those mutations that affect a single base pair. The most common nucleotide mutations are substitutions, in which one base is replaced by another. These can be of two types: transitions or transversions. Transition substitution refers to a purine or pyrimidine being replaced by a base of the same kind; for example, a purine such as adenine may be replaced by the purine guanine. Transversion substitution refers to a purine being replaced by a pyrimidine or vice versa; for example, cytosine, a pyrimidine, is replaced by adenine, a purine. Mutations can also be the result of the addition of a base, known as an insertion, or the removal of a base, known as a deletion. Sometimes a piece of DNA from one chromosome may get translocated to another chromosome or to another region of the same chromosome.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Boundless. Provided by : Boundless Learning. Located at : www.boundless.com//biology/definition/nucleotide-excision-repair. License : CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. November 4, 2013. Provided by : OpenStax CNX. Located at : http://cnx.org/content/m44513/latest/?collection=col11448/latest . License : CC BY: Attribution
- mismatch repair. Provided by : Wikipedia. Located at : en.Wikipedia.org/wiki/mismatch%20repair. License : CC BY-SA: Attribution-ShareAlike
- DNA Repair. Provided by : Wikipedia. Located at : http://en.Wikipedia.org/wiki/File:DNA_Repair.jpg . License : Public Domain: No Known Copyright
ORIGINAL RESEARCH article
Integrated transcriptome and metabolome profiling reveals mechanisms underlying the infection of cytospora mali in “jin hong” branches.

- 1 College of Horticulture and Plant Protection, Inner Mongolia Agricultural University, Hohhot, China
- 2 Beijing Engineering Research Center for Edible Mushroom, Institute of Plant Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China
Introduction: Valsa canker, caused by Cytospora mali , is a destructive disease in apple production. However, the mechanism by which apple defend against C. mali infection remains unclear.
Methods: In this study, the integrative transcriptional and metabolic analysis were used to investigate the responses of the ‘Jin Hong’ apple branches to the invasion of C. mali .
Results and Discussion: Results showed that the differentially expressed genes were mainly enriched in the pathways of carbon metabolism, photosynthesis-antenna proteins, and biosynthesis of amino acids pathways. Additionally, the differentially accumulated metabolites were significantly enriched in aminoacyl-tRNA biosynthesis, fructose and mannose metabolism, and alanine, aspartate, and glutamate metabolism pathways. Conjoint analysis revealed that C. mali infection significantly altered 5 metabolic pathways, 8 highly relevant metabolites and 15 genes of apples. Among which the transcription factors WRKY and basic domain leucine zipper transcription family were induced, the α-linolenic acid and betaine were significantly accumulated in C. mali infected apple stems. This work presents an overview of the changes in gene expression and metabolic profiles in apple under the inoculation of C. mali , which may help to further screen out the mechanism of plant-pathogen interaction at the molecular level.
1 Introduction
Apple Valsa Canker (AVC), also known as Cytospora canker, is caused by the necrotrophic fungus Cytospora mali Grove ( Yin et al., 2015 ; Wang D. L. et al., 2020 ). AVC stands out as one of the most destructive diseases affecting apples ( Malus domestica ) in the world, leading to significant yield losses and decreased fruit quality. Extensive surveys have revealed that the incidence of AVC averaged approximately 53% in the major apple production regions of China, and reached up to 80% in severely affected orchards ( Meng et al., 2019 ).
AVC mainly occurs on the branches of trees, the infected tissues then gradually dry out, collapse slightly, and finally form localized cankers and even kills entire apple trees ( Feng et al., 2023 ). C. mali can extensively penetrate into the host’s phloem and xylem, which is difficult for traditional chemical agents to access, and making it harder to prevent ( Yin et al., 2015 ).
The molecular mechanism of apples in responses to C. mali infection has been explored in recent years. It is reported that apples could resistant against AVC by negatively regulating the production of phloridzin ( Zhou et al., 2019 , 2021 ). Mao et al. (2021) revealed that MdCN11 and MdCN19 genes could negatively regulate the AVC resistance via inducting hypersensitive response. DEGs related to hormonal, Ca 2+ signaling, and phenylpropanoid biosynthesis were highly expressed to enhance the apple’s resistance to C. mali ( Yin et al., 2016 ; Zuo et al., 2018 ). Wen et al. (2023) established an efficient system for screening disease-resistant genes in response to AVC. In addition, the apple activates and accumulates defense-related enzymes and metabolites to protect itself from C. mali , such as phenylalanine ammonia-lyase (PAL), β-1,3-glucanase, and chitinase, dopamine, trans-cinnamic acid, coumarin as well as chlorogenic acid and other phenylpropanoids and flavonoids ( Sung and Lee, 2010 ; Yu et al., 2016 ; Moneo-Sánchez et al., 2018 ; Geng et al., 2020 ; Liu X. M. et al., 2021 ; Liu X. Z. et al., 2021 ; Zhao et al., 2021 ; Du et al., 2023 ; Li C. R. et al., 2023 ). In recent years, the metabolomics and transcriptomics have become a research hotspot to explore the molecular mechanisms of disease resistance in apples against pathogens. Zhao et al. (2022) revealed mechanisms of disease resistance in apples induced by Wickerhamomyces anomalus , using the integrated transcriptomic and metabonomic analysis. The transcriptomics was used to explore the molecular mechanisms of apples in responses to C. mali infection ( Feng et al., 2023 ). However, there are no studies on combined transcriptomics and metabolomics analyses of apples by challenging with C. mali , and some highly relevant genes and metabolites remain unexplored.
This work conducted an integrative analysis of the transcriptome and metabolome of apple branches challenging with C. mali . Significant changes were observed in both the transcriptional and metabolic profiles. A conjoint analysis was then performed to identify co-enrichment pathways in both transcriptional and metabolic data. A number of altered pathways, as well as highly correlated genes and metabolites, were identified. These findings will deepen our understanding of AVC caused by C. mali , and provide valuable insights for further investigation into the molecular mechanisms of apples against AVC.
2 Materials and methods
2.1 plant materials and fungal strains.
C. mali (strain QH2), one of the main species causing AVC in northern China ( Ma et al., 2020 ), was used in this study. The strain was isolated from the infected stems of apple tree in Hohhot, Inner Mongolia. The apple branches used in this study were obtained from the orchard in the Inner Mongolia Agricultural University’s campus, the collected branches were inoculated with C. mali QH2 following Zang’s method ( Zang et al., 2007 ). Briefly, branches from “Jin Hong” apple tree were cut into 30 cm length, immersed in 1% sodium hypochlorite for 10 min, 75% alcohol for 3 min, and washed with sterile water 3 times. The two ends of the twigs were sealed by dipping in heated liquid wax and the middle of the twigs was scalded by a soldering iron 6 mm in diameter. C. mali QH2 disks (Φ = 6 mm) were placed on the scalded area. The fungal disks were wrapped with sterile wet degreasing cotton and preservative film. Healthy branches inoculated with agar were treated as control. Treated branches were placed in a pallet with two players of gauze, and incubated in an illumination incubator with a 16 h light/8 h dark cycle, 85% humidity at 25°C for 9 days ( Figure 1 ). Approximately 2.0 g of bark tissue, including buds, from the borders of infected area was sampled. Each sample was divided into two parts, for transcriptomic and metabolomic analysis, respectively.

Figure 1 . The symptoms of ‘Jin Hong’ apple branches infected with C. mali .
2.2 Total RNA extraction, library construction, and sequencing
The total RNA was extracted using the Takara Mini BEST plant RNA Extraction Kit (Takara, Kyoto, Japan) followed by the instructions. The RNA quality was assessed using the NanoPhotometer ® spectrophotometer (IMPLEN, LA, CA, United States) and the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, Palo alto, CA, United States).
A total of 1 μg RNA per sample was used for RNA sample preparation. The NEBNext ® UltraTM RNA Library Prep Kit for Illumina ® (NEB, Ipswich, MA, USA) was used to generate sequencing libraries. Index codes were added to attribute sequences to each sample.
Briefly, mRNA was enriched by poly A tail selection and chemically fragmented. Subsequently, second strand cDNA synthesis was performed using DNA Polymerase I and RNase H. The synthesized cDNA was then subjected to end-repair and “A” base addition according to Illumina’s library construction protocol. To select cDNA fragments for PCR, we purified the library fragments using AMPure XP beads (Beckman Coulter, Beverly, United States) to ensure a length of 250–300 bp. We also purified the PCR products and assessed the library quality on the Agilent Bioanalyzer 2100 system. Following cluster generation, we sequenced the library preparations on an Illumina Novaseq platform, generating 150 bp paired-end reads.
2.3 Data quality control
We performed splicing and quality control on the raw data. Aligned paired-end clean reads to the reference genome using HISAT2 v2.0.5. Used Feature Counts (1.5.0-p3) to calculate the number of reads mapped to each gene. Then calculated the fragments per kilobase of transcript per million mapped reads (FPKM) for each gene based on gene length and determine the number of reads mapped to that gene.
2.4 Differential expression analysis
Differentially expressed genes (DEGs) were identified using the DESeq2 R package (version 1.16.1) ( Varet et al., 2017 ). Differential expression analysis of two conditions was performed using the edgeR R package (version 3.18.1) ( Dietz et al., 2010 ; McCarthy et al., 2012 ; Varet et al., 2017 ).
The resulting p were adjusted using the Benjamini and Hochberg’s approach ( Yoav and Yosef, 2000 ) to control for false discovery rate. DEGs were determined according to the following general rules: |Log2FoldChange(FC)| > 1 and p -adjust ≤0.05. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway functional enrichment analyses were performed using the clusterProfiler R package (version 3.4.4) ( Wu et al., 2021 ).
2.5 Metabolite extraction
The metabolite was extracted using a solution of methanol/acetonitrile/water in a 2: 2: 1 (v/v) ratio. The mixture was vortexed and subjected to low-temperature sonication for 30 min and stand at −20°C for 10 min, then centrifuged (Eppendorf, 5430R, Hamburg, Germany) at 14,000 g at 4°C for 20 min, the supernatant was collected and vacuum dried. For mass spectrometry analysis, 100 μL of acetonitrile/water solution (acetonitrile: water = 1: 1, v/v) was added for reconstitution, followed by vertexing, the reconstituted sample was then centrifuged at 14,000 g for 15 min at 4°C to obtain the supernatant.
2.6 Non-target metabolomics
Metabolites were chromatographically separated using an ultra-high performance liquid chromatography (UHPLC) system (Agilent 1290 Infinity LC, Agilent Technologies, Palo Alto, California, United States). The UHPLC system was equipped with an ACQUITY BEH C18 column (100 mm × 2.1 mm column, 1.7 μm, Waters, Milford, MA, United States). The mobile phases consisted of solvent A (Water +25 mM ammonium acetate +25 mM ammonia) and solvent B (acetonitrile). The gradient elution program was set as follows to equilibrate the systems: from 0 to 0.5 min, 95% (B); from 0.5 to 7 min, 95 to 65% (B); from 7 to 8 min, 65 to 40% (B); from 8 to 9 min, 40% (B); 9 to 9.1 min, 40 to 95% (B); from 9.1 to 12 min, 95% (B). The sample injection volume was 2 μL, the flow rate was 0.5 mL min −1 , and the column temperature was maintained at 25°C throughout the chromatographic separation.
Mass spectra of the compounds were obtained using an AB Triple TOF 6600 (AB SCIEX, Framingham, United States). ESI source conditions after HILIC chromatographic separation Ion source Gas1 (Gas1): 60, Ion source Gas2 (Gas2): 60, curtain gas (CUR): 30, source temperature: 600°C Ion source voltage floating (ISVF) ± 5,500 V; TOF MS scan m/z range: 60–1,000 Da, product ion scan m/z range: 25–1,000 Da, TOF MS scan accumulation time 0.20 s/spectra, product ion scan accumulation time 0.05 s/spectra; MS2 data were acquired using information dependent acquisition (IDA) and operated in high sensitivity mode, declustering potential (DP): ± 60 V, collision energy: 35 ± 15 eV, exclude isotopes within 4 Da, candidate ions to monitor per cycle: 10. Samples were analyzed in both positive (+) and negative (−) ESI modes.
Raw data in Wiff format were converted to mzXML format using ProteoWizard ( Darren et al., 2008 ) and then peaks were aligned and peak areas extracted using XCMS software ( Domingo-Almenara and Siuzdak, 2020 ). The data extracted by XCMS were used for metabolite structure identification and data pre-processing, quality evaluation, and multi-variate analysis.
2.7 Differentially accumulated metabolites analysis
Orthogonal partial least squares discriminant analysis (OPLS–DA) was used to determine the differentially accumulated metabolites between pairwise groups. The p was estimated using a Hotelling’s T2 test for statistical analysis. Differentially accumulated metabolites (DAMs) (VIP ≥ 1, |Log2FC| > 1) between groups were mapped into biochemical pathways using metabolic enrichment and pathway analysis based on MetaboAnalyst. 1 Those pathways with p ≤ 0.05 were considered significantly enriched.
2.8 Statistical analysis
OPLS–DA was performed using SIMCA 14.1 (Umetrics, Umea, Sweden). The data were log-transformed. The reliability of the predictive models was assessed using the analysis of variance test of cross-validated predictive residuals (CV–ANOVA), the coefficient of determination (R2) and the predicted variance (Q2) which is the proportion of the total variation of X or Y that can be predicted by a component. To test the overall associations between DEGs and DAMs, the DEGs and DAMs co-enriched in KEGG pathways were identified by KEGG analysis. The DEGs and DAMs were subjected to the integration analysis using regularized canonical correlation analysis (rCCA) ( Cruz-Cano and Lee, 2014 ) to explore their relationships. rCCA was implemented with mixOmics ( Rohart et al., 2017 ) using the R program. The regularization parameters (lambda1, lambda2) were optimized using the tune.rcc function. The network communities were determined using the Fast Greedy algorithms with the clusterMaker program ( Morris et al., 2011 ; Utriainen and Morris, 2023 ) and visualized using Cytoscape 3.10.1 ( Zhou et al., 2022 ). Network attributes were calculated using Cytoscape’s built-in network analyzer. Bioinformatics analysis was performed using dynamic real-time interactive online platforms including APPLIED PROTEIN TECHNOLOGY, 2 Metware Cloud, 3 Majorbio, 4 Omicsmart, 5 and bioinformatics. 6
3.1 Transcriptional profiling reveals altered pathways and genes in diseased apple branches
The transcriptome datasets generated in this work are deposited in the NCBI repository with the accession number: PRJNA1074127, following the link: https://www.ncbi.nlm.nih.gov/sra/PRJNA1074127 . The PCA analysis revealed that the two principal components explained 60.9 and 15.7% of the variation and effectively separated samples from different stages ( Figure 2A ). The Venn analysis showed that 1,271 genes were unique to the control group, 1,107 genes were unique to AVC, and 37,511 genes were shared between the two groups ( Figure 2B ). The genes related to cytochrome P450, protein kinase domain, and homeobox domain-like were the most prevalent among control-specific genes. Among AVCs, the highest percentage of genes were for protein kinase domain, leucine-rich repeat, and cytochrome P450 ( Figure 2C ). The most predominant genes shared by both groups were for protein kinase domain, serine–threonine/tyrosine-protein kinase, and catalytic domain ( Figure 2D ). The results indicated that the expression of 5, 413 DEGs was up-regulated and that of 5, 298 DEGs was down-regulated in the AVC group compared to the control group ( Figure 2E ). The DEGs identified were annotated using KEGG enrichment analysis. The up-and down-regulated DEGs were enriched in different pathways. The up-regulated genes were mostly enriched in the proteasome, biosynthesis of amino acids and oxidative phosphorylation. On the other hand, the down-regulated genes were enriched in photosynthesis—antenna proteins, plant hormone signal transduction and photosynthesis ( Figures 2F , G ). The KEGG analysis of all DEGs revealed that the pathways were significantly involved in carbon metabolism, photosynthesis-antenna proteins, and biosynthesis of amino acids ( Supplementary Table S1 ).
Figure 2 . Gene expression analysis of “Jin Hong” in response to AVC infection. (A) Principal component analysis (PCA) of transcriptome data. (B) The venn plot of differentially expressed genes. (C) The unique genes of two groups. (D) The genes shared by both groups. (E) The volcano plot showing the up-and down-regulated genes ( p -adjust < 0.05, |Log2FC| > 1, red represents up-regulated genes, blue represents down-regulated genes, and gray indicates not significantly expressed genes, text highlighted for up-and down-regulated Log2FC top 5 genes). (F,G) The KEGG enriched analysis of the up-and down-regulated genes in AVC _vs_ Control ( p -adjust < 0.05).
After performing GO analysis on the up-regulated genes, the most significantly enriched molecular function (MF) were threonine-type endopeptidase activity and threonine-type peptidase activity. Additionally, the most significantly enriched cellular component (CC) was organelle membrane. Moreover, the down-regulated mRNAs indicate significant enrichment in thylakoid, thylakoid part, and photosynthetic membrane ( Supplementary Table S2 ).
3.2 Metabolic profiling reveals altered pathways and metabolites in diseased apple branches
A summary table highlighting the expressed metabolites can be found in Supplementary Table S3 . The OPLS-DA analysis showed a significant difference between the control and AVC groups ( Figure 3A ). The identified metabolites were annotated using the HMDB database and classified into the following superclass categories: 16 phenylpropanoids and polyketides, 1 organooxygen compound, 16 organoheterocyclic compounds, 43 organic oxygen compounds, 5 organic nitrogen compounds, 40 organic acids and derivatives, 13 nucleosides, nucleotides, and analogs, 1 organonitrogen compound, 17 lipids and lipid-like molecules, 2 lignans, neolignans and related compounds, 11 benzenoids, and 20 metabolites of unknown classification. The top 30 metabolites, as shown in the loading plot (VIP > 1), were classified into 9 superclass categories ( Figure 3B ). The bar charts illustrated the significant regression coefficients of each compound for the different groups ( Figure 3C ). The number of compounds with significant correlations differed between the two groups. The metabolites of benzoic acid, L-threonate and L-gulonic gamma-lactone were positively correlated with the AVC group, while the metabolites of dulcitol, tyramine, and l-phenylalanine showed a negative correlation. Heatmap showed different superclasses of identified metabolites ( Figure 3D ). The volcano plot illustrated the DAMs between AVC and control. There were 2,433 up-regulated and 2,977 down-regulated DAMs ( Figure 3E ). The DAMs between the two groups were mapped to the KEGG database. The identified 48 DAMs were mainly enriched in pathways of aminoacyl-tRNA biosynthesis, fructose and mannose metabolism, alanine, aspartate and glutamate metabolism, galactose metabolism, and isoquinoline alkaloid biosynthesis ( Figure 3F ; Supplementary Table S4 ).
Figure 3 . Metabolic profiling of “Jin Hong” in response to AVC infection. (A) OPLS–DA analysis of the metabolome data of all samples (R2Xcum = 0.764, R2Ycum = 0.980, Q2cum = 0.969, CV–ANOVA, p = 5.577 × 10 −3 ). (B) OPLS–DA loading showing the top 30 metabolites (VIP > 1), color indicates the superclass. (C) Correlation coefficient plots of OPLS–DA showing the relationship between the X and Y variables for the predictive components. The size of the scaled coefficient represents the change in the Y variable when the X variable varies from 0 to 1 in coded units (Selection of the top 5 substances with positive and negative correlation coefficients). (D) The heatmap shows the qualitative names of metabolites, the green bars represent the VIP values of the metabolites and the red dashed line indicates VIP = 1, different colored lines indicate the superclass classification of metabolites. (E) The volcano plot of DAMs in ‘Jin Hong’ upon exposure to AVC ( p < 0.05, |Log2FC| > 1, red represents up-regulated metabolites, blue represents down-regulated metabolites, and gray indicates not significantly expressed genes, text highlighted for up-and down-regulated|Log2FC|top 5 metabolites). (F) The top 10 enriched KEGG pathways of DAMs in “Jin Hong” upon exposure to AVC infection ( p < 0.05).
3.3 Comparative transcriptional and metabolic profiling revealed co-enrichment of pathways
Venn analysis results indicated that DEGs and DAMs were both enriched in 45 KEGG metabolic pathways, among which, 5 were significantly enriched ( p < 0.05) ( Figure 4A ). Figure 4B illustrated pathways associated with plant stress tolerance, including 4 of the 45 co-enriched KEGG pathways and 8 pathways specific to DEGs. The DEGs and DAMs were significantly enriched in 5 KEGG pathways, including galactose metabolism, fructose and mannose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glycine, serine and threonine metabolism, and isoquinoline alkaloid biosynthesis. Some metabolites are associated with plant resistance to adversity stress, and the metabolites in the 5 significantly enriched shared pathways contain sorbitol, D-mannose, tyramine, L-tryptophan, and betaine ( Figures 4C , D ).
Figure 4 . Transcriptome-metabolome-wide association of KEGG pathways. (A) The venn plot of DEGs and DAMs enriched all KEGG pathways and significant KEGG pathways. (B) The pathways associated with plant stress resistance among the 45 commonly enriched pathways as well as those unique to DEGs. (C,D) The heatmap of metabolites and genes among the 5 commonly significant enriched pathways, the triangles are labeled with substances associated with plant stress tolerance and the different colored columns indicate KEEG pathways.
3.4 Combined transcriptional and metabolic profiling revealed relevant genes and metabolites
Strong associations were found between genes and metabolites by procrustes analysis ( Figure 5A ). To identify genes and metabolites that are highly correlated with changes in apples in response to infestation with C. mali , the 45 DEGs and DAMs co-enriched pathways were subjected to a correlation analysis using the rCCA method (threshold > 0.90). The correlation network revealed 3 clear gene-metabolite regulatory communities ( Figure 5B ). In community 1, 94 transcripts exhibited a strong correlation coefficient with L-glutamine and 1-aminocyclopropanecarboxylic acid. Based on the magnitude of the betweenness centrality value, these metabolites were found to be highly correlated with genes MD01G1037400, MD05G1023900, MD14G1102200, MD15G1077000, and MD15G1204200. The interaction networks between the 15 metabolites and 79 transcripts were organized in control and AVC in community 2. The metabolites D-glucuronolactone and alpha-linolenic acid (ALA) had higher connectivity with genes MD00G1112500, MD02G1123600, MD04G1246300, MD10G1063600, and MD00G1061700. Community 3 was the largest gene-metabolite association, consisting of 13 metabolites and 204 genes. The genes MD16G1179500, MD17G1068900, MD07G1159300, MD12G1101800, and MD09G1078700 were found to be more correlated with the metabolites 2-oxoadipate and biotin ( Supplementary Table S5 ). Additionally, we constructed a correlation network of DEGs and DAMs in 5 co-enriched pathways on the Pearson correlation coefficient (PCC) analysis results. Figure 5C showed that 15 DEGs and 8 DAMs in the network were highly correlated (PCC > 0.99). These genes and metabolites may play a crucial role in the defense of apples against AVC.
Figure 5 . Transcriptome-metabolome-wide association network of all samples. (A) Procrustes analysis between all genes and metabolites from all samples. (B) Integration network of highly association genes and metabolites at threshold of 0.9. (C) Correlation analysis of genes and metabolites in 5 commonly significant pathways (threshold>0.99), blue ovals indicate metabolites, pink squares indicate genes, and red line segments indicate positive correlations and blue line segments indicate negative correlations.
4 Discussion
Almost all apple trees can be infected by AVC ( Ke et al., 2014 ; Haxim et al., 2022 ). Plants have developed various mechanisms to cope with pathogenic stresses, such as forming physical barriers and activating their immune system, which mainly includes reinforcing the cell wall, producing reactive oxygen species, altering hormone levels and gene expression, activating pathogenesis-related proteins, accumulating defensive metabolites, and inducing the allergic necrosis of plant cells ( Yin et al., 2015 ; Wang X. L. et al., 2020 ; Liu X. M. et al., 2021 ; Liu X. Z. et al., 2021 ; Wang et al., 2022 ; Xin et al., 2023 ). In this study, the RNA-seq and UHPLC–MS/MS techniques were utilized to investigate the changes in genes and metabolites in apple branches during C. mali infection, 5 metabolic pathways were significantly enriched: galactose metabolism, fructose and mannose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glycine, serine and threonine metabolism, and isoquinoline alkaloid biosynthesis. Furthermore, the 15 key genes and 8 DAMs (tyramine, L-tyrosine, L-phenylalanine, sorbitol, L-tryptophan, L-threonine, melibiose, and betaine) that may be associated with plant’s reaction to pathogen stress were identified.
The cell wall provides initial protection and signal perception against pathogen attacks through sensing and regulating related components on the cell wall ( Sebastian, 2022 ; Xin et al., 2023 ). Fungal pathogens have developed various combinations of plant cell wall degrading enzymes to break them down ( Yin et al., 2015 ; Xin et al., 2023 ). β-galactosidase (β-Gal) is an essential cell wall glycosyl hydrolase in apple ( Yin et al., 2015 ; Li X. L. et al., 2023 ). The gene MD02G1287500 encoding β-Gal was found to be down-regulated in the galactose metabolite pathway in this work, this result is consistent with previous results, that apples may slow down the degradation of the cell wall by decreasing the expression of β-Gal to resist pathogen invasion ( Moneo-Sánchez et al., 2018 ; Zhao et al., 2021 ; Feng et al., 2023 ).
UDP-glucose catalyzes the glycosylation of phloretin to phloridzin, the pathogen could utilize phloridzin to produce toxins that facilitate necrosis in apple bark ( Zhou et al., 2021 ). Our findings showed a 4-fold down-regulation of the UDP-glucose gene MD13G1093700 in the galactose metabolism pathway and decreased accumulation of its metabolite phenothiazine, although this change was not statistically significant. So this result is consistent with the previous finding that the apples could enhance AVC resistance by decreasing the phloridzin biosynthesis, and the down expression of UDP-glucose genes, which may indirectly modulating cell wall deposition and increasing hormone levels ( Zhou et al., 2019 , 2021 ; Feng et al., 2023 ).
Once the pathogen has penetrated the external cell wall, the intracellular immune system is triggered. Several reports have demonstrated that the WRKY and basic domain leucine zipper (bZIP) gene families play significant roles in various biological processes, including physiological metabolism and stress response ( Alves et al., 2013 ; Jiang et al., 2017 ), and are particularly involved in resisting C. mali in apples ( Tsuda and Somssich, 2015 ; Geng et al., 2020 ; Liu X. M. et al., 2021 ; Liu X. Z. et al., 2021 ; Mao et al., 2021 ; Wen et al., 2023 ). Moreover, WRKYs played a crucial role in mediating the communication between jasmonic acid and salicylic acid and are integral to the plant’s defense responses ( Phukan et al., 2016 ; Lui et al., 2017 ; Han et al., 2023 ). Transcriptomic analysis in this study revealed that the invasion of C. mali activated the expression of WRKY and bZIP genes in apple. There were significant increase in the expression of the WRKY genes (MD13G1122100, MD09G1008800, MD17G113810, and MD03G119760) and bZIP gene (MD08G1025800) in the diseased apple branches. These high expression in WRKY and bZIP genes might associated with the apple’s defense response.
The destruction of cellular membranes in pathogen-affected plants releases a large number of polyunsaturated fatty acids, which act as signal molecules to activate plant defense responses ( De Carvalho and Caramujo, 2018 ). For example, α-linolenic acid (ALA) serves as a precursor to jasmonic acid, a key phytohormone and signaling molecule that mediates plant stress response ( Per et al., 2018 ; Bizuneh, 2020 ; Muñoz-Hoyos and Stam, 2023 ; Xin et al., 2023 ). The KEGG analyses of DEGs in this study revealed a significant up-regulation of fatty acid metabolic pathways, which is consistent with previous reports ( Ke et al., 2014 ; Yin et al., 2016 ), and cluster 2 of the network showed a strong association between the up-regulated metabolite ALA and several genes. All these results underscore the crucial roles of the metabolite ALA in apple resistance to AVC.
Betaine could enhance the stability of biomacromolecular structure and function, and reduce ROS accumulation by preserving the activity of ROS scavenging enzymes ( Huang et al., 2020 ), which leads to improved plant resistance against various abiotic stresses such as metal ions, salt, low temperature, and drought ( Li C. Y. et al., 2021 ; Jokinen et al., 2022 ). Moreover, exogenous betaine was added to enhance apple resistance against anthracnose leaf blight ( Liu et al., 2022 ). In our study, we observed a significant accumulation of the betaine in the Glycine, serine, and threonine metabolism pathway. Combining our findings with previous research, we hypothesize that betaine may play a crucial role in apple resistance to C. mali invasion.
The phenylpropanoid pathway leads to synthesis of different types of flavonoid phytoalexins and phenolic compounds, which are involved in plant defense ( Li P. Q. et al., 2021 ). The pathway starts with phenylalanine, which can be converted into aromatic compounds and phenylpropanoids in response to disease resistance ( Geng et al., 2020 ). In addition, phenylalanine ammonia-lyase (PAL) is a crucial enzyme in secondary phenylalanine metabolism and is widely studied in plant responses to biotic stresses ( Kurihara and Yamana, 2022 ; Mansoor et al., 2023 ). We observed that many DEGs and DAMs were enriched in the phenylalanine, tyrosine, and tryptophan biosynthesis pathways in both groups. Within these pathways, the expression of PAL gene MD11G1223500 was significantly increased after being infected. These metabolites and genes in the phenylpropanoid pathway were associated with the resistance of apple to C. mali infestation, and need further exploration.
The infection of apples by C. mali disrupts the cell structure and secretes pathogenic virulence factors. In response to C. mali infection, apples have evolved multiple strategies. Our study indicates that apples primarily resist C. mali infection by mitigating cell wall degradation, activating the PAL activity, and promoting the synthesis of defensive substances.
5 Conclusion
This study conducted a comparative analysis of the metabolome and transcriptome of ‘Jinhong’ apple branches between control and AVC inoculation. Pathway analysis of the apple resistance response revealed significant changes in several metabolic pathways, including galactose metabolism, fructose and mannose metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glycine, serine and threonine metabolism, and isoquinoline alkaloid biosynthesis, during the C. mali inoculation. Moreover, the transcriptional and metabolic patterns of the affected tissues were altered. The production and accumulation of several enzymes and metabolites, including β-Gal, UGP-glucose, ALA, betaine, and PAL may play a crucial role in the presentation of apples when challenged by C. mali . These results shed light on the potential molecular mechanisms underlying the infection of AVC in ‘Jinhong’ branches, further exploration of DEGs and DEMs are in need in future work.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary material .
Author contributions
JZ: Formal analysis, Investigation, Writing – original draft, Methodology. YG: Data curation, Software, Writing – original draft. ZL: Resources, Writing – review & editing, Project administration. YS: Methodology, Writing – original draft. PS: Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant numbers: 32060594 and 31860540); Natural Science Foundation of Inner Mongolia, China (2023LHMS03020); and Start-up program of innovation and entrepreneurship for returned overseas Chinese scholars in the Inner Mongolia autonomous region (DC2100001765).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1394447/full#supplementary-material
1. ^ https://www.metaboanalyst.ca
2. ^ https://bio-cloud.aptbiotech.com
3. ^ https://cloud.metware.cn
4. ^ https://www.majorbio.com
5. ^ http://www.omic smart.com
6. ^ http://www.bioinformatics.com.cn
Alves, M. S., Dadalto, S. P., Goncalves, A. B., Barros, V. A., and Fietto, L. G. (2013). Plant bZIP transcription factors responsive to pathogens: a review. Int. J. Mol. Sci. 14, 7815–7828. doi: 10.3390/ijms14047815
PubMed Abstract | Crossref Full Text | Google Scholar
Bizuneh, G. K. (2020). The chemical diversity and biological activities of phytoalexins. Adv. Tradit. Med. 21, 1–13. doi: 10.1007/s13596-020-00442-w
Crossref Full Text | Google Scholar
Cruz-Cano, R., and Lee, M. L. T. (2014). Fast regularized canonical correlation analysis. Comput. Stat. Data Anal. 70, 88–100. doi: 10.1016/j.csda.2013.09.020
Darren, K., Matt, C., Robert, B., David, A., and Parag, M. (2008). ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics (Oxford, England) 24, 2534–2536. doi: 10.1093/bioinformatics/btn323
De Carvalho, C. C. R., and Caramujo, M. J. (2018). The various roles of fatty acids. Molecules (Basel, Switzerland) 23:2583. doi: 10.3390/molecules23102583
Dietz, K. J., Vogel, M. O., and Viehhauser, A. (2010). AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 245, 3–14. doi: 10.1007/s00709-010-0142-8
Domingo-Almenara, X., and Siuzdak, G. (2020). Metabolomics data processing using XCMS. Methods Mol. Biol. (Clifton, N.J.) 2104, 11–24. doi: 10.1007/978-1-0716-0239-3_2
Du, Y. W., Jia, H. C., Yang, Z., Wang, S. H., Liu, Y. Y., Ma, H. Y., et al. (2023). Sufficient coumarin accumulation improves apple resistance to Cytospora mali under high potassium status. Plant Physiol. 192, 1396–1419. doi: 10.1093/plphys/kiad184
Feng, H., Wang, C. L., He, Y. T., Tang, L., Han, P. L., Liang, J. H., et al. (2023). Apple Valsa canker: insights into pathogenesis and disease control. Phytopathol. Res. 5:45. doi: 10.1186/s42483-023-00200-1
Geng, D. L., Shen, X. M., Xie, Y. P., Yang, Y. S., Bian, R. L., Gao, Y. Q., et al. (2020). Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Horticult. Res. 7:102. doi: 10.1038/s41438-020-0324-2
Han, P. L., Zhang, R. T., Li, R., Li, F. D., Nie, J. J., Xu, M., et al. (2023). MdVQ12 confers resistance to Valsa Mali by regulating MdHDA19 expression in apple. Mol. Plant Pathol. 25:e13411. doi: 10.1111/mpp.13411
Haxim, Y., Kahar, G., Zhang, X. C., Si, Y., Waheed, A., Liu, X., et al. (2022). Genome-wide characterization of the chitinase gene family in wild apple ( Malus sieversii ) and domesticated apple ( Malus domestica ) reveals its role in resistance to Valsa Mali. Front. Plant Sci. 13, –1007936. doi: 10.3389/fpls.2022.1007936
Huang, S., Zuo, T., and Ni, W. Z. (2020). Important roles of glycinebetaine in stabilizing the struture and function of the photosystem II complex under abiotic stersses. Planta 251:36. doi: 10.1007/s00425-019-03330-z
Jiang, J. J., Ma, S. H., Ye, N. H., Jiang, M., Cao, J. S., and Zhang, J. H. (2017). WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 59, 86–101. doi: 10.1111/jipb.12513
Jokinen, K., Salovaara, A. K., Wasonga, D. O., Edelmann, M., Simpura, I., and Mäkelä Pirjo, S. A. (2022). Root-applied glycinebetaine decreases intrate accumulation and improves quality in hydroponi cally grown lettuce. Food Chem. 366:130558. doi: 10.1016/j.foodchem.2021.130558
Ke, X. W., Yin, Z. Y., Song, N., Dai, Q. Q., Voegele, R. T., Liu, Y. Y., et al. (2014). Transcriptome profiling to identify genes involved in pathogenicity of Valsa Mali on apple tree. Fungal Genet. Biol. 68, 31–38. doi: 10.1016/j.fgb.2014.04.004
Kurihara, J., and Yamana, T. (2022). Detection of apple Valsa canker based on hyperspectral imaging. Remote Sens. 14:1420. doi: 10.3390/rs14061420
Li, C. R., Jing, Y. Y., Cheng, L., Si, Z. G., Mou, Z. Y., Niu, D. S., et al. (2023). The antifungal activity of trans-cinnamic acid and its priming effect in apple in response to Valsa Mali. Plant Pathol. 72, 1595–1603. doi: 10.1111/ppa.13786
Li, P. Q., Ruan, Z., Fei, Z. X., Yan, J. J., and Tang, G. H. (2021). Integrated transcriptome and metabolome analysis revealed that flavonoid biosynthesis may dominate the resistance of Zanthoxylum bungeanum against stem canker. J. Agric. Food Chem. 69, 6360–6378. doi: 10.1021/acs.jafc.1c00357
Li, X. L., Su, Q. F., Jia, R. J., Wang, Z. D., Fu, J. H., Guo, J. H., et al. (2023). Comparison of cell wall changes of two different types of apple cultivars during fruit development and ripening. J. Integr. Agric. 22, 2705–2718. doi: 10.1016/j.jia.2023.07.019
Li, C. Y., Zhang, T. P., Feng, P. W., Li, D. X., Brestic, M., Liu, Y., et al. (2021). Genetic engineering of glycinebetaine synthesis enhances cadmium tolerance in BADH-transgenic tobacco plants viareducing cadmium stress damage. Environ. Exp. Bot. 191:104602. doi: 10.1016/j.envexpbot.2021.104602
Liu, Y. T., Xu, R. X., Wang, H. T., Shi, Y. J., Li, C. Y., Ma, F. W., et al. (2022). Exogenous glycine betaine improved the resistance of apple to Glomerella leaf spot. J. Fruit Sci. 39, 1252–1261. doi: 10.13935/j.cnki.gsxb.20210566
Liu, X. M., Yuan, X., Zhang, Z. J., Wang, Y. P., Ma, F. W., and Li, C. (2021). Dopamine enhances the resistance of apple to Valsa Mali infection. Phytopathology 112, 1141–1151. doi: 10.1094/PHYTO-08-21-0328-R
Liu, X. Z., Zhang, Y. H., Zhou, T., Li, X. S., Wen, X. J., and Zhang, D. Y. (2021). Full-length transcriptome-wide characteristic and functional identification of WRKY family in Malus sieversii during the Valsa canker disease response. Forests 12:790. doi: 10.3390/f12060790
Lui, S., Luo, C. G., Zhu, L. M., Sha, R. H., Qu, S. C., Cai, B. H., et al. (2017). Identification and expression analysis of WRKY transcription factor genes in response to fungal pathogen and hormone treatments in apple ( Malus domestica ). J. Plant Biol. 60, 215–230. doi: 10.1007/s12374-016-0577-3
Ma, Q., Ju, M. Y., Liu, Q., Zhang, L., Li, Z. N., and Sun, P. P. (2020). Identification of the pathogenic fungi associated with apple valsa cancer in Inner Mongolia, China. J. Fruit Sci. 37, 714–722. doi: 10.13925/j.cnki.gsxb.20190474
Mansoor, S., Sakina, A., Mir, M. A., Mir, J. I., Wani, A. A., Un Nabi, S., et al. (2023). Elucidating the role of reactive oxygen species metabolism and phenylpropanoid pathway during an incompatible interaction between apple-Venturia inaequalis host-pathosystem. S. Afr. J. Bot. 160, 428–436. doi: 10.1016/j.sajb.2023.07.020
Mao, X., Wang, C., Lv, Q., Tian, Y., Wang, D., Chen, B., et al. (2021). Cyclic nucleotide gated channel genes (CNGCs) in Rosaceae: genome-wide annotation, evolution and the roles on Valsa canker resistance. Plant Cell Rep. 40, 2369–2382. doi: 10.1007/s00299-021-02778-2
McCarthy, D. J., Chen, Y., and Smyth, G. K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. doi: 10.1093/nar/gks042
Meng, X. L., Qi, X. H., Han, Z. Y., Guo, Y. B., Wang, Y. N., Hu, T. L., et al. (2019). Latent infection of Valsa Mali in the seeds, seedlings and twigs of crabapple and apple trees is a potential inoculum source of Valsa canker. Sci. Rep. 9, 1–10. doi: 10.1038/s41598-019-44228-w
Moneo-Sánchez, M., Izquierdo, L., Martín, I., Hernández-Nistal, J., Albornos, L., Dopico, B., et al. (2018). Knockout mutants of Arabidopsis thaliana β-galactosidase. Modifications in the cell wall saccharides and enzymatic activities. Biol. Plant. 62, 80–88. doi: 10.1007/s10535-017-0739-2
Morris, J. H., Apeltsin, L., Newman, A. M., Baumbach, J., Wittkop, T., Su, G., et al. (2011). ClusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinf. 12:436. doi: 10.1186/1471-2105-12-436
Muñoz-Hoyos, L., and Stam, R. (2023). Metabolomics in plant pathogen-defence: from single molecules to large scale analysis. Phytopathology 113, 760–770. doi: 10.1094/PHYTO-11-22-0415-FI
Per, T. S., Khan, M. I. R., Anjum, N. A., Masood, A., Hussain, S. J., and Khan, N. A. (2018). Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ. Exp. Bot. 145, 104–120. doi: 10.1016/j.envexpbot.2017.11.004
Phukan, U. J., Jeena, G. S., and Shukla, R. K. (2016). WRKY transcription factors: molecular regulation and stress responses in plants. Front. Plant Sci. 7:760. doi: 10.3389/fpls.2016.00760
Rohart, F., Gautier, B., Singh, A., and Lê Cao, K. A. (2017). Mix omics: an R package for 'omics feature selection and multiple data integration. PLoS Comput. Biol. 13:e1005752. doi: 10.1371/journal.pcbi.1005752
Sebastian, W. (2022). Cell Wall signaling in plant development and defense. Annu. Rev. Plant Biol. 73, 323–353. doi: 10.1146/annurev-arplant-102820-095312
Sung, W. S., and Lee, D. G. (2010). Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 82, 219–226. doi: 10.1351/PAC-CON-09-01-08
Tsuda, K., and Somssich, I. E. (2015). Transcriptional networks in plant immunity. New Phytol. 206, 932–947. doi: 10.1111/nph.13286
Utriainen, M., and Morris, J. H. (2023). Cluster maker 2: a major update to cluster maker, a multi-algorithm clustering app for Cytoscape. BMC Bioinformatics 24:134. doi: 10.1186/s12859-023-05225-z
Varet, H., Brillet-Guéguen, L., Coppée, J., and Dillies, M. (2017). SARTools: a DESeq2-and edge R-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS One 11:e0157022. doi: 10.1371/journal.pone.0157022
Wang, S. S., Chen, K., Zhang, J. Y., Wang, J. Q., Li, H. S., Yang, X. Y., et al. (2022). Genome-wide characterization of MATE family members in Cucumis melo L. and their expression profiles in response to abiotic and biotic stress. Horticult. Plant J. 8, 474–488. doi: 10.1016/j.hpj.2022.05.004
Wang, D. L., Liu, B., Ma, Z. Q., Feng, J. T., and Yan, H. (2020). Reticine A, a new potent natural elicitor: isolation from the fruit peel of Citrus reticulate and induction of systemic resistance against tobacco mosaic virus and other plant fungal diseases. Pest Manag. Sci. 77, 354–364. doi: 10.1002/ps.6025
Wang, X. L., Shi, C. M., Gleason, M. L., and Huang, L. L. (2020). Fungal species associated with apple Valsa canker in East Asia. Phytopathol. Res. 2:14. doi: 10.1186/s42483-020-00076-5
Wen, X. Z., Yuan, J. X., Bozorov, T. A., Waheed, A., Kahar, G., Haxim, Y., et al. (2023). An efficient screening system of disease-resistant genes from wild apple, Malus sieversii in response to Valsa Mali pathogenic fungus. Plant Methods 19:138. doi: 10.1186/s13007-023-01115-w
Wu, T. Z., Hu, E. Q., Xu, S. B., Chen, M. J., Guo, P. F., Dai, Z. H., et al. (2021). Cluster profiler 4.0: a universal enrichment tool for interpreting omics data. Innovations 2:100141. doi: 10.1016/j.xinn.2021.100141
Xin, L., Zhang, R., Wang, X. P., Liu, X. X., Wang, Y. C., Qi, P. R., et al. (2023). Extracellular and intracellular infection of Botryosphaeria dothidea and resistance mechanism in apple cells. Horticult. Plant J. 9, 209–223. doi: 10.1016/j.hpj.2022.05.001
Yin, Z. Y., Ke, X. W., Kang, Z. S., and Huang, L. L. (2016). Apple resistance responses against Valsa Mali revealed by transcriptomics analyses. Physiol. Mol. Plant Pathol. 93, 85–92. doi: 10.1016/j.pmpp.2016.01.004
Yin, Z. Y., Liu, H. Q., Li, Z. P., Ke, X. W., Dou, D. L., Gao, X. N., et al. (2015). Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol. 208, 1202–1216. doi: 10.1111/nph.13544
Yoav, B., and Yosef, H. (2000). On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Stat. 25:60. doi: 10.2307/1165312
Yu, X. Y., Bi, Y., Yan, L., Liu, X., Wang, Y., Shen, K. P., et al. (2016). Activation of phenylpropanoid pathway and PR of potato tuber against fusarium sulphureum by fungal elicitor from Trichothecium roseum . World J. Microbiol. Biotechnol. 32:142. doi: 10.1007/s11274-016-2108-2
Zang, R., Huang, L., Kang, Z., and Wang, X. (2007). Biological characteristics and pathogenicity of different isolates of Cytospora spp. isolated from apple trees in Shaanxi province. Acta Phytopathol. Sin. 37, 343–351. doi: 10.3321/j.issn:0412-0914.2007.04.002
Zhao, D., Duo, H., Lv, Q. Q., Tian, Y. Z., and Zuo, C. W. (2021). β-Galactosidase gene family responds to the signal of Valsa mail in apple. Plant Physiol. J. 57, 1319–1328. doi: 10.13592/j.cnki.ppj.2020.0515
Zhao, L. N., Shu, Y. L., Liang, L. Y., Wang, Y. J., Esa, A. G., Zhang, X. Y., et al. (2022). Integrated transcriptomic and metabonomic analysis reveal mechanisms of disease resistance in apples induced by Wickerhamomyces anomalus. Biol. Control 173:105005. doi: 10.1016/j.biocontrol.2022.105005
Zhou, K., Hu, L. Y., Li, Y. T. S., Chen, X. F., Zhang, Z. J., Liu, B. B., et al. (2019). MdUGT88F1-mediated Phloridzin biosynthesis regulates apple development and Valsa canker resistance. Plant Physiol. 180, 2290–2305. doi: 10.1104/pp.19.00494
Zhou, K., Hu, L. Y., Yue, H., Zhang, Z. J., Zhang, J. Y., Gong, X. Q., et al. (2021). MdUGT88F1-mediated phloridzin biosynthesis coordinates carbon and nitrogen accumulation in apple. J. Exp. Bot. 73, 886–902. doi: 10.1093/jxb/erab410
Zhou, G. Y., Pang, Z. Q., Lu, Y., Jessica, E., and Xia, J. G. (2022). Omics net 2.0: a web-based platform for multi-omics integration and network visual analytics. Nucleic Acids Res. 50, W527–W533. doi: 10.1093/nar/gkac376
Zuo, C. W., Mao, J., Chen, Z. J., Chu, M. Y., Duo, H., and Chen, B. H. (2018). RNA sequencing analysis provides new insights into dynamic molecular responses to Valsa Mali pathogenicity in apple ‘Changfu no. 2’. Tree Genet. Genomes 14, 1–10. doi: 10.1007/s11295-018-1288-3
Keywords: apple, Cytospora mali , transcriptome, metabolome, conjoint analysis
Citation: Zhao J, Guo Y, Li Z, Shi Y and Sun P (2024) Integrated transcriptome and metabolome profiling reveals mechanisms underlying the infection of Cytospora mali in “Jin Hong” branches. Front. Microbiol . 15:1394447. doi: 10.3389/fmicb.2024.1394447
Received: 01 March 2024; Accepted: 08 April 2024; Published: 24 April 2024.
Reviewed by:
Copyright © 2024 Zhao, Guo, Li, Shi and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingping Sun, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
• The photolyase mechanism is no longer working in humans and other placental mammals who instead rely on the less efficient nucleotide excision repair mechanism 23. • Photolyases bind complementary DNA strands and break certain types of pyrimidine dimers that arise when a pair of thymine or cytosine bases on the same strand of DNA become ...
The most common means of repairing damage or a mismatch is to cut it out of the duplex DNA and recopy the remaining complementary strand of DNA, as outlined in Figure 7.12. Three different types of excision repair have been characterized: nucleotide excision repair, base excision repair, and mismatch repair.
Replication errors, DNA base mismatches and topoisomerase-DNA complexes. Every time a human cell replicates, approximately 3 X 10 9 bases are copied over by high fidelity replicative polymerases (δ and ε). However, a battery of other DNA polymerases (α, β, σ, γ, λ, REV1, ζ, η, ι, κ, θ, ν, μ, Tdt and PrimPol) can carry out lower fidelity DNA synthesis during DNA replication or ...
Repairing Damaged Bases. Damaged or inappropriate bases can be repaired by several mechanisms: Direct chemical reversal of the damage; Excision Repair, in which the damaged base or bases are removed and then replaced with the correct ones in a localized burst of DNA synthesis.There are three modes of excision repair, each of which employs specialized sets of enzymes.
Nucleotide Excision repair. Nucleotide excision repair. UvrA bulky. Two excinucleases (excision endonucleases) bind DNA at the site of bulky lesion. One cleaves the 5' side and the other cleaves the 3' side of the lesion, and the DNA segment is removed by a helicase. (c) DNA polymerase fills in the gap and (d) DNA ligase seals the nick.
The DNA Double Helix Is Readily Repaired. The double-helical structure of DNA is ideally suited for repair because it carries two separate copies of all the genetic information—one in each of its two strands. Thus, when one strand is damaged, the complementary strand retains an intact copy of the same information, and this copy is generally used to restore the correct nucleotide sequences to ...
DNA damage is a critical factor contributing to genetic alterations, directly affecting human health, including developing diseases such as cancer and age-related disorders. DNA repair mechanisms play a pivotal role in safeguarding genetic integrity and preventing the onset of these ailments. Over the past decade, substantial progress and ...
In contrast, during HRR, the homologous chromosome itself is used as a template for repair. Mutations in an organism's DNA are a part of life. Our genetic code is exposed to a variety of insults ...
Figure 13.7.1 13.7. 1: Proofreading by DNA polymerase corrects errors during replication. Some errors are not corrected during replication, but are instead corrected after replication is completed; this type of repair is known as mismatch repair (Figure 13.7.2 13.7. 2 ). The enzymes recognize the incorrectly added nucleotide and excise it; this ...
Direct reversal repair is a DNA repair mechanism that directly fixes specific types of DNA damage without the need for excision or replacement. Two examples of DNA damage that can be reversed are UV-induced lesions and alkylated bases. UV-induced lesions, caused by UV light, can be reversed through a process called photoreactivation, which uses ...
cellular mechanisms—DNA replication and DNA repair—that are responsible for keeping these changes to a minimum. Finally, we consider some of the most intriguing ways in which DNA sequences are altered by cells, with a focus on DNA recombination and the movement of special DNA sequences in our chro-mosomes called transposable elements.
DNA damage response Sensors in the DNA damage response. To avoid DNA damage, cells have evolved numerous interacting mechanisms for ensuring genomic stability or have even used DNA damage to ...
The genetic information stored in DNA is under continuous threat by endogenous and environmental sources of DNA damage. Cells have evolved multiple DNA repair pathways that function in overlapping manners, with principles shared across species. Here, we depict the main DNA repair pathways cells rely on, with the primary lesions they are tackling, along with key players and main DNA transactions.
with each DNA repair mechanism. 2.3.1 Base Excision Repair (BER) BER, as the name implies, is the predominant mechanism responsible for the repair of damaged DNA bases that, in contrast to NER (see Sect. 2.3.3), do not signifi-cantly distort the overall structure of the DNA helix (for detailed review of BER, see [34]).
Where DNA repair fails, additional mechanisms have evolved to enable cells to cope with the damage.Figure 5.19Spontaneous damage to DNAThere are two major forms of spontaneous DNA damage: (A) deamination of adenine, cytosine, and guanine, and (B) depurination (loss of purine bases) resulting from cleavage of the bond between the purine bases ...
DNA damage resulting in multiple broken chromosomes. DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA damage, resulting in tens of thousands of individual molecular lesions per cell per day.
Figure 9.21 illustrates nucleotide excision repair for a pyrimidine dimer. Figure 9.21: In nucleotide excision repair, (1) a fragment containing the damaged DNA, e.g., a pyrimidine (C-T) dimer is excised; (2) a polymerase fills in the missing nucleotides in the gap, and (3) a DNA ligase seals the remaining nick, resulting repaired DNA strands.
Action of DNA glycosylases. These enzymes hydrolyze the glycosidic bond of their corresponding altered base (red) Nucleotide excision repair • Mechanism used to replace DNA upto 30 base pairs. • Excision endonuclease (exinuclease) identifies and cleaves a fragment of DNA of around 20 -25 base pairs. • G1 phase of cell cycle.
Researchers have discovered a DNA repair mechanism that advances understanding of how human cells stay healthy, and which could lead to new treatments for cancer and premature aging.
From the archives: On DNA maintenance - SWI/SNF chromatin remodeling complexes, DNA damage repair, and transposon excision repair mechanisms The H3K4 demethylase JMJ1 is required for proper timing of flowering in Brachypodium distachyon
Cockayne syndrome: New insights into cellular DNA repair mechanism. ScienceDaily. Retrieved April 19, 2024 from www.sciencedaily.com / releases / 2024 / 04 / 240410112659.htm.
This review summarizes common molecular strategies viruses deploy to exploit host DNA repair mechanisms. Viruses are obligate intracellular pathogens that hijack a myriad of host cell processes to facilitate replication and suppress host antiviral defenses. In its essence, a virus is a segment of foreign nucleic acid that engages host cell ...
No headers. Strictly defined, the simplest repair mechanism does not use an enzyme. Dealkylation, or removal of alkyl groups (like —CH 3 or —C 2 H 5) involves only the transfer of an alkyl group from an O6-methylguanine or O6-ethylguanine onto O6-alkylguanyl-DNA alkyltransferase.Despite the name, the alkyltransferase is not really an enzyme, since it is permanently altered and inactivated ...
Uveal melanoma (UVM) is the most common primary intraocular tumor in adults, with a median survival of 4-5 months following metastasis. DNA damage response (DDR) upregulation in UVM, which could be linked to its frequent activation of the PI3K/AKT pathway, contributes to its treatment resistance. We have reported that embryonic stem cell microenvironments (ESCMe) can revert cancer cells to ...
Persistent damage to the DNA over time results in an increased risk of mutation and an accumulation of mutations with age. Loss of efficient DNA damage repair can lead to accelerated ageing phenotypes or an increased cancer risk, and the trade-off between cancer susceptibility and longevity is often driven by the cell's response to DNA damage.
Replication Protein A (RPA), a single-stranded DNA (ssDNA) binding protein, is vital for various aspects of genome maintenance such as replication, recombination, repair and cell cycle checkpoint activation. Binding of RPA to ssDNA protects it from degradation by cellular nucleases, prevents secondary structure formation and illegitimate recombination. In our current study, we identified the ...
mismatch repair: a system for recognizing and repairing some forms of DNA damage and erroneous insertion, deletion, or mis-incorporation of bases that can arise during DNA replication and recombination. nucleotide excision repair: a DNA repair mechanism that corrects damage done by UV radiation, including thymine dimers and 6,4 photoproducts ...
Briefly, mRNA was enriched by poly A tail selection and chemically fragmented. Subsequently, second strand cDNA synthesis was performed using DNA Polymerase I and RNase H. The synthesized cDNA was then subjected to end-repair and "A" base addition according to Illumina's library construction protocol.